Abstract
Rabbit antithymocyte globulin-Genzyme™ is used to prevent graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Common disadvantages of treatment are infectious complications. The effects of rabbit antithymocyte globulin-Genzyme™ on thymic function have not been well-studied. Multicolor flow cytometry was used to analyze the kinetics of conventional and regulatory T cells in adult patients treated (n=12) or not treated (n=8) with rabbit antithymocyte globulin-Genzyme™ during the first 6 months after allogeneic hematopoietic stem cell transplantation. Patients treated with rabbit antithymocyte globulin-Genzyme™ had almost undetectable levels of recent thymic emigrants (CD45RA+CD31+) of both conventional and regulatory CD4T cells throughout the 6 months after allogeneic hematopoietic stem cell transplantation whereas CD4+CD45RA-memory T cells were less affected, but their levels were also significantly lower than in patients not treated with rabbit antithymocyte globulin-Genzyme™. In vitro, rabbit antithymocyte globulin-Genzyme™ induced apoptosis and cytolysis of human thymocytes, and its cytotoxic effects were greater than those of rabbit antithymocyte globulin-Fresenius™. Rabbit antithymocyte globulin-Genzyme™ in combination with a conditioning regimen strongly impairs thymic recovery of both conventional and regulatory CD4+ T cells. The sustained depletion of conventional and regulatory CD4+T cells carries a high risk of both infections and graft-versus-host disease. Our data indicate that patients treated with rabbit antithymocyte globulin-Genzyme™ could benefit from thymus-protective therapies and that trials comparing this product with other rabbit antithymocyte globulin preparations or lymphocyte-depleting compounds would be informative.
Introduction
The thymus is required for de novo generation of naïve T cells with a broad T-cell receptor repertoire.1 Although thymic function in humans decreases after puberty, the adult thymus still contributes substantially to the restoration of immune competence after myeloablative therapy.2 Factors that inhibit thymic function after allogeneic hematopoietic stem cell transplantation (HSCT) include thymic damage by the conditioning regimen3,4 as well as graft-versus-host disease (GvHD), in which cell damage is mediated by donor alloreactive T cells.5,6 The consequence of delayed thymic recovery is profound T-cell deficiency and repertoire restriction after allogeneic HSCT.1,7 In consequence, the delay in thymic recovery is associated with a higher incidence of infections8 and disease relapse;9 furthermore, it hinders success of immunotherapeutic strategies.
CD31 (PECAM-1) has been demonstrated to characterize the T-cell receptor excision circles (TREC)-rich CD4 recent thymic emigrants (RTE)10,11 and is already adopted in clinical practice as an excellent marker for thymic function in solid organ transplantation12 and after autologous HSCT.13,14 Junge et al. found a correlation between CD31+ CD4 T cells and the recurrence of TREC during aging of healthy individuals and in six lymphopenic children after allogeneic HSCT.15
Rabbit antithymocyte globulin-Genzyme™ (ATG-G, Thymoglobulin®, Genzyme™ Transplant, Cambridge, MA, USA) therapy is commonly used for in vivo T-cell depletion in the context of allogeneic HSCT to prevent GvHD.16 Different rabbit ATG preparations are currently available of which ATG-G and ATG-Fresenius™ (ATG-F, Fresenius™ Biotech GmbH, Munich, Germany) are frequently used. ATG-F is manufactured by immunization of rabbits with a Jurkat cell line whereas ATG-G is manufactured by immunization of rabbits with human thymocytes. In this study ATG-G was used, which contains a diverse spectrum of antibody specificities depleting various T-cell subsets.17 Clinical data regarding the effect of ATG-G on thymopoiesis after allogeneic HSCT are scarce, and the effect of ATG-G on thymic output of regulatory T cells (Treg), in particular after allogeneic HSCT, has not been studied yet.
The aim of this study was to assess the impact of ATG-G on the reconstitution of RTE, naïve and memory T cell subsets of both conventional (Tcon) and regulatory (Treg) CD4 T cells in adult patients undergoing allogeneic HSCT. Considering a possible direct effect of ATG-G on the thymus, we also tested the toxicity of ATG-G to human thymocytes in vitro and compared to ATG-F as well as to the toxicity to peripheral blood mononuclear cells (PBMC).
