Abstract
Despite advances in immunosuppressive regimens, acute graft-versus-host disease remains a frequent complication of allogeneic hematopoietic cell transplantation. Pathogenic donor T cells are dependent on correct attachment of small GTPases to the cell membrane, mediated by farnesyl- or geranylgeranyl residues, which, therefore, constitute potential targets for graft-versus-host disease prophylaxis. A mouse model was used to study the impact of a farnesyl-transferase inhibitor and a geranylgeranyl-transferase inhibitor on acute graft-versus-host disease, anti-cytomegalovirus T-cell responses and graft-versus-leukemia activity. Treatment of mice undergoing allogeneic hematopoietic cell transplantation with farnesyl-transferase inhibitor and geranylgeranyl-transferase inhibitor reduced the histological severity of graft-versus-host disease and prolonged survival significantly. Mechanistically, farnesyl-transferase inhibitor and geranylgeranyl-transferase inhibitor treatment resulted in reduced alloantigen-driven expansion of CD4 T cells. In vivo treatment led to increased thymic cellularity and polyclonality of the T-cell receptor repertoire by reducing thymic graft-versus-host disease. These effects were absent when squalene production was blocked. The farnesyl-transferase inhibitor and geranylgeranyl-transferase inhibitor did not compromise CD8 function against leukemia cells or reconstitution of T cells that were subsequently responsible for anti-murine cytomegalovirus responses. In summary, we observed an immunomodulatory effect of inhibitors of farnesyl-transferase and geranylgeranyl-transferase on graft-versus-host disease, with enhanced functional immune reconstitution. In the light of the modest toxicity of farnesyl-transferase inhibitors such as tipifarnib in patients and the potent reduction of graft-versus-host disease in mice, farnesyl-transferase and geranylgeranyl-transferase inhibitors could help to reduce graft-versus-host disease significantly without having a negative impact on immune reconstitution.
Introduction
Currently applied prophylaxis against graft-versus-host disease (GvHD) significantly reduces the beneficial effects of donor T cells, especially the control of opportunistic viral infections and graft-versus-leukemia activity. Strategies which promote tolerance and at the same time have antitumor activity may help to improve outcome after allogeneic hematopoietic cell transplantation (HCT). Activation, cytokine production and expansion of pathogenic donor T cells are hallmarks of GvHD. The process of T-cell activation is dependent on functional GTPases that transmit transmembrane signaling. Among isoprenylated proteins the GTPases Ras, Rheb, Rho and Rab are crucial in biochemical signaling cascades controlling pro-inflammatory gene expression and T-cell phenotype.1 In order to be active, GTPases must associate with the T-cell membrane, a process which is dependent on their covalent attachment to isoprenoids.2In vivo studies in different murine models demonstrated that farnesyltransferase inhibitors (FTI) can reduce the severity of collagen-induced arthritis3 and that this effect is paralleled by a decreased expression of the pro-inflammatory cytokines tumor necrosis factor (TNF) and interleukin (IL)-1β, which also play a central role in GvHD. There are several lines of evidence indicating that allogeneic immune responses can be modified by the inhibition of protein farnesylation or geranylation. By using a mouse model for the rejection of MHC class II-disparate skin allografts it was observed that treatment with FTI delayed the rejection process.4 Cinnamaldehyde, which inhibits farnesyltransferase,5 was shown to modulate T-cell differentiation and proliferation.6 The anti-inflammatory activity of protein FTI was also examined in various in vitro and in vivo studies.7,8 Measurement of FTI-induced inhibition of nuclear factor kappa B (NFκB)-mediated pro-inflammatory effects revealed significant inhibition of mRNA and protein production of the chemokines CCL2 and CCXL1 with nanomolar doses of the FTI R115777.9 Inhibition of Ras has been shown to increase FoxP3 expression in a human T-cell line,10 suggesting that FTI treatment could increase the number of T regulatory cells which can effectively contribute to the control of GvHD.11 A large clinical study showed that the incidence of rejection after cardiac transplantation was reduced by pravastatin.12 Pravastatin is an inhibitor of the HMG-CoA reductase, which inhibits protein farnesylation and geranylation; its protective effect against cardiac allograft rejection was independent of the cholesterol level which indicated a second mechanism that was not related to the lipid-lowering effects of pravastatin and was most likely an immunomodulatory mechanism. Although these data suggest an immunoregulatory potential of FTI and geranylgeranylation inhibitors (GGTI), the impact of these inhibitors on GvHD was unclear.
