Abstract
Dapper homolog 1 (DACT1) is a disheveled partner in the planar cell polarity pathway. By using genome-wide promoter methylation screening, dapper homolog 1 (DACT1) was found to be frequently methylated in gastric cancer. We aim to clarify its epigenetic inactivation, biological function and clinical implication in gastric cancer. We demonstrated that DACT1 was silenced in 7 of 10 gastric cancer cell lines and in primary gastric cancers. Transcriptional gene silence of DACT1 was mainly regulated by promoter hypermethylation. Ectopic expression of DACT1 in silenced gastric cancer cell lines (AGS, BGC823 and MGC803) by stable transfection suppressed colony formation (P < 0.001), induced cell apoptosis (P < 0.01) and retarded tumorigenesis in nude mice (P < 0.001). The tumor suppressive effect of DACT1 was further confirmed by loss of DACT1 function experiment. The proapoptotic and antiproliferative effect by DACT1 was associated with inhibition of nuclear factor (NF)-κB activation and its downstream factors, including B-cell CLL/lymphoma-2, Bcl-X, interleukin-8 and tumor necrosis factor-α. Moreover, promoter methylation of DACT1 was detected in 29.3% (60/205) of primary gastric tumors. DACT1 methylation was significantly associated with tumor metastasis (P < 0.05), invasion (P < 0.05) and advanced tumor stage (P < 0.0005). These findings provided insight into the role of DACT1 as a novel functional tumor suppressor in gastric cancer through inhibiting NF-κB signaling pathway. Promoter methylation of DACT1 is associated with tumor aggressiveness.
INTRODUCTION
Gastric cancer is one of the most common malignancies and remains the second leading cause of cancer-related death worldwide (1). However, the mechanism leading to gastric cancer development remains elusive. Hypermethylation of CpG island in the promoter region of tumor suppressor gene is associated with transcriptional gene silence and contributes to the development and progression of gastric carcinogenesis (2–4). Thus, identification of novel genes silenced by methylation may shed light on the mechanisms for the inactivation of tumor suppressive pathways and provide epigenetic biomarkers for tumor diagnosis and prognosis prediction. We used a novel approach by genome-wide promoter methylation analysis to identify hypermethylation silenced genes in tumors and identified dapper homolog 1 (DACT1) as a potential tumor suppressor gene frequently silenced by methylation in gastric cancer (5).
Dapper homolog 1 (DACT1), a member of DACT family, is an important regulator in the planar cell polarity (PCP) pathway (6–8). As a critical branch of noncanonical WNT signaling, PCP pathway is activated by the binding of noncanonical Wnt proteins to transmembrane receptors (Frizzled), which leads to the recruitment of cytoplasmic Dishevelled (Dvl) to the plasma membrane. Dvl converts upstream PCP signaling into specific downstream programs, leading to cytoskeleton reorganization in cell movement and polarity (9). Upstream PCP components include transmembrane Frizzled, Vang-like (Vangl) and cytoplasmic Dvl and Prickle, which control the downstream PCP executors (for example, Ras-related C3 botulinum toxin substrate [Ras], c-Jun N-terminal kinases [JNKs] and Profilin) engaged in cytoskeleton regulation (9). The PCP pathway affects diverse cellular processes and plays an important role in cancer development (9). DACT1 is a cytoplasmic protein that interacts and posttranslationally regulates central PCP components Dvl2 and Vangl2 and modulates PCP downstream of the Rac1/JNK cascade (6–8). It was reported that DACT family member DACT3 exerted proapoptotic and tumor suppressor effects and was epigenetically downregulated by histone modification in colon cancer (10). Aberrant down regulation of DACT1 was observed in non–small cell lung cancer and hepatocellular carcinoma (11,12). However, little is known about the molecular mechanisms and effects of DACT1 in tumorigenicity. In this study, we studied the epigenetic inactivation, biological function, molecular mechanisms and clinical significance of DACT1 in gastric cancer.
MATERIALS AND METHODS
Cell Lines
Ten gastric cancer cell lines (AGS, Kato III, MKN28, MKN45, NCI-N87, SNU1, SNU16, SNU719, BGC823 and MGC803) and one immortalized human gastric epithelial mucosa cell line (GES1) were obtained from the American Type Culture Collection (Manassas, VA, USA) and Cancer Research Institute of Beijing, Beijing University (Beijing, China). Cell lines were maintained in RPMI-1640 (Gibco; Life Technologies, Carlsbad, CA, USA) or Dulbecco’s modified Eagle medium (DMEM) (Gibco; Life Technologies) with 10% fetal bovine serum. Human normal adult tissue RNA samples were purchased commercially (Stratagene, La Jolla, CA, USA).
RNA Extraction, Semiquantitative Reverse Transcriptase–Polymerase Chain Reaction (PCR) and Real-Time PCR (RT-PCR) Analyses
Total RNA was extracted from cell pellets or tissues by using Quizol reagent (Qiagen, Valencia, CA, USA), and cDNA was synthesized by using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Life Technologies). Semiquantitative RT-PCR was performed by using the Go-Taq DNA polymerase (Promega, Madison, WI, USA). Real-time PCR was performed by using an SYBR Green master mixture on an HT7900 system (Applied Biosystems; Life Technologies). Primer sequences are listed in Supplementary Table S1.
Methylation-Specific PCR and Bisulfite Genomic Sequencing
DNA bisulfite treatment, methylation-specific PCR (MSP) and bisulfite genomic sequencing (BGS) were performed as described previously (11). The bisulfite-modified DNA was amplified by using primer pairs that specifically amplify either methylated or unmethylated sequences of the DACT1 gene (Supplementary Table S1). Amplified BGS products were sequenced.
Demethylation Treatment with 5-Aza-2′-Deoxycytidine and Trichostatin A
Gastric cancer cells (1 × 105/mL) were seeded. After 24 h, cells were treated with 2 μmol/L of the DNA demethylating agent 5-Aza-2′-deoxycytidine (5-Aza) (Sigma-Aldrich, St. Louis, MO, USA) for 96 h. Some cell lines were further treated with the histone deacetylase inhibitor trichostatin A (300 nmol/L) for an additional 24 h. Cells then were harvested for DNA and RNA extractions.