Design and Methods
Patients and treatment
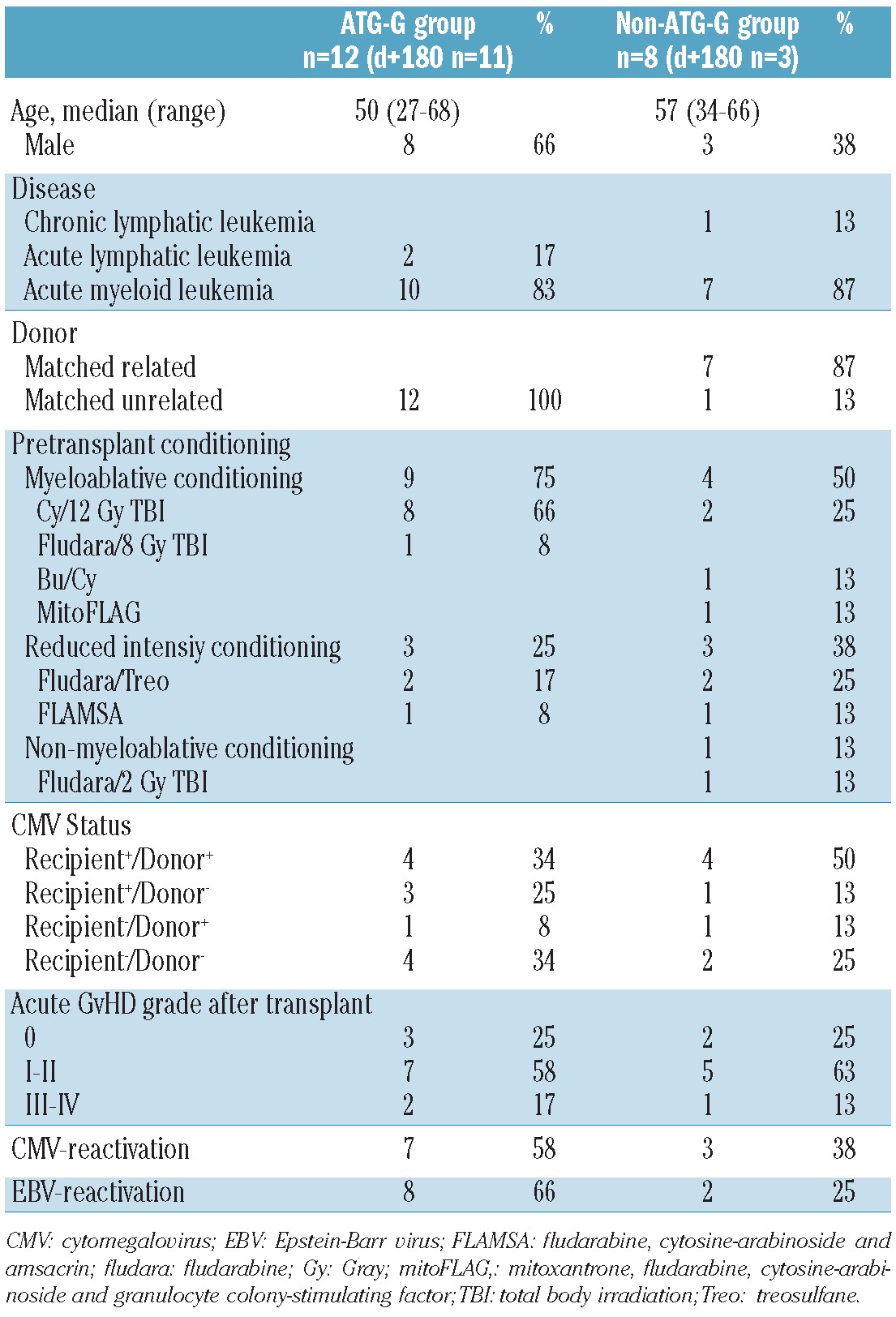
Blood samples for the kinetic study were obtained from 20 adult patients, who received myeloablative conditioning (n=13), reduced intensity conditioning (n=6) or non-myeloablative conditioning (n=1, a patient diagnosed with chronic lymphocytic leukemia) according to standard protocols. The patients' characteristics are listed in Table 1. All hematopoietic grafts were obtained by apheresis after stem cell mobilization with granulocyte-colony stimulating factor and were provided from healthy HLA-matched related donors (n=7) and unrelated (n=13) donors. GvHD prophylaxis consisted of cyclosporine A in combination with either methotrexate (n=15) or mycophenolate mofetil (n=5). ATG-G (2 mg/kg/day) was additionally administered in the case of matched unrelated donor transplants on days -3, -2 and -1 before transplantation according to standard protocols in 12 patients. Patients' samples were obtained once before transplantation and on days 15, 30, 60, 90 and 180 after transplantation. Analyses were performed on fresh blood samples in order to avoid possible influences caused by cycles of freezing and thawing. Human thymocytes were obtained from fresh thymic tissue of patients, who underwent cardiac surgery. Studies were approved by the local ethics committee of the Charité Berlin (approval n. EA4/128/09 and n. EA1/233/09) and patients gave informed consent.
Table 1.
Patients' characteristics.
