Abstract
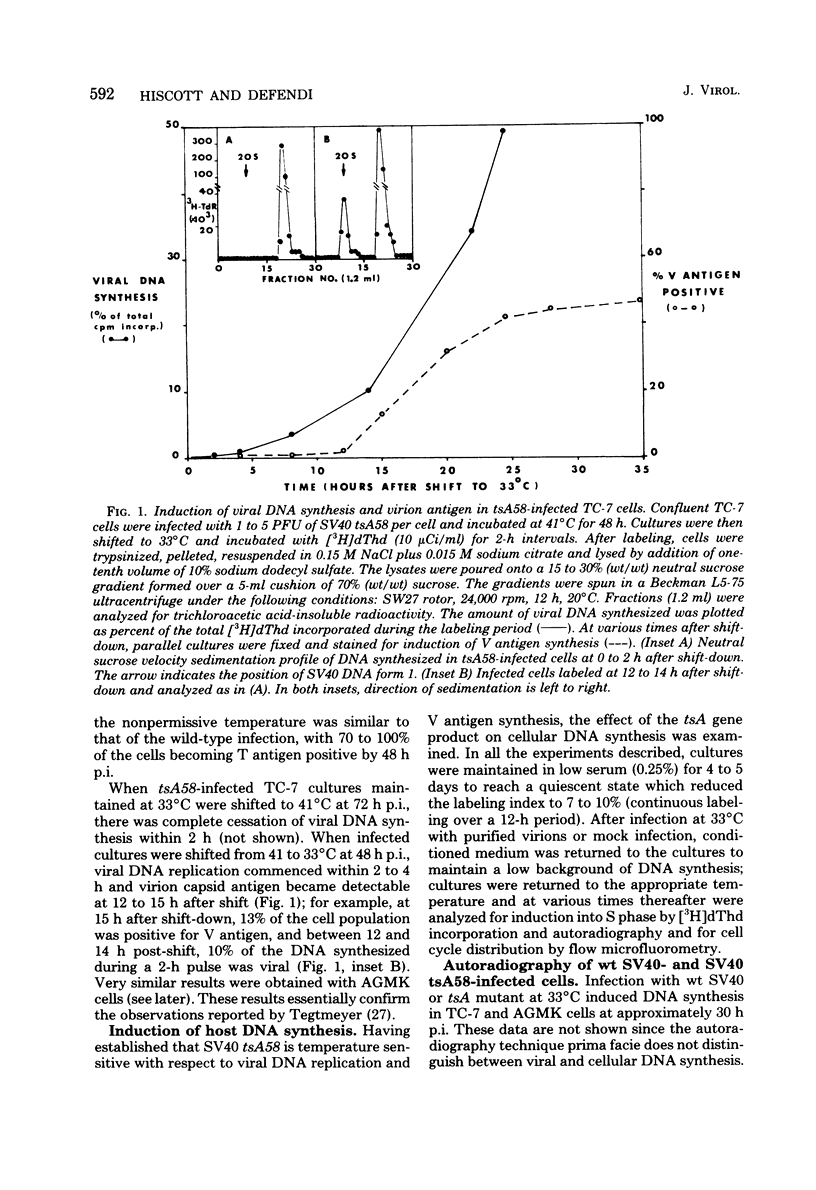
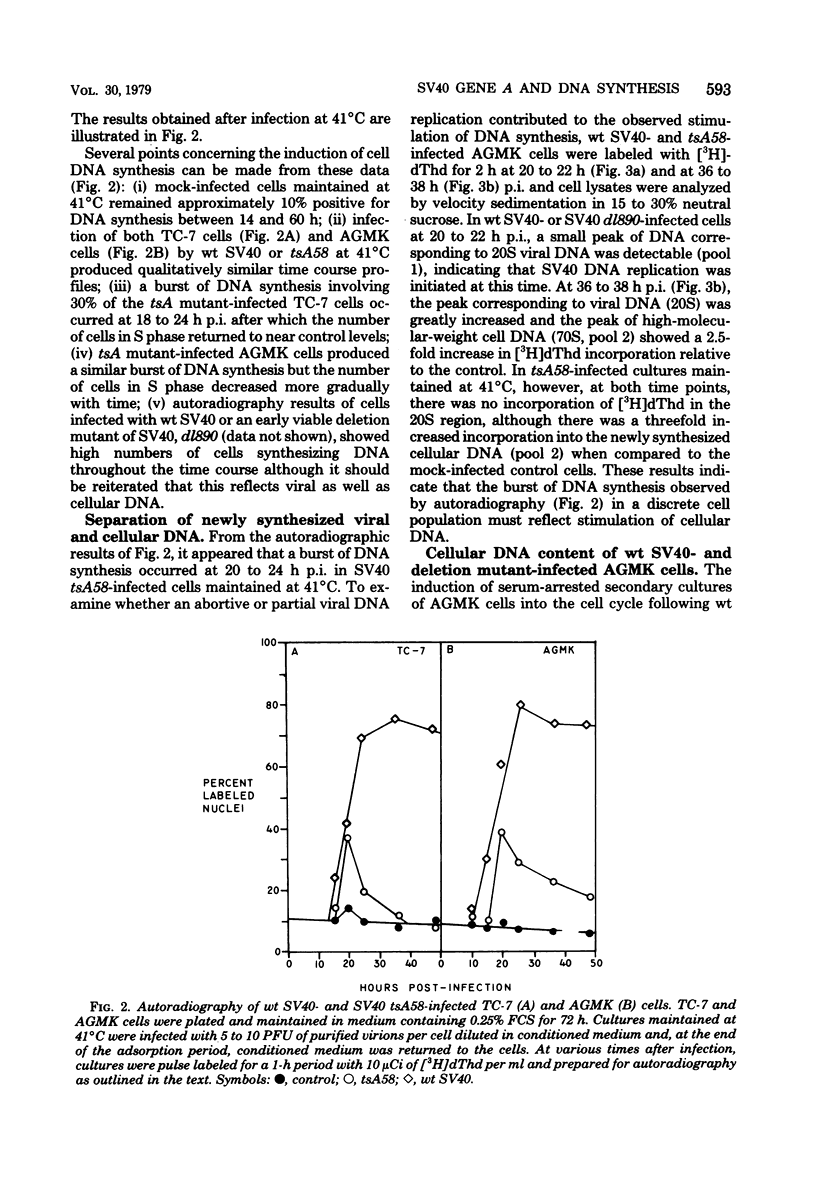
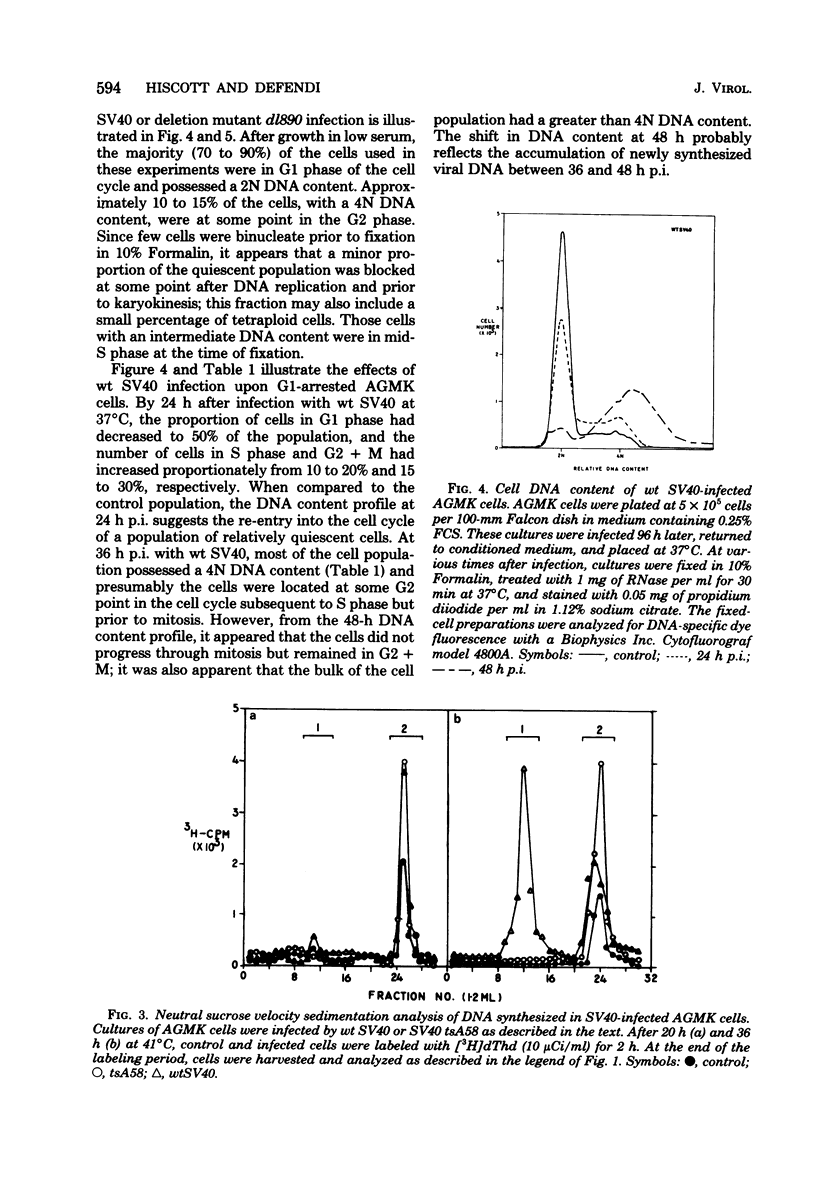
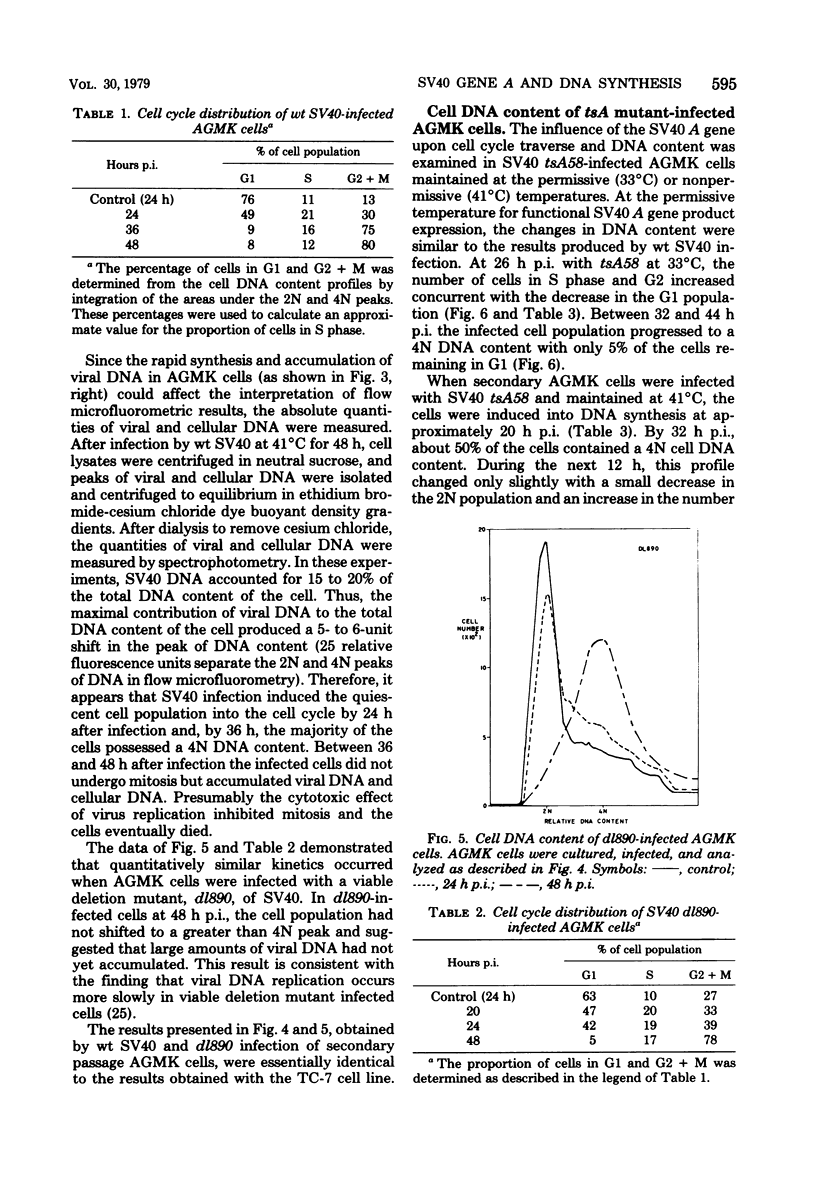
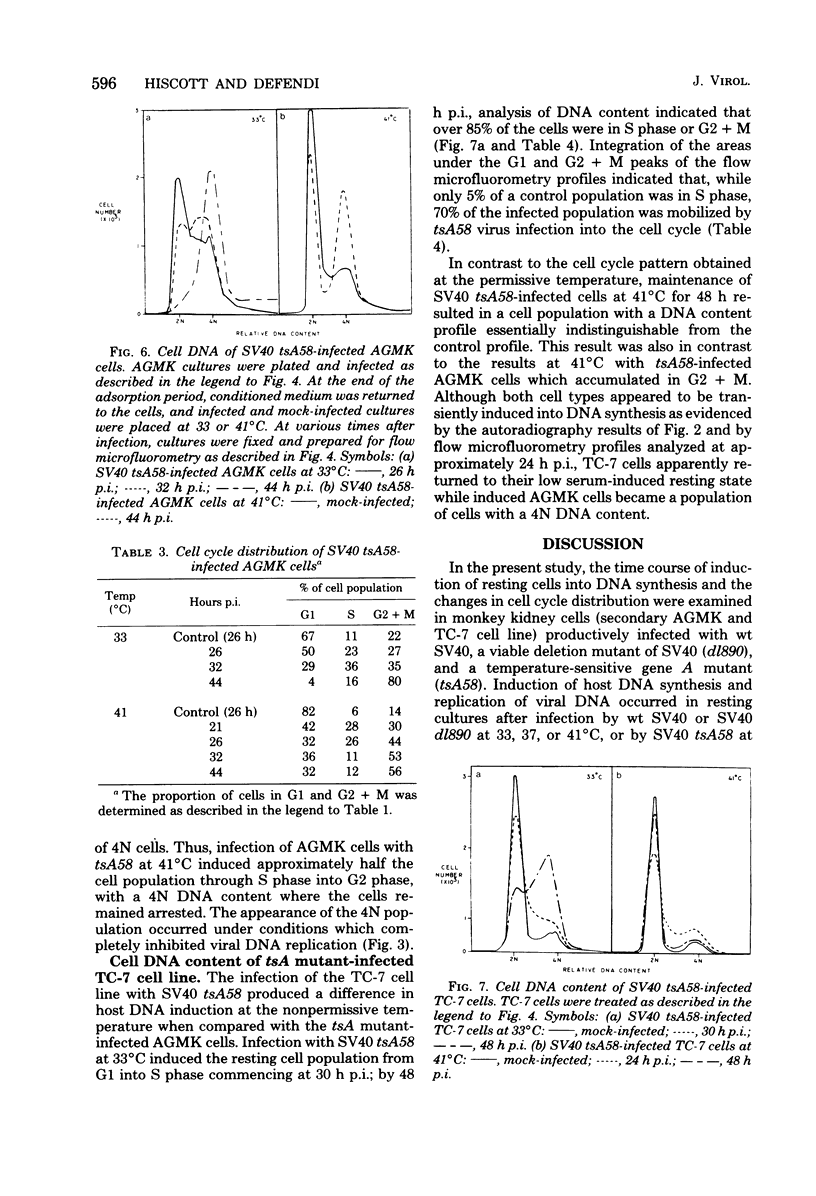
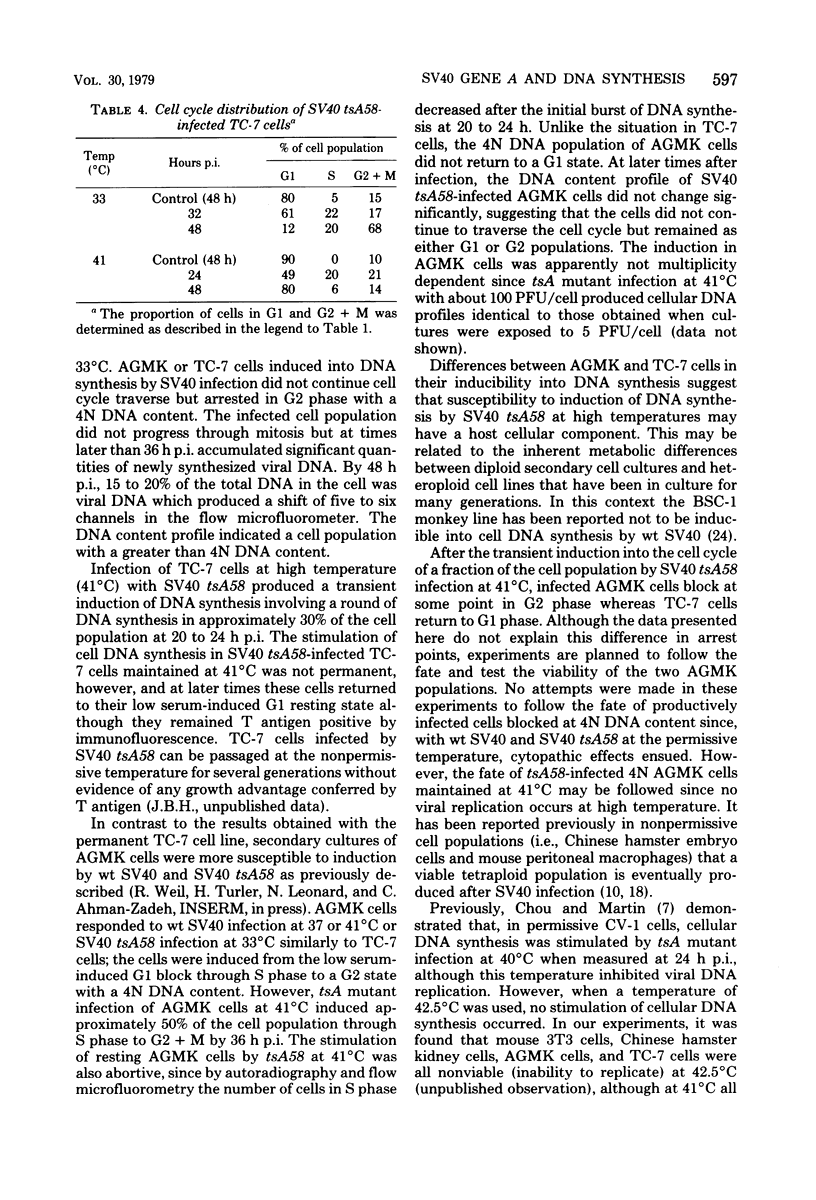
The kinetics of host cellular DNA stimulation by simian virus 40 (SV40) tsA58 infection was studied by flow microfluorometry and autoradiography in two types of productively infected monkey kidney cells (AGMK, secondary passage, and the TC-7 cell line). Prior to infection, the cell populations were maintained predominantly in G0-G1 hase of the cell cycle by low (0.25%) serum concentration. Infection of TC-7 or AGMK cells by wild-type SV40, viable