Array Comparative Genomic Hybridization (Array-CGH)
DNA from five gastric cancer cell lines (MKN45, MKN28, Kato III, NCI-N87 and SNU1) and pooled normal gastric tissues are labeled differentially, by using different fluorophores, and hybridized to Array-CGH (Agilent Technologies, Santa Clara, CA, USA). The results were analyzed by Agilent G4175AA CGH Analytics 3.4 (Agilent Technologies). The ratio of the fluorescence intensity of the gastric cancer cell line to the normal reference DNA is then calculated to assess the copy number changes for a particular location in the genome. Two probes were used to detect the copy number change of the DACT1 gene: locus I was located at chromosome 14, from 58177255 to 58177309, and locus II was located at chromosome 14, from 58179290 to 58179349.
Gene Cloning and Plasmid Construction
The full-length DACT1 cDNA was amplified and cloned into the pCDNA3.1 + expression vector (Invitrogen; Life Technologies). pIRES2-ZsGreen1-DACT1 or pBABE-puro-DACT1 was generated by inserting the cloned full-length DACT1 into the pIRES2-ZsGreen1 empty vector (Clontech, Mountain View, CA, USA) or pBABE-puro empty vector (Addgene, Cambridge, MA, USA). DACT1 shRNA constructs and scrambled control shRNA in plasmid pGFP-V-RS were purchased from Origene (Rockville, MD, USA): shDACT1-1, 5′-AGAGC ACAAC CACCA GCGAC TCTGA AGAA-3′ (GI328121); shDACT1-2, 5′-GATGG CTACA TTCTG AGCCT GGTCC AGAA-3′ (GI328123); and scrambled control shRNA: 5′-GCACT ACCAG AGCTA ACTCA GATAG TACT-3′ (TR30013).
Colony Formation Assay
For overexpression assay, cells (AGS, BGC823 and MGC803) were transfected with pcDNA3.1-DACT1 or empty pcDNA3.1. For knockdown assay, GES1 cells were transfected with shControl (TR30013) or ShDACT1-1 (GI328121) or ShDACT1-2 (GI328123) (Origene). After 48 h transfection, cells were selected with G418 (Calbiochem, Darmstadt, Germany) for 10–14 d. Colonies (≥50 cells/colony) were counted.
Cell Growth Curve
Cells stably transfected with pcDNA3.1-DACT1 or empty pcDNA3.1 were seeded into a 12-well plate. The number of viable cells was counted every day for 4 d.
Apoptosis Assay
Apoptosis was determined by APC Annexin V and 7-aminoactinomycin (7-AAD) staining (BD Biosciences, San Jose, CA, USA) and was assessed by flow cytometry. Sample fluorescence of 10,000 cells was analyzed by using an FACSCalibur System (BD Biosciences). Cell populations were divided as viable (APC Annexin V negative, 7-AAD negative), early apoptotic (APC Annexin V positive, 7-AAD negative), necrotic (APC Annexin V negative, 7-AAD positive) and late apoptotic cells (APC Annexin V positive, 7-AAD positive). The relative proportion of cells at each quadrant was determined by using the ModFitLT software (BD, Franklin Lakes, NJ, USA).
Apoptosis was also determined by terminal deoxynucleotidyl transferase–mediated nick end labeling (TUNEL) staining (Promega) in xenograft tumor tissue sections from nude mice. Nuclei with clear brown staining were regarded as TUNEL-positive apoptotic cells.
Dual-Luciferase Reporter Assay
To elucidate the signaling pathways modulated by DACT1, several signaling pathway luciferase reporters were examined in DACT1-transfected AGS and BGC823 cells, including nuclear factor (NF)-κB-luc (5 × NF-κB binding sites), AP1/JNK-luc (7 × AP1 binding sites) and the T-cell factor (TCF)-responsive luciferase construct TOPFlash as reported previously (13). TOPFlash was kindly provided by Q Tao, the Chinese University of Hong Kong. Reporter activity was analyzed by the dual-luciferase reporter assay system and normalized to the control Renilla (Promega).
Determination of NF-κB Nuclear DNA-Binding Activity
Nuclear extracts were obtained from BGC823 cells stably transfected with pcDNA3.1-DACT1 or pcDNA3.1 empty vector by using a Nuclear Extract kit (Millipore, Billerica, MA, USA). NF-κB binding activity was determined by using NF-κB (human p50/p65) combo transcription factor assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
cDNA Expression Array
Gene expression profiles of BGC823 cells stably transfected with pcDNA3.1-DACT1 or pcDNA3.1 empty vector were analyzed by a Human Cancer Path-wayFinder™ RT2 Profiler™ PCR Array (SABiosciences, Frederick, MD, USA), which contains 84 functionally well-characterized genes involved in human tumorigenesis. Ct was measured during the exponential amplification phase, and the amplification plots were analyzed using SDS 1.9.1 software (Applied Biosystems; Life Technologies). Fold-change (ΔΔCt) = ΔCtDACT1-expressing group − ΔCtcontrol group (ΔCt = ΔCttarget − ΔCtthe average housekeeping genes used for normalization). Gene expression with fold-change ≥1.5 or ≤ −1.5 was considered to be of biological significance and was further validated.
In Vivo Tumorigenicity
BGC823 cells were infected with retro-virus pBABE-puro-DACT1 or pBABE-puro control and selected with puromycin (Invitrogen; Life Technologies) to generate stable DACT1-expressing or control BGC823 cells. Cells (5 × 105 cells in 0.2 mL phosphate-buffered saline [PBS]) were injected subcutaneously into the dorsal flank of four 4-wk-old male Balb/c nude mice separately (four per group). Tumor diameter was measured every 3 d until 3 wks. All experimental procedures were approved by the Animal Ethics Committee of the Chinese University of Hong Kong.