In this study we examined the effects of FTI and GGTI on acute GvHD severity, immune reconstitution, the graft-versus-leukemia effect and response to murine cytomegalovirus (MCMV) in a murine model.
Design and Methods
Mice
The animals used in this study are described in the Online Supplementary Design and Methods.
Bone marrow transplantation model and histopathology of graft-versus-host disease
Bone marrow transplantation experiments were performed as previously described.13 The severity of GvHD was determined according to a previously published histopathology scoring system.14
B-cell leukemia model and in vitro cytotoxicity assay
To investigate the graft-versus-leukemia activity of transferred donor T cells, we employed an A20-luc B-cell leukemia that had been previously demonstrated to migrate primarily to the bone marrow, with secondary infiltration of the spleen and other lymphoid organs when injected intravenously at the time of bone marrow transplantation.11 Animals were injected with A20-luc-cells (BALB/c background, 2×105) 2 days prior to administration of the T cells to allow the tumor to home and establish.
To evaluate anti-tumor responses in vitro, CD4+ and CD8+ T cells from C57BL/6 splenocytes were purified by negative selection and co-cultured for 6 h with L1210 leukemia cells (BALB/c background). Cytotoxicity was quantified by flow cytometry-based analysis of propidium iodide-positive cells.
Murine cytomegalovirus model
To evaluate antiviral T-cell responses, the MCMV (Smith strain) was employed. MCMV was produced by tissue culture with mouse embryonic fibroblasts. On day 30 after bone marrow transplantation, 1×105 plaque-forming units of MCMV were injected intraperitoneally. Further details on the MCMV model are provided in the Online Supplementary Design and Methods.
In vivo and in vitro treatment with farnesyl-transferase and geranylgeranyl-transferase inhibitors
FTI-276, GGTI-2133 and zaragocic acid A (ZA) (all from Sigma Aldrich, Munich, Germany) were administered intraperitoneally at a previously reported dosage of 20 mg/kg (FTI, GGTI) or 10 mg/kg (ZA) daily from day -1 to day +10 after allogeneic HCT.
Cells were incubated with 10 μM FTI-276, GGTI-2133 or ZA for the periods indicated for each individual experiment.
T-cell receptor Vβ spectratyping
RNA was extracted from splenic single-cell suspensions using the RNeasy Mini Kit (Qiagen, Düsseldorf, Germany) and then reverse-transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Munich, Germany). T-cell receptor (TCR) CDR3 spectratyping was performed as previously described.15 Aliquots of the run-off reactions were analyzed on an ABI 3130 XL capillary sequencer (Applied Biosystems, Darmstadt, Germany).
In vivo bioluminescence imaging
Briefly, mice were injected intraperitoneally with luciferin (150 μg/g bodyweight). Ten minutes later the mice were imaged using an IVIS100 charge-coupled device imaging system (Xenogen, Alameda, CA, USA) for 5 min.16 Cell expansion was quantified in photons/second/mouse. Imaging data were analyzed and quantified with Living Image 3.0 software (Calipers, Rüsselsheim, Germany).
In vitro proliferation assays
CD4+ or CD8+ T cells from BALB/c mice were purified by positive selection. CFSE labeling of cells was with Vybrant CFDA SE (Molecular Probes, Eugene, OR, USA), as previously described,17 with 5 days of incubation.
Generation of bone marrow-derived dendritic cells
Bone marrow dendritic cells were prepared as described elsewhere.18 Bone marrow cells were cultured at 5×106 cells/10 mL in the presence of 40 ng/mL granulocyte-monocyte colony-stimulating factor (supernatant from the producer line X63-Hybridom). On days 3 and 5, 10 mL of fresh medium containing granulocyte-monocyte colony-stimulating factor were added.
Flow cytometry
All antibodies were purchased from BD Biosciences, Biolegend, (Fell, Germany) and eBiosciences (Hatfield, UK) and used conjugated with fluorescein isothiocyanate, phycoerythrin, Alexa647 or Pacific Blue conjugates. The antibodies used are listed in the Online Supplementary Design and Methods.
Data were acquired with a CyanADP (Beckman Coulter, Krefeld, Germany) and then analyzed with FlowJo 7/8 software (Tree Star, Ashland, OR, USA).