deletion mutant dl890, or by SV40 tsA58 at 33 degrees C induced cells through S phase after which they were blocked with a 4N DNA content in the G2 phase. The infection of TC-7 cells by tsA58 at 41 degrees C, which was a nonpermissive temperature for viral DNA replication, induced a round of cell DNA synthesis in approximately 30% of the cell population. These cells proceeded through S phase but then re-entered the G1 resting state. In contrast, infection of AGMK cells by tsA58 at 41 degrees C induced DNA synthesis in approximately 50% of the cells, but this population remained blocked in the G2 phase. These results indicate that the mitogenic effect of the A gene product upon cellular DNA is more heat resistant than its regulating activity on viral DNA synthesis and that the extent of induction of cell DNA synthesis by the A gene product may be influenced by the host cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Chang C., Mora P. T., Martin R. G. Expression and thermal stability of simian virus 40 tumor-specific transplantation antigen and tumor antigen in wild type- and tsA mutant-transformed cells. J Virol. 1977 Feb;21(2):459–467. doi: 10.1128/jvi.21.2.459-467.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Soule H. R. Role of the simian virus 40 gene A product in regulation of DNA synthesis in transformed cells. J Virol. 1978 Jun;26(3):584–594. doi: 10.1128/jvi.26.3.584-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Mullaney P. F., Steinkamp J. A. Methods and applications of flow systems for analysis and sorting of mammalian cells. Methods Cell Biol. 1975;9(0):179–246. doi: 10.1016/s0091-679x(08)60076-x. [DOI] [PubMed] [Google Scholar]
- Ide T., Whelly S., Baserga R. Stimulation of RNA synthesis in isolated nuclei by partially purified preparations of simian virus 40 T-antigen. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3189–3192. doi: 10.1073/pnas.74.8.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino T., Yamaguchi N. Characterization of T antigen in cells infected with a temperature-sensitive mutant of simian virus 40. J Virol. 