Immunohistochemistry
Immunohistochemistry was performed by using Histostain®-Plus Bulk Kit, Invitrogen® Second Generation, LAB-SA Detection System (Invitrogen; Life Technologies). The paraffin-embedded tissue sections were deparaffinized and rehydrated. The endogenous peroxidase activity was blocked with 3% H2O2 for 10 min. For the antigen retrieval, slides were immersed in 10 mmol/L citrate buffer (pH 6.0) and boiled for 15 min in a microwave oven. Nonspecific binding was blocked by 5% normal goat serum for 10 min. The slides were incubated with monoclonal antibody against DACT1 (1:100 dilution, ab42547; Abcam, Cambridge, MA, USA), Ki-67 (1:200 dilution, Clone SP6; Lab Vision, Fremont, CA, USA), NF-κB p65 (1:50 dilution, sc-109; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. Biotinylated secondary antibody and enzyme conjugate was applied consequently to the sections and washed with PBS. 3,3′-Diaminobenzidine (DAB) staining (Invitrogen; Life Technologies) was used to visualize antibody and was finally counterstained with hematoxylin. The number of NF-κB p65 or Ki-67 nuclear positive cells was counted per 1,000 tumor cells in five fields.
Western Blot
Total protein was extracted from the cell pellet. Protein (30 μg) from each sample was separated on 12% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) and transferred onto nitrocellulose membranes (GE Healthcare, Piscataway, NJ, USA). Blots were immunostained with primary antibodies against DACT1 (ab42547; Abcam); cleaved poly-(ADP-ribose) polymerase (PARP); and cleaved caspase-3, -7 and -9 (#5625, #9664, #9491, #9501; Cell Signaling), followed by anti-rabbit or anti-mouse secondary antibody, and developed with enhanced chemiluminescence (Amersham, Arlington Heights, IL, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a loading control.
Gastric Tissue Samples
Paraffin-embedded tumor tissue samples were obtained from 205 gastric cancer patients diagnosed in the First Affiliated Hospital of Sun Yatsen University, Guangzhou, China, from January 1999 to December 2006. In addition, 20 age-matched subjects with normal upper gastroscopy were recruited as control. All cancer patients were treated according to a standard protocol, with surgery being the mainstay of treatment. The study protocol was approved by the Clinical Research Ethics Committee of the Sun Yatsen University of Medical Sciences. Patients and control subjects gave informed consent for participation in this study, and the principles set forth by the Declaration of Helsinki in the applicable version have been obeyed. In BGS analysis, more than three out of total nine CpG islands showed methylation level over 30%, which was defined as partial or dense methylated. Others were defined as unmethylated or mild methyalted.
Statistical Analysis
The independent Student t test was used to compare the difference between two preselected groups. The difference in cell growth curve or the tumor growth rate in mice was determined by repeated-measures analysis of variance. Relationship between DACT1 methylation and the clinicopathologic characteristics of gastric cancer patients were compared by using the Pearson χ2 test. P < 0.05 was statistically significant.
All supplementary materials are available online at www.molmed.org.
RESULTS
Silence or Downregulation of DACT1 by Promoter Methylation of DACT1 in Gastric Cancer
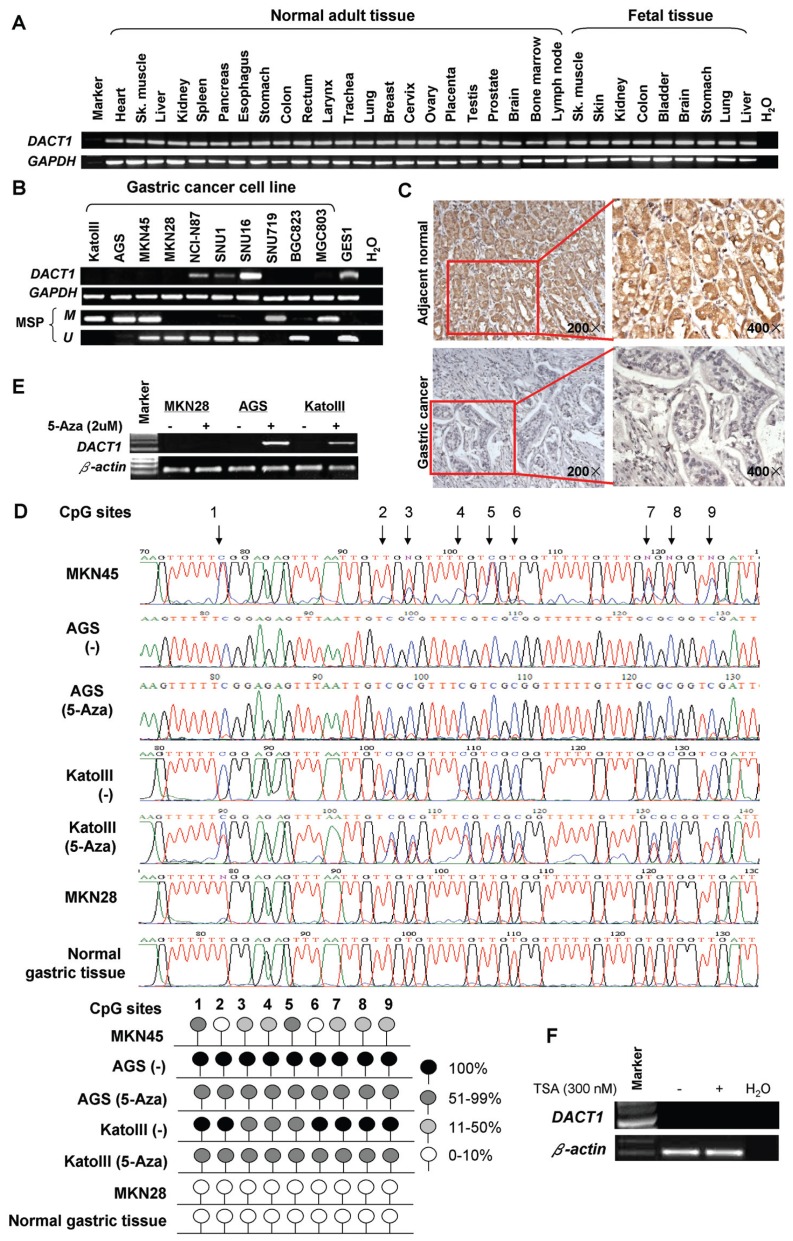
DACT1 was expressed in all normal adult tissues and fetal tissues examined as well as in immortalized normal human gastric epithelial mucosa cell line (GES1) (Figure 1A). In contrast, the mRNA expression of DACT1 was silenced in 7 of 10 gastric cancer cell lines (Figure 1B). In addition, downregulation or silence of DACT1 was detected in 19 primary gastric cancers compared with the adjacent nontumor tissues by immunohistochemistry (P = 0.001) (Figure 1C).