Antibody staining and flow cytometry
PBMC were isolated by density gradient centrifugation (Ficoll-Hypaque; GE Healthcare) from fresh heparinized blood samples in order to avoid possible influences caused by freeze-thaw cycles. PBMC were distributed into different tubes. The PBMC in one tube were incubated for 15 min at room temperature with the following conjugated monoclonal antibodies for later assessment of central and effector memory CD4 T cells as well as RTE CD4 T cells: anti-CD3 APC/Cy7 (clone UCHT1; BioLegend), anti-CD4 Alexa Fluor 700 (clone RPA-T4; BioLegend), anti-CD8 V500 (clone RPA-T8; BD Biosciences), anti-CD45RA PerCP/Cy5.5 (clone HI100; BioLegend), anti-CD45RO Pacific Blue (clone U HL1; BioLegend), anti-CD197 (CCR7) Alexa Fluor 488 (clone TG8/CCR7; BioLegend), anti-CD62L APC (clone DREG-56; BioLegend), CD27 PE/Cy7 (clone O323; eBioscience) and anti-CD31 PE (clone WM59, BioLegend). PBMC were washed and resuspended for flow-cytometric analysis. The PBMC in a separate tube were incubated for 15 min at room temperature with the following conjugated monoclonal antibodies for later assessment of RTE of Tcon and Treg CD4 cells: anti-CD3 APC/Cy7 (clone UCHT1; BioLegend), anti-CD4 Alexa Fluor 700 (clone RPA-T4; BioLegend), anti-CD8 V500 (clone RPA-T8; BD Horizon), anti-CD25 PE/Cy7 (clone 2A3; BD Horizon), anti-CD31 APC (clone A 128; Miltenyi), anti-CD45RA eFlour605 (clone HI100; eBioscience) and CD45RO Pacific Blue (clone UCHL1; BioLegend). After washing and resuspension PBMC from the second tube were treated with fixation buffer and permeabilization buffer (eBioscience) and then incubated with anti-FOXP3 PE (clone PCH101; eBioscience) for 30 min at room temperature. The PBM were then washed and resuspended for flow cytometric analysis. The phenotype of the samples was analysed using a BD™ LSRII (Becton Dickinson, Palo Alto, CA, USA) supported by FlowJo 9.3 software (TreeStar, Ashland, OR, USA).
For in vitro experiments, thymocytes and PBMC were washed with phosphate-buffered saline after stimulation and resuspended in phosphate-buffered saline/Flebogamma solution for blocking on ice for 20 min. Cells were stained for 15 min at room temperature with propidium iodide staining solution and annexin V according to the manufacturer's instructions (BD Pharmingen). After washing, thymocytes and PBMC were resuspended in annexin V binding buffer and analyzed by flow cytometry using a BD™ Fortessa flow cytometer (Becton Dickinson, Palo Alto, CA, USA) supported by FlowJo 9.3 software (TreeStar, Ashland, OR, USA).
Cultures of thymocytes and peripheral blood mononuclear cells
Fresh thymic tissue was obtained from patients who were partially thymectomized in the context of heart surgery. The tissue was manually prepared in sterile buffer solution (phosphate-buffered saline with 2 mM EDTA and 2% bovine serum albumin) for dissociation with the gentle MACS Dissociator (Miltenyi Biotech, Bergisch Gladbach, Germany). After dissociation, single cell suspensions were obtained by passing through 70 μm and 40 μm cell strainers with washing in between. Cells were then counted and resuspended in stimulation medium (RPMI-1640, 1% P/S, 10% FCS, 1% NEAA and 0.05 mM b-mercaptoethanol). PBMC were isolated by density gradient centrifugation (Ficoll-Hypaque; GE Healthcare) from fresh heparinized blood samples from healthy donors and resuspended in stimulation medium (RPMI-1640, 1% P/S, 10% F S). Thymocytes and PBMC were then supplemented with either ATG-F or ATG-G at the following concentrations:2.5 μg/mL, 25 μg/mL and 100 μg/mL. No treatment was given to controls. Cells were redistributed in 96-well plates with 2x106 cells in 500 μL stimulation medium per two wells and incubated for 20 h at 37°C Cells were then co-stained with propidium iodide solution and annexin V and immediately analyzed by flow cytometry, in order to determine the level of necrosis and apoptosis.
Complement-dependent cytotoxicity assay
Freshly isolated human thymocytes or PBMC were resuspended at a density of 5x106 cells/mL in culture medium containing 2.5 μg/mL, 25 μg/mL or 100 μg/mL ATG-F or ATG-G, respectively. One hundred microliters of each suspension were added to round-bottomed microtiter plates and incubated for 60 min at 4°C. After addition of 100 μL freshly prepared human serum the cells were incubated for 60 min at 37°C. Finally the samples were stained with propidium iodide solution and immediately analyzed by flow cytometry.
Statistics and software
Calculations were performed in GraphPad Prism v5.0 (GraphPad Software, La Jolla, CA, USA). Group comparisons were made with the Mann-Whitney U test.
Results
Treatment with antithymocyte globulin-Genzyme™ impairs CD4 but not CD8 T-cell recovery
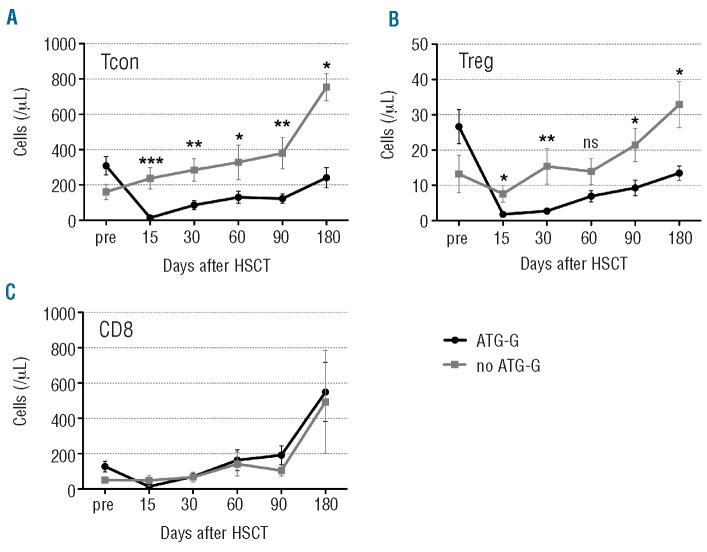
Within the CD3 T-cell compartment we analyzed the recovery of CD4 Tcon (Figure 1A) and Treg (Figure 1B). In the ATG-G group the total peripheral CD4+ Tcon and Treg cell counts were diminished by roughly 95% at day +15, and their recovery from day 30 to day 180 was significantly delayed compared to that observed in the cohort of patients not treated with ATG-G. Mean CD4+ Tcon cell counts did not exceed 250 cells/μL at any time point after transplantation in patients treated with ATG-G, while mean Treg cell counts did not exceed 15 cells/μL. In contrast, there was no significant difference in CD8 T-cell recovery at any of the defined time points between the cohorts of patients treated or not treated with ATG-G (Figure 1C)
Figure 1.