Conventional histology and immunohistochemistry
Fresh-frozen sections of 5 μm thickness were mounted on microscope slides (Superfrost/Plus; R. Langenbrink). For morphological analysis the tissues were stained with hematoxylin (Dako, Hamburg, Germany) and eosin (Thermo scientific, München, Germany) (H/E) and analyzed on a Zeiss Axioplan 2 microscope (Zeiss, Jena, Germany). The standard objectives used were 20x/numerical aperture 0.45 and 40x/numerical aperture 0.60. For immunoenzymatic staining the tissue was fixed for 10 min in acetone (Sigma) and the primary biotinylated antibody was applied. For visualization either streptavidin alkaline phosphatase-coupled antibody and corresponding substrate (Vector labs, Peterborough, UK) or the DAB-system (Dako Cytomation) was used.
Histopathological analysis of the thymus
Thymi were fixed using 4% paraformaldehyde and later embedded in paraffin. Sections 4-μm thick were stained with H/E. To distinguish between the cortex and the medulla by immunofluorescence, paraffin sections were stained using cytokeratin 5 (CK5) (catalog n. PRB-160P; Covance). The detection system on the secondary antibodies was alkaline phosphatase and the corresponding substrate (Vector labs). Sections were analyzed using a Zeiss Axioplan 2 Imaging microscope. For objective analysis of cortical thickness, the percentage of cortical area indicated by CK5-negative areas was determined. Each individual thymus was sectioned four times through various parts of the organ, 50 μm apart from each other. To assess cortical area, the percentage of the cortex as an index of cortical thinning was determined for all four sections using the following formula: ([total area - medullary area]/total area) ×100. Each percentage was averaged for the individual thymus.
Cytokine measurements
The levels of IL-6, IL-10, monocyte chemotactic protein 1 (MCP-1), TNF-α and interferon (IFN)-γ were analyzed with the flow cytometry-based CBA Inflammation kit (BD, Germany) in serum collected on day 7 by cardiac puncture.
Western blotting
After sodium dodecylsulfate polyacrylamide gel electrophoresis and transfer of proteins onto nitrocellulose membranes (Amersham Biosciences, Munich, Germany), ERK (cell signaling #9102), ribosomal protein S6 (cell signaling #2217), phosphorylated ERK (cell signaling #9101) and phosphorylated ribosomal protein S6 (cell signaling #4856) were analyzed using electrochemoluminescence (GE Healthcare). Densitometric analysis was performed using ImageJ software.
Statistical analysis
Data are reported as mean values ± standard error of mean. We compared pairs by the Student's t test with the Welch correction. Differences in animals' survival were analyzed by the log-rank test. A P value <0.05 was considered statistically significant.
Results
Farnesylpyrophosphate and geranylgeranylpyrophosphate but not squalenes are relevant for protection from graft-versus-host disease by L-mevalonate pathway blockade
In order to better define the relevance of the downstream L-mevalonate pathway products farnesylpyrophosphate, geranylgeranylpyrophosphate and squalene with regards to the severity of GvHD, we employed several pathway inhibitors. These included ZA, GGTI-2133 and FTI-276 (Figure 1A). Survival studies in two different major MHC class I and II mismatch models indicated that both FTI and GGTI reduced GvHD severity and prolonged survival following allogeneic HCT (Figure 1B and Online Supplementary Figure S1). Conversely, treatment with vehicle or ZA did not improve survival (Figure 1B).
Figure 1.