Figure 1.
DACT1 is inactivated by promoter methylation in gastric cancer. (A) Robust mRNA expression of DACT1 in normal adult tissues and fetal tissues. (B) DACT1 is frequently silenced in gastric cancer cell lines by promoter methylation. Methylation of DACT1 was determined by MSP. M, methylated; U, unmethylated. (C) DACT1 protein expression in gastric cancer and their adjacent normal tissues by immunohistochemistry (magnification [left], 200×; magnification [right], 400×). (D) Methylation status of the DACT1 promoter was confirmed by BGS. (E) Pharmacological demethylation with 5-Aza restored DACT1 expression. (F) Histone deacetylases inhibitor trichostatin A (TSA, 300 nmol/L) treatment failed to restore DACT1 expression.
To elucidate the role of promoter methylation in the downregulation of DACT1, DACT1 methylation status was first examined by MSP. Methylation of DACT1 was detected in five silenced cell lines (Kato III, AGS, MKN45, SNU719 and MGC803), but not in GES1 (see Figure 1B). The DACT1 methylation status was further validated by BGS. The BGS results were consistent with the results of MSP, in which dense methylation was found in methylated cell lines (AGS, MKN45 and Kato III), but not in unmethylated MKN28 and normal gastric tissues (Figure 1D). To test whether methylation directly mediates DACT1 silencing, we treated three silenced cell lines (MKN28, AGS and Kato III) with demethylation agent 5-Aza. This treatment reduced DACT1 promoter methylation level (see Figure 1D) and restored DACT1 expression in AGS and Kato III cell lines (Figure 1E), inferring transcriptional gene silence of DACT1 was mediated by promoter methylation in GC cells. However, both 5-Aza and trichostatin A failed to restore the expression of DACT1 in an unmethylated and silenced cell line MKN28 (Figures 1E, F), indicating the transcriptional gene silence of DACT1 in MKN28 is due to other mechanisms but not DNA methylation and histone modification.
Copy Number Loss of DACT1 in MKN28
Array-CGH was performed to detect genomic copy number variations in five gastric cancer cell lines (Kato III, MKN45, MKN28, NCI-N87 and SNU1) compared with pooled normal gastric tissue samples (as shown in Table 1). MKN28 showed copy number loss in both loci (0.754 in locus I and 0.684 in locus II) of the DACT1 gene. A relative low-level copy number loss in one locus of the DACT1 gene was seen in MKN45 (0.797 in locus I) with partial methylation. However, there were no copy number changes in Kato III (DACT1 silenced cell line with full methylation), NCI-N87 and SNU1 (DACT1 expressing cell lines without methylation).
Table 1.
Relative copy number change of DACT1 gene in gastric cancer cell lines.
| Relative copy number change | ||||
|---|---|---|---|---|
|
|
||||
| Cell line | Locus I | Locus II | DACT1 expression | DACT1 methylation |
| Kato III | 0.930 | 0.919 | None | Full |
| MKN45 | 0.797 | 0.983 | None | Partial |
| MKN28 | 0.754 | 0.684 | None | None |
| NCI-N87 | 1.047 | 0.953 | Yes | None |
| SNU1 | 1.052 | 0.990 | Yes | None |
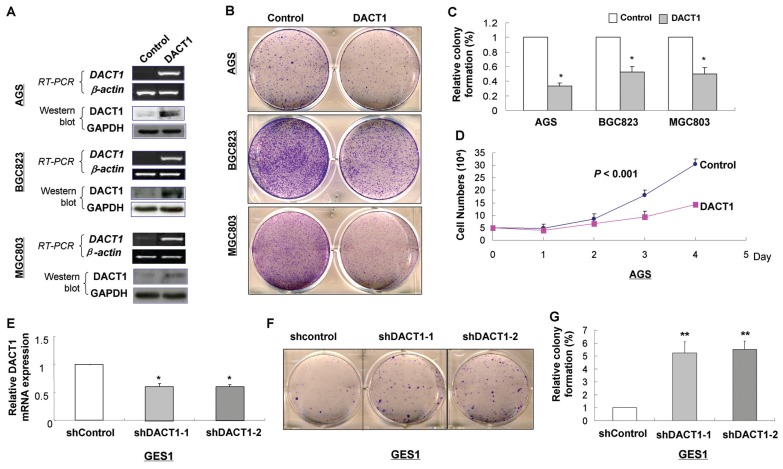
DACT1 Inhibits Gastric Cancer Cell Growth
To elucidate the function of DACT1 in gastric cancer, we examined the effect of DACT1 re-expression and knockdown on growth characteristics of gastric cancer cells by colony formation and growth curve assays. DACT1-expressing plasmid was stably transfected into AGS, BGC823 and MGC803 cells without DACT1 expression (Figure 2A1). The colonies formed in three DACT1-transfected cells were statistically significantly fewer in number and smaller in size than in control vector–transfected cells in AGS (1 versus 33.47% ± 4.01%) and BGC823 (1 versus 52.33% ± 7.95%) and in MGC803 (1 versus 49.57% ± 9.14%) (Figures 2B, C). Keeping with this finding, ectopic expression of DACT1 significantly reduced cell growth curve in AGS (P < 0.001) (Figure 2D). On the other hand, shRNA-mediated knockdown of DACT1 in normal gastric epithelial GES1 cell (Figure 2E) significantly promoted cell growth by colony formation assay (shcontrol versus shDACT1-1 versus shDACT1-2: 1 versus 5.24 ± 0.87 versus 5.51 ± 0.65) (Figures 2F, G).
Figure 2.
DACT1 inhibits tumor cell growth and clonogenicity. (A) Effect of DACT1 overexpression on colony formation in AGS, BGC823 and MGC803 cells (B and C). (D) Cell growth curve by DACT1 overexpression in AGS cells. (E) Effect of DACT1 knockdown on colony formation in an immortalized human gastric epithelial mucosa cell line GES1 cells (F and G).