CD4 T lymphopenia in ATG-G-treated patients. The graphs illustrate (A) conventional CD4 (Tcon;CD3+CD4+CD25lowFOXP3- lymphocytes), (B) regulatory CD4 (Treg; CD3+CD4+CD25highFOXP3+ lymphocytes) and (C) CD8 (CD3+CD8+ lymphocytes) T-cell subsets [mean values of absolute numbers of cells/μL ± standard error of mean (SEM)] before (pre) and after transplantation. Differences in cell counts between patients treated or not with ATG-G were statistically evaluated using the Mann-Whitney test. *P<0.05; **P<0.01; ***P<0.005.
T-cell subset-dependent recovery after allogeneic hematopoietic stem cell transplantation
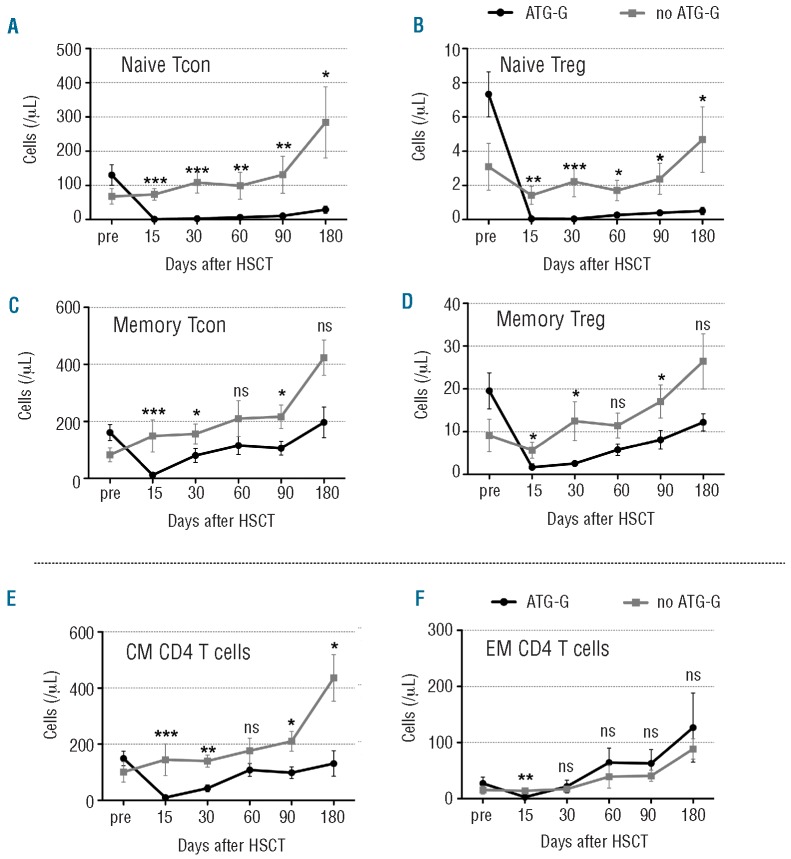
We next differentiated naïve (CD45RO CD45RA+) and memory (CD45RO+ CD45RA-) CD4+ Tcon and Treg. The effect of ATG-G was most pronounced in the naïve subsets of both Tcon (Figure 2A) and Treg (Figure 2B). At all time points after transplantation the naïve cell counts were significantly lower in the ATG-G group than in the group not treated with ATG-G.
Figure 2.
Reconstitution of the CD4 T-cell naïve and memory compartments. The graphs illustrate (A) naïve conventional CD4 T cells (naïve Tcon; CD25lowFOXP3-CD45RA+), (B) naïve regulatory CD4 T cells (naïve Treg; CD25highFOXP3+CD45RA+), (C) memory Tcon (CD25lowFOXP3-CD45RO+CD45RA-) and (D) memory Treg(CD25highFOXP3+CD45RO+CD 45RA-). Graphs (E) and (F) illustrate central memory (CM, CD45RO+CD62L+CCR7+) and effector memory (EM, CD45RO+CD62L-CCR7-) subsets of total CD4 T cells, respectively. Data were collected before (pre) and after transplantation. Mean values of absolute numbers of cells/μL ± standard error of mean (SEM) are given. Differences in cell counts between patients treated or not with ATG-G were statistically evaluated using the Mann-Whitney test. *P<0.05; **P<0.01; ***P<0.005.