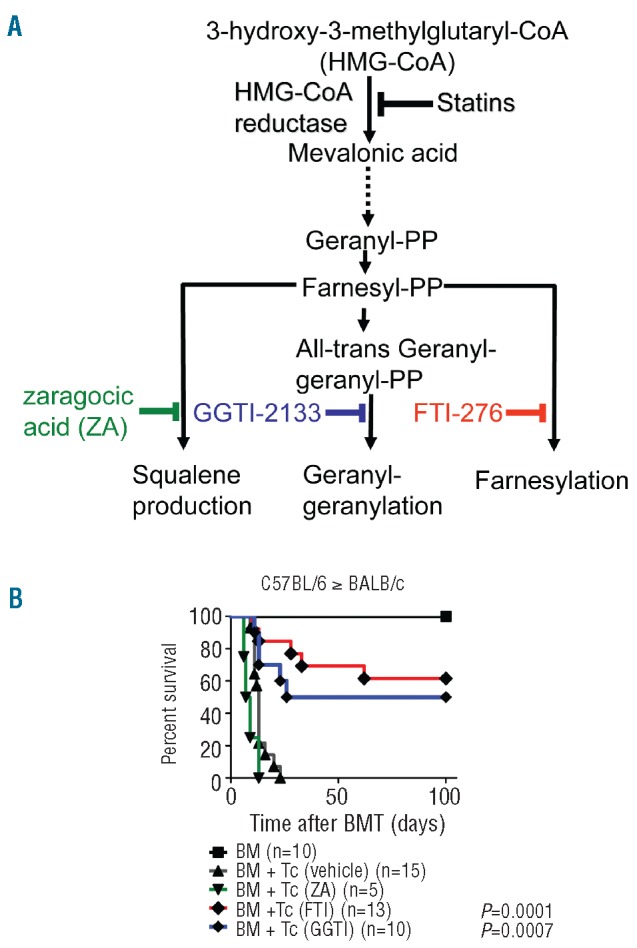
Pretreatment with FTI/GGTI improves survival after major MHC mismatched bone marrow transplantation (BMT). (A) The L-mevalonate pathway. Metabolites and enzymes in the pathway are shown in black, inhibitors of squalene production (green), geranylgeranylation (blue) and farnesylation (red) are shown in color. (B) Survival of mice receiving bone marrow alone (BM, black line, square), or with T cells and treatment with vehicle (black line, triangle), FTI (red line), GGTI (blue line), or zaragocic acid (ZA, green line) in the C57BL/6 into BALB/c model. The following dosage was applied: zaragocic acid, 10 mg/kg day -1 to +10, GGTI-2133, 20 mg/kg day -1 to +10 and FTI-276, 20 mg/kg day -1 to +10. Percentage survival of BMT recipients is significantly higher than that of the vehicle-treated group when FTI or GGTI were given (number of animals and P-values for the comparison with the vehicle group are indicated next to each treatment group). Data are pooled from at least three experiments.
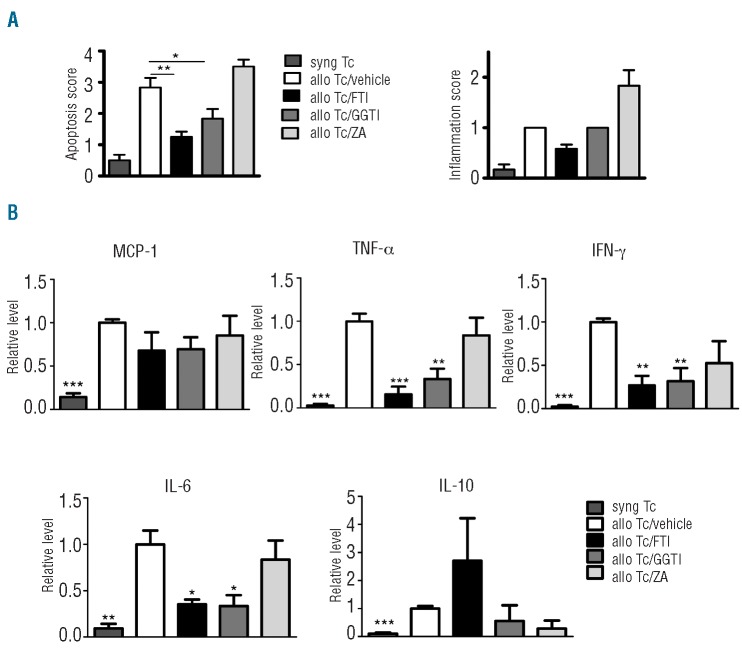
Consistent with its impact on survival, FTI and GGTI treatment led to a decrease in apoptosis and GvHD-related histopathology score for the small and large intestines (Figure 2A and Online Supplementary Figure S2). Compared to treatment with vehicle, FTI and GGTI treatment led to significantly reduced serum levels of TNF-α, IFN-γ and IL-6 (Figure 2B).
Figure 2.