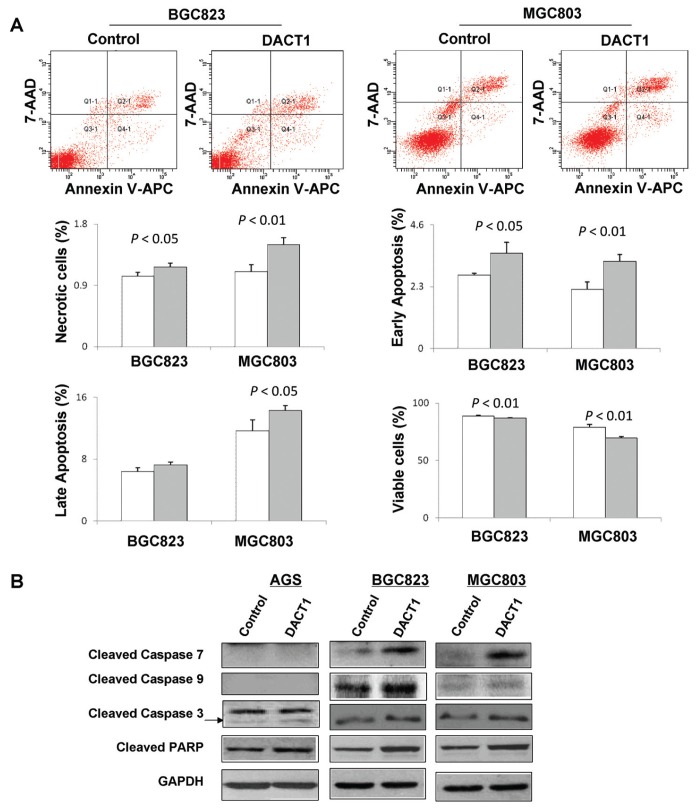
DACT1 Induces Cell Apoptosis
To examine the contribution of apoptosis to the observed growth suppression by DACT1, cell apoptosis was evaluated by using APC Annexin V and 7-AAD double staining. Ectopic expression of DACT1 led to a modest increase of early apoptotic cells in both BGC823 (2.73 ± 0.058% versus 3.60 ± 0.46%, P < 0.05) and in MGC803 (2.36 ± 0.25% versus 3.63 ± 0.25%, P < 0.01) compared with control (Figure 3A). Induction of apoptosis was further confirmed by analysis the expression of apoptosis-related proteins. Re-expression of DACT1 enhanced the protein expression of cleavage of caspase-3, -7 and -9 and PARP in stably transfected AGS, BGC823 and MGC803 cells compared with controls (Figure 3B).
Figure 3.
DACT1 induces apoptosis of gastric cancer cells. (A) Cell apoptosis was examined by flow cytometry analysis of APC Annexin V and 7-AAD double-staining. Region Q1 shows the necrotic cells, Q2 shows the late apoptotic cells, Q3 shows the live cells and Q4 shows the early apoptotic cells. (B) Protein expression of cleaved caspase-3, -7 and -9 and PARP was evaluated by Western blot.
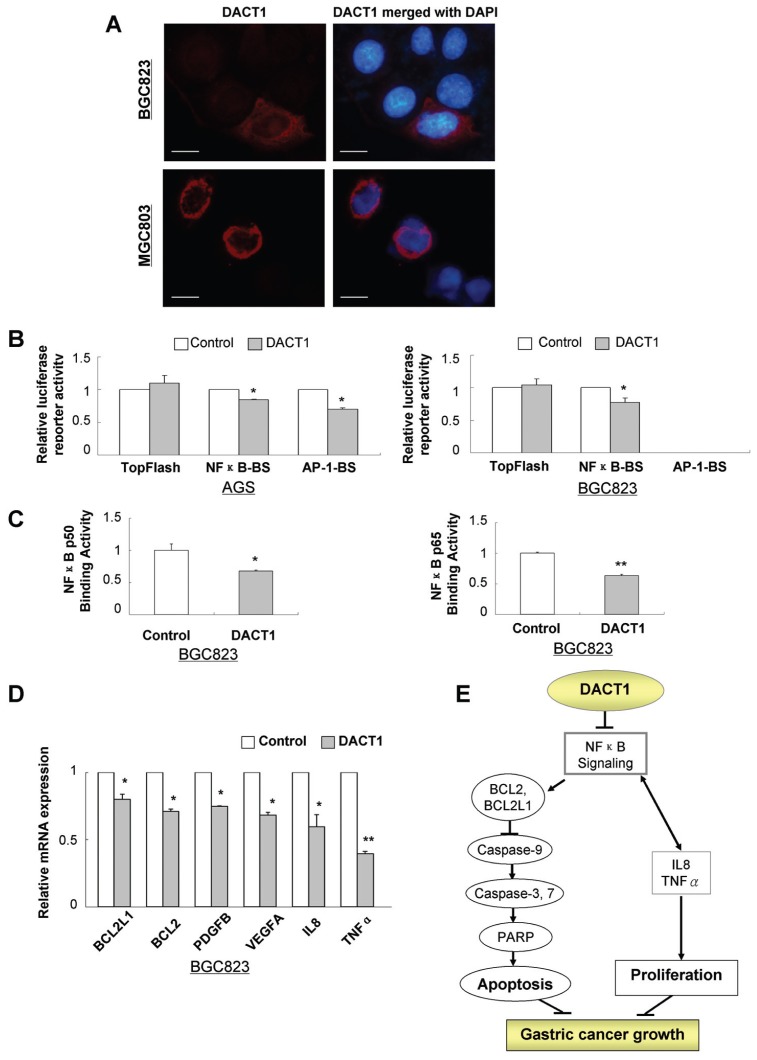
DACT1 Is a Cytoplasmic Protein Inhibiting the NF-κB Signaling Pathway
The subcellular localization of DACT1 was examined in pIRES-ZsGreen1-DACT1 transfected gastric cancer cells. Direct DACT1 immunofluorescent staining showed that DACT1 was a cytoplasmic protein (Figure 4A).
Figure 4.