Memory CD4 Tcon (Figure 2C) and Treg (Figure 2D) were also more severely depleted in the group treated with ATG-G than in the group not treated with ATG-G, but showed an overall more rapid recovery starting from day 30 after transplantation than the respective naïve subsets. Memory T cells were further differentiated into central memory CD4 T cells and effector memory CD4 T cells. Central memory CD4 T cells were almost completely depleted on day 15 in ATG-G-treated patients. Their recovery was delayed until day 180, whereas they recovered faster and to higher levels in the group not treated with ATG-G (Figure 2E). Levels of effector memory CD4 T cells were significantly lower only on day 15; otherwise their levels were comparable in the two groups (Figure 2F).
Impaired thymic generation of both naïve conventional and naïve FOXP3+ regulatory CD4+ T cells
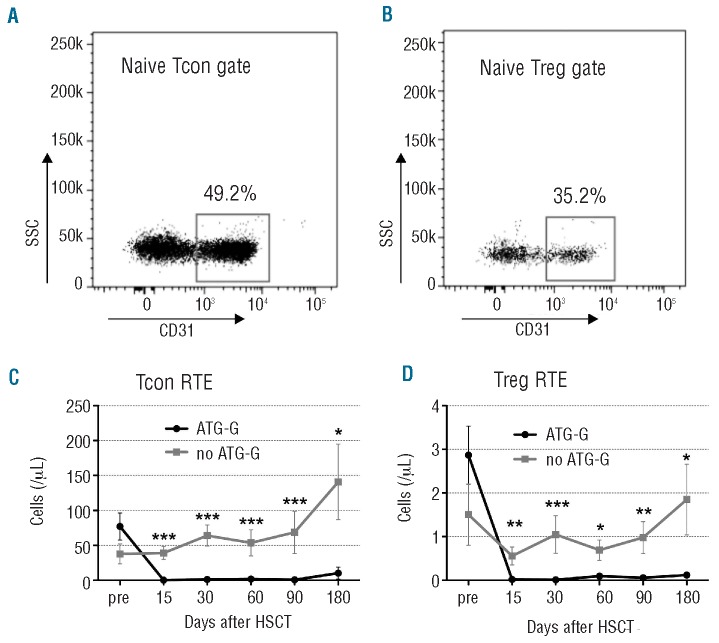
Since the naïve cell subset in particular was depleted, we delineated the presence of RTE, in order to determine the thymic function and output of thymic T cells. We analyzed the recovery of the RTE CD4+ T cells based on CD31 expression. Representative gates of Tcon RTE and Treg RTE are shown in Figure 3A and 3B, respectively. In the ATG-G-treated group a nearly complete lack of RTE of both Tcon and Treg cells was observed from day 15 until day 180. The Tcon RTE cell count initially decreased by 99.5% and its recovery did not start until day 180 (Figure 3C). Similarly, Treg RTE cells were strongly depleted and remained stable at very low levels throughout the whole time to day 180 in the ATG-G-treated group (Figure 3D). In contrast, no significant decrease was observed in the group not treated with ATG-G.
Figure 3.
Reduced thymic generation of Tcon and Treg in ATG-G treated patients. Representative gate of (A) naïve conventional CD4 T cells (naïve Tcon; CD25lowFOXP3-CD45RA+) and (B) naïve regulatory CD4 T cells (naïve Treg; CD25highFOXP3+CD45RA+) for identification of CD31+ recent thymic emigrants (RTE). The graphs illustrate mean values of absolute numbers of cells/μL ± standard error of mean (SEM) before (pre) and after transplantation of (C) TconRTE and (D) TregRTE. Differences in cell counts between patients treated or not with ATG-G were statistically evaluated using the Mann-Whitney test. *P<0.05; **P<0.01; ***P<0.005.