FTI and GGTI treatment reduces GvHD severity and inhibits proinflammatory cytokine production. (A) Ten days after transplantation, mice from the indicated groups were sacrificed and analyzed. Histopathological scoring was performed as described in the Design and Methods section. Data are pooled from two experiments, representing six animals per group. The P-values are shown when a significant difference as compared to the vehicle group was found (*P<0.05, **P<0.01). (B) Serum was collected by cardiac puncture on day 7 after bone marrow transplantation and analyzed for the indicated cytokines. Data are pooled from three independent experiments, representing at least six animals per group. P-values are in comparison to the vehicle group and are defined as follows: *P<0.05, **P<0.01, ***P<0.001.
Inhibiting protein prenylation reduces the expansion of CD4+ but not CD8+ T cells
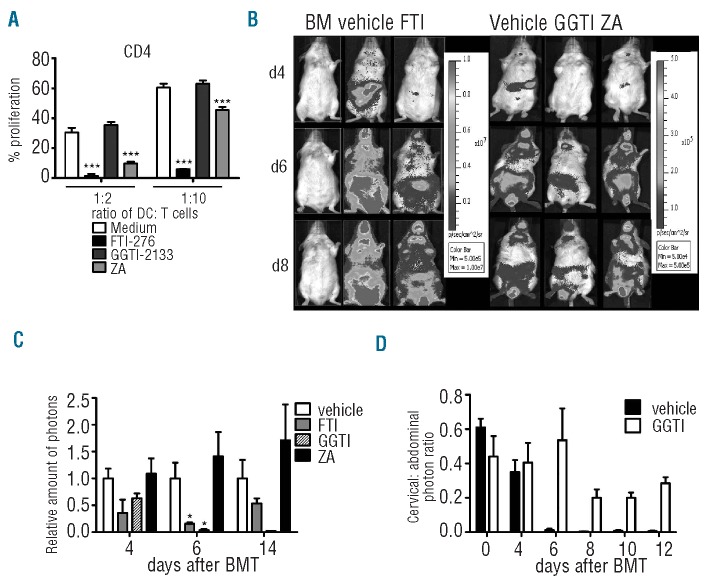
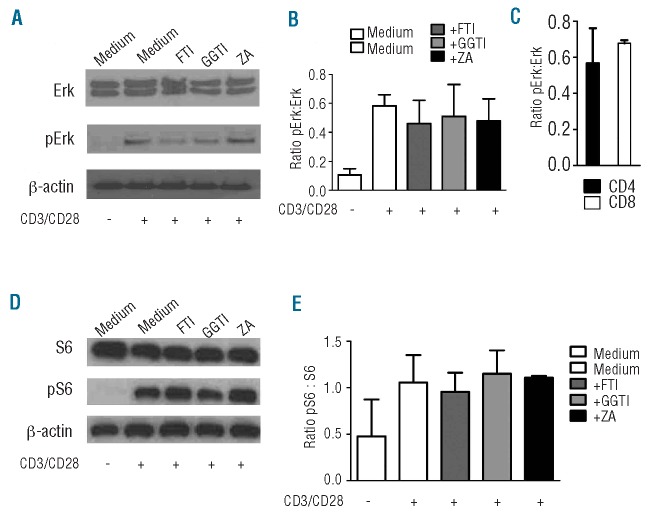
We hypothesized that FTI and GGTI may affect T-cell function based on the robust protective effect against GvHD observed with both drugs in the survival studies. Expansion of alloreactive T cells is a typical characteristic of acute GvHD. FTI and ZA reduced expansion of CD4+ cells in response to allogeneic antigen-presenting cells in vitro (Figure 3A). Compatible with this in vitro finding, we observed that FTI, GGTI and ZA did not significantly inhibit CD8 T-cell expansion in vivo (Online Supplementary Figure S3A,B). Conversely, FTI and GGTI treatment reduced the expansion of unseparated luciferase transgenic T cells after allogeneic HCT, quantified by photons over the total body area, as shown for representative time points (Figure 3B) and when quantified in photons (Figure 3C). As the photon signal seemed to be reduced in the abdominal region but not in the cervical region in the GGTI-treated group, we divided the signal intensity derived from the cervical lymph node area by the signal intensity from the abdominal area. The ratio in the GGTI-treated group was higher than that in the vehicle-treated group (Figure 3D). These differences were not seen when FTI/vehicle and ZA/vehicle ratios were calculated (data not shown). This may indicate that T cells were not able to leave the lymph nodes and migrate towards the gastrointestinal tract as a GvHD target organ in the GGTI-treated group. To evaluate the mechanism for this potent reduction of T-cell expansion in vivo and in vitro, we investigated the roles of squalene production and protein farnesylation and geranylation in the signaling steps involved in the extracellular signal-related kinase (ERK1/2) signaling pathway. This pathway was previously shown to be relevant for statin-mediated effects in other disease models.19 When FTI or GGTI was included in the culture 24 h before, there was a trend towards reduced ERK1/2 phosphorylation in T cells after stimulation with CD3/CD28, as determined by western blot (Figure 4A, B). The reduction in Erk activation seen with both drugs is most likely secondary to decreases in cytokine production and autocrine signaling by cytokine receptors that occur when farnesylation and geranylgeranylation are blocked. To study different phosphorylation patterns in CD4 and CD8 T cells we isolated and stimulated them separately and also detected a trend towards lower phosphorylation in CD4 T cells than in CD8 T cells when FTI (but not GGTI) was given (Figure 4C and data not shown). We also investigated phosphorylation of ribosomal protein S6, because FTI have previously been reported to inhibit signaling by Rheb,20 but could not find any difference when T cells were incubated with inhibitors (Figure 4 D, E). T-cell viability was not affected by exposure to FTI or GGTI in vitro (Online Supplementary Figure S4).