DACT1 is a cytoplasmic protein regulating NF-κB signaling pathway and molecular targets. (A) DACT1 localized in cytoplasm by immunofluorescence. Scale bar, 5 μm. (B) The effects of DACT1 on several signaling pathways (NF-κB, JNK/AP-1 and β-catenin/TOPFlash). Gastric cancer cell lines (MGC803, BGC823) were cotransfected with luciferase reporter plasmid and pRL-CMV vector with DACT1-pcDNA3.1 or pc-DNA3.1 for 48 h, and luciferase activities were analyzed by the dual-luciferase reporter assay system. (C) The effects of DACT1 on NF-κB p50 and p65 transcription factor binding activity. (D) Real-time PCR validation for genes screened by cDNA microarray in DACT1 stably transfected cells. Data are means ± standard deviation (SD); *P < 0.05; **P < 0.01. (E) Schematic diagram for the molecular basis of DACT1 as a tumor suppressor gene in gastric cancer.
To clarify the downstream signaling pathways modulated by DACT1 in tumor inhibition, we screened three key cancer-related signaling pathways (Wnt/β-catenin pathway, JNK pathway and NF-κB pathway) by using promoter-luciferase activity assays (TOPFlash, AP-1-Luc and NF-κB-Luc) in AGS and BGC823 cells. DACT1 significantly suppressed NF-κB reporter activity in both cell lines and inhibited AP-1 reporter activity in JNK- activated AGS cells, whereas the reporter activity of the TOPFlash pathway was not changed (Figure 4B). Besides, JNK activity was present at a very low level and hard to detect in BC823 cells. Inhibition of NF-κB activity was further confirmed by the NF-κB transcription factor binding activity assay; both NF-κB-p50 and -p65 binding activity was significantly inhibited by DACT1 (Figure 4C).
Identification and Validation of DACT1 Target Genes
Because BGC823 has the ability to grow tumors in nude mice and is used for tumorigenicity assay in this study, the molecular mechanism by DACT1 was examined in this line to gain insight into the molecular mechanisms underlying the tumor suppression of DACT1. The gene expression profile in DACT1 stably transfected BGC823 was analyzed by Human Cancer PathwayFinder RT2 Profiler PCR Array. The antitumorigenesis effect by DACT1 was mediated by regulating important genes in apoptosis, cell proliferation, angiogenesis, adhesion, migration and invasion (Table 2), which were confirmed by real-time PCR (Figure 4D). DACT1 decreased the expression of NF-κB signaling downstream factors including antiapoptotic B-cell CLL/lymphoma 2 (BCL2), apoptosis regulator Bcl-XL, oncogenic interleukin-8 (IL-8) and tumor necrosis factor-α (TNFα). DACT1 also suppressed oncogenic cytokine platelet-derived growth factor β polypeptide (PDGFB) and vascular endothelial growth factor A (VEGFA) (Table 2, Figures 4D, E).
Table 2.
Effect of DACT1 on the gene expression profiles of cancer pathways.
| Genbank accession | Gene symbol | Gene name | Gene function | Fold change (DACT1/control) |
|---|---|---|---|---|
| NM_000633 | BCL2 | B-cell CLL/lymphoma 2 | Antiapoptosis | −1.9 |
| NM_138578 | BCL-XL | BCL2-like 1 | Antiapoptosis | −1.6 |
| NM_000584 | IL8 | Interleukin-8 | Angiogenesis | −1.6 |
| NM_000594 | TNFα | Tumor necrosis factor (TNF superfamily, member 2) | Angiogenesis | −1.5 |
| NM_002608 | PDGFB | Platelet-derived growth factor β polypeptide (simian sarcoma viral [v-sis] oncogene homolog) | Proliferation and angiogenesis | −1.5 |
| NM_003376 | VEGFA | Vascular endothelial growth factor A | Proliferation and angiogenesis | −1.5 |
DACT1 Inhibits In Vivo Tumor Growth
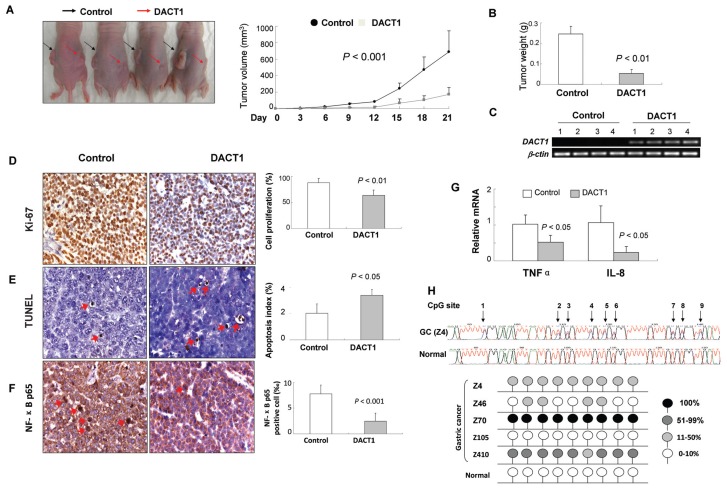
We also tested whether DACT1 could suppress the growth of gastric cancer cells in nude mice in vivo. The subcutaneous tumor growth of BGC823 stably transfected with DACT1 was significantly suppressed compared with BGC823 transfected with vector in nude mice (P < 0.001) (Figures 5). The mean tumor weight was significantly less in the DACT1 group than in the control group (0.25 ± 0.036 versus 0.054 ± 0.020, P < 0.01) (Figure 5B).
Figure 5.
Antitumor effect of DACT1 in vivo. (A) Tumor growth curve of DACT1-expressing BGC823 cells in nude mice was compared with control cells. (B) The mean tumor weights of the DACT1 and vector groups were compared. (C) DACT1 expression was examined by reverse transcriptase PCR. (D) Ki-67 staining in xenograft tumors of nude mice. Brown nuclear signals indicate the Ki-67–positive nuclei-stained cell (magnification 200×). (E) Apoptotic cells in tumor tissues from nude mice are detected by TUNEL staining. The red arrows indicate the apoptotic cell (400×). (F) Immunohistochemistry for NF-κB p65. Red arrows indicate the p65-positive cells (×400). (G) The mRNA expression of TNFα and IL-8 in DACT1-expressing xenograft tumors and control tumors by real-time PCR. (H) Methylation status of DACT1 in primary gastric cancers and in normal tissue by BGS.