Differential effects of Genzyme™ and Fresenius™ rabbit antithymocyte globulins on human thymocytes and peripheral blood mononuclear cells in vitro
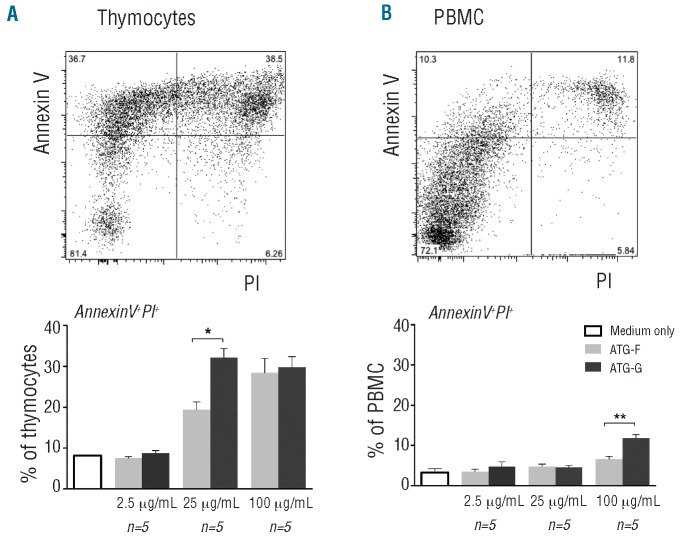
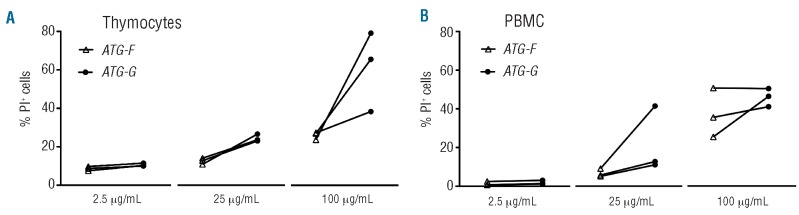
We hypothesized, that ATG-G might have a strong cytotoxic effect on human thymocytes as ATG-G-treated patients had significantly lower RTE counts and ATG-G is manufactured by immunization of rabbits with human thymocytes. We compared ATG-G with ATG-F, as ATG-F is manufactured by immunization of rabbits with Jurkat cells and not thymocytes. The in vitro cytotoxic effect of both ATG preparations on cultured human thymocytes (Figure 4A) and PBMC (Figure 4B) was studied at concentrations of 2.5 μg/mL, 25 μg/mL and 100 μg/mL. In order to determine levels of necrosis and apoptosis, we co-stained for propidium iodide and annexin V. An untreated population was used to define the basal level of apoptotic and dead cells. The cytotoxic effect of ATG was generally greater for thymocytes than for PBMC and most prominent in the propidium iodide+/annexin V+ cells, characterizing the late apoptotic and necrotic cells shown in Figure 4. ATG-G treatment of thymocytes with 25 μg/mL (P<0.05) and PBMC with 100 μg/mL (P<0.01) led to significantly more propidium iodide+/annexin V+ cells than ATG-F treatment. The difference between the two types of ATG for the propidium iodide-/annexin V+ and propidium iodide+/annexin V- cells was not significant at any concentration for thymocytes and PBMC (data not shown).
Figure 4.
Differential effect of ATG-G and ATG-F on human thymocytes and PBMC. Percentage of propidium iodide (PI)+/annexin V+ (A) thymocytes and (B) PBMC [mean values with standard error of mean (SEM)] after treatment with either ATG-G (dark gray) or ATG-F (light gray) for 20 h at different concentrations. Representative dot plots for thymocytes and PBMC are shown (upper images). Differences in percentages of PI+/annexin V+ cells were statistically evaluated using the Mann-Whitney test. *P<0.05; **P<0.01; ***P<0.005. Different ATG concentrations were tested in three independent experiments.
In order to investigate complement-mediated lysis as another important mechanism, we performed complement-dependent cytotoxicity assays, as described by Popow et al.18 These assays showed that both types of ATG caused dose-dependent specific lysis of thymocytes (Figure 5A) as well as PBMC (Figure 5B). Compared to ATG-F, ATG-G, at the concentrations of 25 and 100 μg/mL for thymocytes and PBMC, respectively, caused more complement-mediated lysis of both types of cells.
Figure 5.
Dose-dependent complement-mediated lysis of thymocytes and PBMC by ATG-G and ATG-F. Percentage of propidium iodide-positive (PI+) (A) thymocytes and (B) PBMC [mean values with standard error of mean (SEM), n=3] after complement-mediated specific lysis after treatment with either ATG-G (filled circles) or ATG-F (open triangles) at different concentrations. The percentages of PI+ cells were tested in two independent experiments.
Discussion
In our study, we analyzed the effect of ATG-G on thymic function and reconstitution of the T-cell compartment early after allogeneic HSCT. Our initial findings demonstrated a significantly delayed recovery of total CD4 T-cell counts in ATG-G-treated patients, whereas total CD8 T-cell counts were similar in patients treated or not treated with ATG-G. A number of studies showed a more rapid recovery of CD8 T cells than CD4 T cells in allogeneic HSCT recipients,19,20 and various factors, such as older age and toxicity of the conditioning regimen have been associated with delayed CD4 T-cell recovery.21-23 A functional thymus has been shown to be necessary for the reconstitution of naïve CD4 T cells, whereas thymic-independent pathways seem to exist to regenerate naïve CD8 T cells.24 Our study suggests that ATG-G treatment is an additional factor that is associated with severe and prolonged thymic dysfunction after allogeneic HSCT.
ATG-G-treated patients exhibited a reconstitution failure of RTE of both Tcon and Treg in the peripheral blood, with hardly any recovery by day 180 after allogeneic HSCT. With regards to Tcon our results support previous findings by Sairafi et al., who reported reduced TREC levels in ATG-G-treated patients in a retrospective study.25 Regarding Treg we found an impairment of thymic generation of Treg, which had not been previously reported. Matsuoka et al.26 found abnormal Treg homeostasis after allogeneic HSCT among patients not treated with ATG-G; these patients had limited thymic generation of Treg and disturbed proliferative and apoptotic patterns. Our study showed that ATG-G treatment aggravates the imbalance by further limiting thymic output. Normal Treg homeostasis may be crucial especially after allogeneic HSCT, as Treg regulate responses to allogeneic target antigens27 and are, therefore, important in controlling GvHD.28-30
Results from in vitro studies31,32 and solid organ transplantation research33 suggested that ATG-G is capable of promoting Treg expansion thus contributing to GvHD prophylaxis. On the other hand, impaired thymic generation of Treg, as found in our study, may favor GvHD. Total body irradiation is also known to influence thymocyte viability negatively.6 A possible potentiation of irradiation and the effects of ATG-G cannot be excluded and might have additionally influenced RTE recovery in this study (Table 1).