Figure 3.
Impact of FTI and GGTI on T-cell expansion in vitro and in vivo. (A) In vitro expansion of CFSE-stained CD4 T cells (H2b) stimulated with DC (H2d). Comparison of the absolute values when FTI-276, GGTI-2133 or ZA was included as compared to medium alone as indicated. Proliferation was assessed as reduced CFSE intensity by flow cytometry. Ratios represent dendritic cells: T cells. The results of one of three independent experiments with comparable results are shown (**P<0.01; ***P<0.001). (B) Allogeneic HCT was performed as described in the Design and Methods section with at least four mice in each group. In vivo expansion of luc transgenic T cells (H2b) in allogeneic recipients (H2d) is displayed for serial time points. Representative time points for BALB/c recipients with expanding luc transgenic T cells (H2b). (C) Where indicated FTI/GGTI as compared to vehicle treatment led to lower signal derived from expanding T cells (*P<0.05). (D) The signal derived from the cervical area divided by the signal from the abdominal area. The resultant ratio is displayed. Experiments were performed twice.
Figure 4.
FTI and GGTI treatment leads to reduced ERK signaling. (A) T cells (H2b) were preincubated with inhibitors for 24 h. Cells were then stimulated with CD3/CD28 beads and incubated with inhibitors for 4 h. Phosphorylated ERK protein was detected by western blot. (B) Quantification of the signal derived from phosphorylated ERK. Pooled data from three experiments are shown. (C) CD4+ or CD8+ T cells were separately pretreated with inhibitors for 24 h. Cells were then stimulated with CD3/CD28 beads and incubated with inhibitors for 4 h. Quantification of the signal derived from phosphorylated ERK for FTI-treated cells is shown. (D) T cells (H2b) were preincubated with inhibitors for 24 h and then stimulated with CD3/CD28 beads and incubated with inhibitors for 4 h. Phosphorylated ribosomal protein S6 was detected by western blot. (E) Quantification of the signal derived from phosphorylated ribosomal protein S6. Pooled data from three experiments are shown.