Cell proliferation, apoptosis and NF-κB activation mediated by DACT1 in the xenograft tumors were validated by Ki-67, TUNEL and NF-κB staining, respectively. Consistent with the result obtained in gastric cancer cell lines in vitro, DACT1- expressing tumors displayed significantly less proliferative cells (P < 0.01) (Figure 5D), more apoptotic cells (P < 0.05) (Figure 5E) and fewer NF-κB p65-positive cells (P < 0.001) (Figure 5F) compared with the control group. Moreover, expression of NF-κB downstream effectors TNFα (P < 0.05) and IL-8 (P < 0.05) were also decreased in DACT1-expressing tumors (Figure 5G).
Promoter Methylation of DACT1 Is Associated with Advanced Tumor Stage and Tumor Aggressiveness
The clinical application of DACT1 methylation was evaluated in 205 primary gastric cancers and in 20 healthy gastric tissue samples. Among 205 gastric cancer cases, partial and dense promoter methylation of DACT1 was detected in 29.3% (60/205) of cases, but none in 20 healthy gastric tissue samples (Figure 5H). DACT1 methylation was associated with advanced tumor size (P < 0.05), lymph node metastasis (P < 0.05) and distant metastasis (P = 0.05) (Table 3). DACT1 methylation was more frequent in tumor, node, metastasis (TNM) stage III/IV than in stage I/II cases (P < 0.0005) (Table 3).
Table 3.
Clinicopathologic features of gastric cancer patients according to DACT1 methylation status.
| Variable | Nonmethylated (n = 145) | % | Methylated (n = 60) | % | P |
|---|---|---|---|---|---|
| Age (mean ± SD) | 58 ± 11.5 | 55 ± 13.9 | 0.07 | ||
| Sex | |||||
| M | 102 | 70.3 | 37 | 61.7 | 0.23 |
| F | 43 | 29.7 | 23 | 38.3 | |
| Helicobacter pylori infection | |||||
| Negative | 66 | 66.7 | 20 | 64.5 | 0.83 |
| Positive | 33 | 33.3 | 11 | 35.5 | |
| Lauren classification | |||||
| Diffuse or Mixed | 25 | 17.4 | 8 | 13.6 | 0.51 |
| Intestinal | 119 | 82.6 | 51 | 86.4 | |
| Location | |||||
| Cardiac | 30 | 20.7 | 11 | 18.3 | 0.70 |
| Non-cardiac | 115 | 79.3 | 49 | 81.7 | |
| Differentiation | |||||
| Poor | 78 | 64.5 | 40 | 76.9 | 0.11 |
| Well or moderate | 43 | 35.5 | 12 | 23.1 | |
| Lymph node metastasis | |||||
| Negative | 37 | 31.1 | 5 | 11.9 | 0.015 |
| Positive | 82 | 68.9 | 37 | 88.1 | |
| Tumor Size | |||||
| T1–T2 | 34 | 29.6 | 4 | 10.0 | 0.013 |
| T3–T4 | 81 | 70.4 | 36 | 90.0 | |
| Distant metastasis | |||||
| Negative | 95 | 77.9 | 27 | 62.8 | 0.05 |
| Positive | 27 | 22.1 | 16 | 37.2 | |
| TNM stage | |||||
| I–II | 51 | 38.1 | 6 | 11.3 | <0.0005 |
| III–IV | 83 | 61.9 | 47 | 88.7 | |
DISCUSSION
In this study, we demonstrated for the first time that DACT1 was silenced in 7 of 10 gastric cancer cell lines and was down-regulated in primary gastric cancers. The downregulation was mostly attributed to promoter methylation by MSP and BGS. Restored DACT1 expression was achieved by demethylation treatment, which suggested that promoter methylation was a major mechanism for the transcriptional silencing of DACT1 in gastric cancer. In addition, there were also unmethylated alleles in the gastric cancer cell line MKN28 with no DACT1 expression. Genomic copy number loss in two loci of the DACT1 gene was observed in MKN28 by Array-CGH, implying that apart from promoter methylation, genetic alteration such as breakage of a chromosome, a non-reciprocal translocation event or loss of heterozygosity, might also play a role in transcriptional gene silence of DACT1 in gastric cancer. In keeping with this, loss of heterozygosity or genomic copy number loss at DACT1 locus was reported in liver cancer and gastrointestinal stromal tumor (12,14).
Because downregulation of DACT1 was observed in gastric cancer and not in normal gastric tissues, DACT1 may function as a potential tumor suppressor. We therefore tested the putative tumor suppressor function of DACT1 in gastric cancer cell lines both in vitro and in vivo. Ectopic expression of DACT1 in silenced gastric cancer cell lines (AGS, BGC823 and MGC803) significantly suppressed clonogenicity (Figure 2B) and cell growth curve (Figure 2D) and induced apoptosis (Figure 3). The diminution of tumor growth in DACT1-reexpressed cells was further confirmed in the tumorigenesity in nude mice (Figure 5A). The observation of decreased cell proliferation by Ki-67 nuclei immunostaining and increased apoptosis by TUNEL staining induced by DACT1 in vivo (Figures 5D, E) was entirely consistent with the in vitro effects, adding further weight to the potential significance of these findings. On the other hand, shRNA-mediated knockdown of DACT1 in an immortalized human gastric epithelial mucosa cell line GES1 significantly increased clonogenicity (Figures 2F, G). Collectively, these results indicated for the first time that DACT1 functions as a tumor suppressor in gastric cancer.
The molecular mechanisms in which DACT1 exerts its tumor suppressor function were further elucidated. To identify key regulators of DACT1-mediated anti-tumorigenic effect in gastric cancer, we used Western blot and cDNA microarray to study gastric cancer cells transfected with DACT1; genes that were significantly altered in expression levels by cDNA microarray were then validated by RT-PCR. We revealed that the induction of apoptosis by DACT1 was mediated through caspase-dependent pathways, including activation of caspase-9, followed by cleavage of downstream caspase effectors caspase-3 and caspase-7, ultimately stimulating the activation of PARP and cellular disassembly and apoptosis. Moreover, DACT1 downregulated antiapoptotic genes BCL2 and BCL-XL, which contributed to the prevention of mitochondrial apoptosis (Figure 4D). The antiproliferative function of DACT1 appeared to be associated with the down-regulation of oncogenic cytokines TNFα, IL-8, PDGFB and VEGFA by cDNA array (see Figure 4D).