With regards to the duration of the effects of ATG-G, Waller et al. performed pharmacokinetic analyses showing that active ATG-G is rapidly cleared (half-life, 6 days) to sub-therapeutic levels (<1 μg/mL) by a median of 17±9 days after transplantation in comparable treatment conditions.34 They postulated that slow and prolonged T-cell recovery was not, therefore, due to effects of ATG-G, assuming ATG-G activity only if the product was present at therapeutic levels. However, in view of our results for Tcon RTE and Treg RTE recovery we suggest that ATG-G also targets the thymus with long-term consequences for thymic T-cell recovery beyond week 3 after transplantation. ATG-G is prepared by immunizing rabbits with cells derived from the thymus organ, so ATG-G should contain antibodies potentially targeting all cells within the thymus. The complex architecture of the human thymus is difficult to mimic in an experimental set-up and it is not, therefore, possible to determine in vitro concentrations of ATG that realistically reflect those concentrations of ATG that would be found within the thymus in vivo in the clinical situation of allogeneic HSCT. Additionally, human thymic tissue is difficult to obtain, because it cannot be readily biopsied. We, therefore, decided to test different concentrations of ATG-G in a simplified experimental approach with fresh cultured human thymocytes. While it has already been shown that ATG-G can induce apoptosis of PBMC,35 we showed that this ATG preparation also has an apoptotic effect on cultured human thymocytes. Furthermore, thymocytes appeared to be more susceptible than PBMC to ATG-G-induced cell death. As ATG-F is produced by immunization of rabbits against a Jurkat cell line and not against human thymocytes, we also compared the effect of ATG-F and ATG-G on thymocytes. We found that at a dose of 25 μg/mL, ATG-G had a significantly stronger pro-apoptotic effect (late apoptosis) than ATG-F on thymocytes. In a recent study Popow et al. showed that complement-dependent lysis is an important mechanism in ATG-mediated cytotoxicity.18 Our results suggest that complement-dependent lysis is of equal importance in thymocyte toxicity of ATG-G.
Our in vitro data appear consequential in view of the different methods of producing the two ATG preparations and support the assumption that ATG-G affects the thymus directly. In this respect it would be interesting to perform comparative clinical studies with other T-cell-depleting therapeutic approaches such as alemtuzumab or ATG-F, all differing in their biological activity. However, there are only a few studies comparing the different in vivo T-cell depletion treatments in allogeneic HSCT patients: Soiffer et al. investigated ATG-containing and alemtuzum-ab-containing regimens,36 while Basara et al. analyzed the effects of ATG-G and ATG-F on GvHD and relapse risk.37 In the light of our results, thymic function should be examined in detail in future comparative studies, as this might help with the choice of T-cell-depleting treatment for different clinical indications.
While recovery of naïve T cells was almost completely suppressed in the first 6 months in ATG-G-treated patients, memory T-cell counts increased continuously, although later than those in patients not treated with ATG-G. Looking at the memory cell compartment, our results suggest a preferential effect of ATG-G on central memory CD4 T cells compared to effector memory CD4 T cells in the setting of allogeneic HSCT. To our knowledge this effect has so far only been shown in the setting of solid organ transplants.33,38 It has been suggested that CD4 memory T cells can cause a graft-versus-leukemia effect without causing GvHD in mice,39 a phenomenon that has more recently been ascribed to effector memory CD4 T cells in murine models.40,41 On the other hand, from clinical studies it is known that ATG-G contributes to prevention of GvHD without causing higher relapse rates in humans. The shift in the CD4 memory compartment after ATG-G treatment in favor of effective memory CD4 T cells in our study might thus be interpreted as an indication for one of the mechanisms underlying this mode of action of ATG-G.
Taken together, our findings suggest that ATG-G, which is raised against human thymocytes, may directly affect thymic cells and result in functional deficits. However, given the rather small sample number, our data need to be confirmed in larger studies. In this respect, we suggest further comparative trials of different T-cell-depleting approaches. Another possible implication of our study is that thymus-protective and/or-supportive therapies, such as keratinocyte growth factor, interleukin 7, growth hormone and others which are already being discussed as treatment approaches to limit thymic damage by conditioning regimens (irradiation/chemotherapy) or GvHD, should be considered, especially when ATG-G is used as GvHD prophylaxis.
Acknowledgments
We thank Sandra Bauer for her technical support and Professors Hetzer and Hübler from the German Heart Institute Berlin (Deutsches Herzzentrum Berlin, DHZB) for making human thymocytes available to us.
Funding: This work was supported by a grant from the Experimental and Clinical Research Center and BMBF (0315848A, Charité).