1975 Jun;15(6):1302–1307. doi: 10.1128/jvi.15.6.1302-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Defendi V. Changes in deoxyribonucleic acid synthesis regulation in Chinese hamster cells infected with simian virus 40. J Virol. 1970 Dec;6(6):738–749. doi: 10.1128/jvi.6.6.738-749.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M., Mauel J., Defendi V. Regulation of DNA synthesis in macrophages infected with semian virus 40. Exp Cell Res. 1971 Jul;67(1):230–233. doi: 10.1016/0014-4827(71)90644-6. [DOI] [PubMed] [Google Scholar]
- Mauel J., Defendi V. Infection and transformation of mouse peritoneal macrophages by simian virus 40. J Exp Med. 1971 Aug 1;134(2):335–350. doi: 10.1084/jem.134.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Levine A. J. The requirement of simian virus 40 gene A product for the stimulation of cellular thymidine kinase activity after viral infection. Virology. 1976 Aug;73(1):206–215. doi: 10.1016/0042-6822(76)90075-1. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The cell cycle and the control of cellular reproduction. Adv Genet. 1976;18:99–177. doi: 10.1016/s0065-2660(08)60438-1. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. 3. Comparison of simian virus 40 lytic infection in three different monkey kidney cell lines. J Virol. 1970 Jun;5(6):686–692. doi: 10.1128/jvi.5.6.686-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]