We elucidated the downstream signaling pathways modulated by DACT1. We reported that DACT1 inhibited the NF-κB pathway with adequate evidence from the luciferase reporter assay, NF-κB DNA-binding activity assay in nuclear extracts and NF-κB p65 immunohistochemistry. NF-κB signaling pathway is a key link between inflammation and cancer and plays a critical role in cancer development and progression by regulating the transcription of genes involved in cell proliferation and suppression of apoptosis (15,16). Genetic inactivation of NF-κB decreases tumor multiplicity or size in inflammation-driven cancer mice models by down-regulating antiapoptotic gene expression and dampened production of growth-stimulating cytokines (17,18). In keeping with this, ectopic expression of DACT1 inhibited the expressions of NF-κB downstream targets including BCL2 and BCL-XL and oncogenic cytokines TNFα and IL-8. BCL2 and BCL-XL are two critical antiapoptotic genes induced by NF-κB activation in cancer development (16). The promoter of BCL2 and BCL-XL could be directly activated by NF-κB, contributing to decreased apoptosis and tumor incidence (17,19,20). BCL2 and BCL-XL sequester proapoptotic Bcl-2 members prevent Bax/Bak activation and consequently inhibit mitochondrial proapoptotic events including blocking the release of cyto chrome c and the formation of cyto chrome c–dependent Apaf-1/caspase-9 complex (21). Thus, inhibition of NF-κB by DACT1 decreased BCL2 and BCL-XL, leading to activation of the downstream apoptotic protease cascade (Figure 4E). TNFα and IL-8 were key mediators in NF-κB–associated cancer progression (17,18,22,23). Interference with TNFα or genetic blockade of NF-κB inhibited tumor cell growth with increased apoptosis in the mice liver cancer model (18,22). On the other hand, both TNFα and IL-8 served as a potent inducer of NF-κB signaling activation, thus augmenting NF-κB signaling in an autocrine manner. Collectively, through modulating the NF-κB signaling pathway and its downstream targets, DACT1 could function as a tumor suppressor by promoting apoptosis and growth inhibition. In addition, all DACT family proteins form complexes with themselves and with each other and interact with members of the Dvl and Vangl protein families in a conservative way (24). Thus, the possibility is raised that overexpression of this family protein can cause nonspecific or nonphysiological apoptosis and cell death by association and interference with wild-type DACT proteins and their partners. Promoter methylation of DACT1 was evaluated in 205 primary gastric cancers by BGS and 29.3% (60/205) of gastric cancers detected promoter methylation. DACT1 methylation was associated with tumor aggressiveness including lymph node metastasis (P < 0.05), distant metastasis (P = 0.05) and advanced stage of gastric cancers (P < 0.0005). Thus, in keeping with the biological function of DACT1 identified in this study, the inactivation of this gene by promoter methylation would favor tumor progression.
CONCLUSION
In summary, we identified a novel functional tumor suppressor gene DACT1 mainly inactivated by promoter methylation in gastric cancer. DACT1 contributes to the suppression of tumorigenesis by promoting cell apoptosis, decreasing cell proliferation through inhibiting NF-κB signaling pathway. DACT1 methylation is associated with advanced stage and tumor aggressiveness.
Supplemental Data
ACKNOWLEDGMENTS
This project was supported by the Shenzhen Basic Research Program (JC20110520111A), National High-Tech R&D Program (863 Program, 2012AA02A504 and 2012AA02A203), National Basic Research Program of China (973 Program, 2010CB529305), the Chinese University of Hong Kong (CUHK) Focused Investment Scheme C (1903026) and Research Fund for the Control of Infectious Diseases (RFCID) (10090942, 11100022).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, et al. Methylation of protocadherin 10, a novel tumor suppressor, is associated with poor prognosis in patients with gastric cancer. Gastroenterology. 2009;136:640–51. doi: 10.1053/j.gastro.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, et al. Promoter methylation of the Wnt/beta-catenin signaling antagonist Dkk-3 is associated with poor survival in gastric cancer. Cancer. 2009;115:49–60. doi: 10.1002/cncr.23989. [DOI] [PubMed] [Google Scholar]
- 4.Cheung KF, et al. Characterization of the gene structure, functional significance, and clinical application of RNF180, a novel gene in gastric cancer. Cancer. 2012;118:947–59. doi: 10.1002/cncr.26189. [DOI] [PubMed] [Google Scholar]
- 5.Ying J, et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors. Clin Cancer Res. 2005;11:6442–9. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, et al. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem. 2006;281:8607–12. doi: 10.1074/jbc.M600274200. [DOI] [PubMed] [Google Scholar]
- 7.Wen J, et al. Loss of Dact1 disrupts planar cell polarity signaling by altering dishevelled activity and leads to posterior malformation in mice. J Biol Chem. 2010;285:11023–30. doi: 10.1074/jbc.M109.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suriben R, Kivimäe S, Fisher DA, Moon RT, Cheyette BN. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009;41:977–85. doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–9. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–41. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang ZQ, et al. Downregulation of HDPR1 is associated with poor prognosis and affects expression levels of p120-catenin and beta-catenin in nonsmall cell lung cancer. Mol Carcinog. 2010;49:508–19. doi: 10.1002/mc.20622. [DOI] [PubMed] [Google Scholar]
- 12.Yau TO, et al. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607–14. doi: 10.1038/sj.onc.1208340. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, et al. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 2011;53:843–53. doi: 10.1002/hep.24124. [DOI] [PubMed] [Google Scholar]
- 14.Astolfi A, et al. A molecular portrait of gastrointestinal stromal tumors: an integrative analysis of gene expression profiling and high-resolution genomic copy number. Lab Invest. 2010;90:1285–94. doi: 10.1038/labinvest.2010.110. [DOI] [PubMed] [Google Scholar]
- 15.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 17.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Pikarsky E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 19.Khoshnan A, et al. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165:1743–54. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 20.Tamatani M, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 21.Gross A, et al. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 22.Popivanova BK, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–70. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Kivimäe S, et al. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33. doi: 10.1186/1471-2091-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.