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org
References
- 1.Dumont-Girard F, Roux E, van Lier RA, Hale G, Helg C, Chapuis B, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92(11):4464-71 [PubMed] [Google Scholar]
- 2.Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875-81 [DOI] [PubMed] [Google Scholar]
- 3.Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113(25):6477-84 [DOI] [PubMed] [Google Scholar]
- 4.Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99(12):4592-600 [DOI] [PubMed] [Google Scholar]
- 5.Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985; 88:107-33 [DOI] [PubMed] [Google Scholar]
- 6.Na IK, Lu SX, Yim NL, Goldberg GL, Tsai J, Rao U, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. J Clin Invest. 2010;120(1):343-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux E, Dumont-Girard F, Starobinski M, Siegrist CA, Helg C, Chapuis B, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000; 96(6):2299-303 [PubMed] [Google Scholar]
- 8.Ochs L, Shu XO, Miller J, Enright H, Wagner J, Filipovich A, et al. Late infections after allogeneic bone marrow transplantations: comparison of incidence in related and unrelated donor transplant recipients. Blood. 1995;86(10):3979-86 [PubMed] [Google Scholar]
- 9.Maraninchi D, Gluckman E, Blaise D, Guyotat D, Rio B, Pico JL, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias. Lancet. 1987;2(8552):175-8 [DOI] [PubMed] [Google Scholar]
- 10.Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Mowes B, Radbruch A, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195(6):789-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31-human naive CD4+ T-cell subsets. Blood. 2009;113(4):769-74 [DOI] [PubMed] [Google Scholar]
- 12.Nickel P, Kreutzer S, Bold G, Friebe A, Schmolke K, Meisel C, et al. CD31+ naive Th cells are stable during six months following kidney transplantation: implications for post-transplant thymic function. Am J Transplant. 2005;5(7):1764-71 [DOI] [PubMed] [Google Scholar]
- 13.Duszczyszyn DA, Beck JD, Antel J, Bar-Or A, Lapierre Y, Gadag V, et al. Altered naive CD4 and CD8 T cell homeostasis in patients with relapsing-remitting multiple sclerosis: thymic versus peripheral (non-thymic) mechanisms. Clin Exp Immunol. 2006;143(2):305-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201(5):805-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37(11):3270-80 [DOI] [PubMed] [Google Scholar]
- 16.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387-94 [DOI] [PubMed] [Google Scholar]
- 17.Mueller T. Mechanisms of action of thymoglobulin. Transplantation. 2007;84(11S):S5-S10 [Google Scholar]
- 18.Popow I, Leitner J, Majdic O, Kovarik JJ, Saemann MD, Zlabinger GJ, et al. Assessment of batch to batch variation in polyclonal antithymocyte globulin preparations. Transplantation. 2012;93(1):32-40 [DOI] [PubMed] [Google Scholar]
- 19.Ashihara E, Shimazaki C, Yamagata N, Hirata T, Okawa K, Oku N, et al. Reconstitution of lymphocyte subsets after peripheral blood stem cell transplantation: two-color flow cytometric analysis. Bone Marrow Transplant. 1994;13(4):377-81 [PubMed] [Google Scholar]
- 20.Shenoy S, Mohanakumar T, Todd G, Westhoff W, Dunnigan K, Adkins DR, et al. Immune reconstitution following allogeneic peripheral blood stem cell transplants. Bone Marrow Transplant. 1999;23(4):335-46 [DOI] [PubMed] [Google Scholar]
- 21.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18(1):10-8 [DOI] [PubMed] [Google Scholar]
- 22.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89(10):3700-7 [PubMed] [Google Scholar]
- 23.Hoepfner S, Haut PR, O'Gorman M, Kletzel M. Rapid immune reconstitution following autologous hematopoietic stem cell transplantation in children: a single institution experience. Bone Marrow Transplant. 2003;31(4):285-90 [DOI] [PubMed] [Google Scholar]
- 24.Heitger A, Neu N, Kern H, Panzer-Grumayer ER, Greinix H, Nachbaur D, et al. Essential role of the thymus to reconstitute naive (C45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90(2):850-7 [PubMed] [Google Scholar]
- 25.Sairafi D, Mattsson J, Uhlin M, Uzunel M. Thymic function after allogeneic stem cell transplantation is dependent on graft source and predictive of long term survival. Clin Immunol. 2012;142(3):343-50 [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144-50 [DOI] [PubMed] [Google Scholar]
- 29.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002; 99(10):3493-9 [DOI] [PubMed] [Google Scholar]
- 30.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+ CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17(10):2844-53 [DOI] [PubMed] [Google Scholar]
- 33.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymo-cyte globulin. Am J Transplant. 2010;10(9):2132-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ, et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2003;9(7):460-71 [DOI] [PubMed] [Google Scholar]
- 35.Genestier L, Fournel S, Flacher M, Assossou O, Revillard JP, Bonnefoy-Berard N. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91(7):2360-8 [PubMed] [Google Scholar]
- 36.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basara N, Baurmann H, Kolbe K, Yaman A, Labopin M, Burchardt A, et al. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant. 2005; 35(10):1011-8 [DOI] [PubMed] [Google Scholar]
- 38.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, Mc oy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465-74 [DOI] [PubMed] [Google Scholar]
- 39.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L-memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534-41 [DOI] [PubMed] [Google Scholar]
- 41.Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008; 111(4):2476-84 [DOI] [PMC free article] [PubMed] [Google Scholar]