Reduced graft-versus-host disease after pharmacological inhibition of farnesyl- and geranylgeranyltranferase diminished thymic stroma damage and led to a more diverse T-cell receptor repertoire
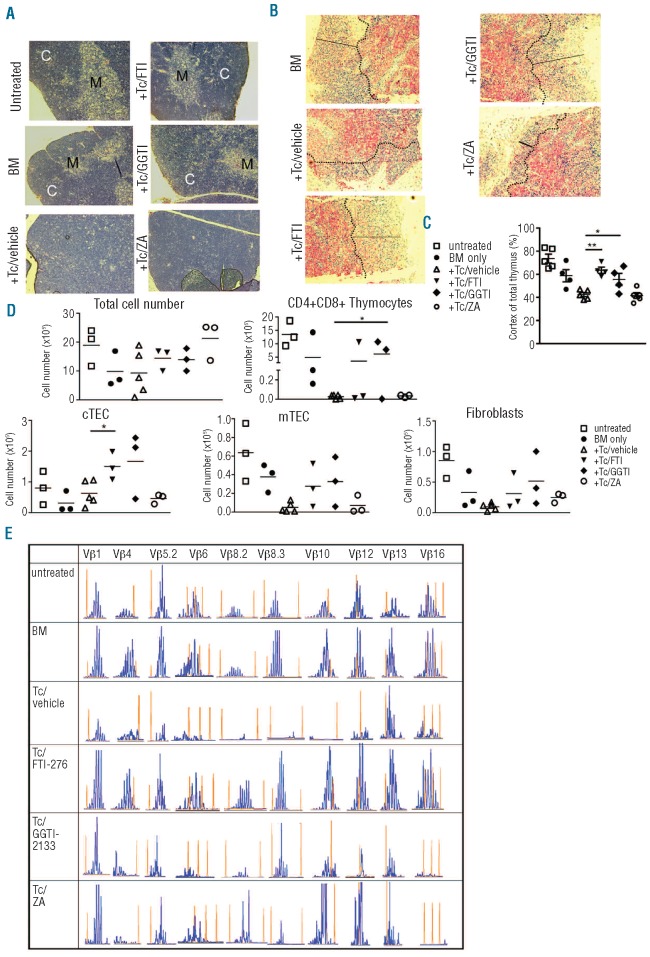
Since T-cell immunodeficiency is a central causative factor for the high morbidity and mortality after clinical allogeneic HCT, we next analyzed the impact of reduced GvHD on thymic structure and function in FTI/GGTI-treated mice. Conventional histology demonstrated an intact corticomedullary demarcation in the group of animals that had received bone marrow alone, while recipients of additional T cells and vehicle displayed a disturbed thymic architecture with no distinguishable cortical region (Figure 5A). Treatment of bone marrow/T-cell recipients with FTI or GGTI allowed recipients to survive the acute phase of GvHD and these animals later showed repaired thymic damage and a normal corticomedullary demarcation (Figure 5A). Immunhistochemical analysis with the thymic medullary marker CK5 demonstrated that cortical thinning, normally a key feature of thymic GvHD, was reduced when FTI/GGTI but not ZA treatment was given (Figure 5B). Quantitative analysis of the cortical area in relation to the total area of the thymus showed that the statistically significant loss of cortical area in recipients of T cells compared to in recipients of bone marrow alone was antagonized when FTI or GGTI was administered but not when ZA was given (Figure 5C). Cortical thinning was associated with significantly reduced numbers of CD4+CD8+ thymocytes (Figure 5D), which would indicate a disturbance in thymopoiesis. The number of CD4+CD8+ thymocytes was significantly higher when GGTI was administered than when T cells/vehicle was given (Figure 5D). In order to study host thymic stroma we determined the number of medullary thymic epithelial cells (mTEC), cortical thymic epithelial cells (cTEC) and fibroblasts after allogeneic HCT and found them to be dramatically reduced in the T-cell/vehicle group (Figure 5D). Thymi of mice treated with FTI or GGTI tended to have higher numbers of cells than thymi of animals treated with the vehicle (Figure 5D). These observations indicate that pharmacological inhibition of farnesyl- and geranylgeranyltranferase reduces hallmark features of thymic GvHD.
Figure 5.
FTI and GGTI reduce thymic damage and favor a polyclonal TCR repertoire. (A) Thymi were removed on day 10 from three mice in each group (vehicle; ZA)/d100 (others) and paraffin-fixed thymus sections (untreated, BM only, +T cells (Tc)/vehicle, +Tc/FTI, +Tc/GGTI and Tc/ZA) were analyzed for detection of the corticomedullary junction. C, cortex; M, medulla. Original magnification ×100. Representative sections are shown. (B) Representative paraffin-fixed thymus sections collected as described above (a) from the indicated groups were stained for K5-positive (red) medullary regions and analyzed by conventional microscopy. The dotted lines indicate the corticomedullary junction and solid lines represent cortical thickness. Original magnification, ×100. Representative images are shown. (C) The cortical area (% of total area) was quantified by using the CK5 negative region. Symbols represent individual animals, the horizontal bars indicate the mean. P-values: untreated versus vehicle P=0.039; BM only versus vehicle P=0.41. (D) Thymic cell subsets were assessed by flow cytometry on day 12 from three mice in each group. Absolute numbers are displayed. mTec: CD45-MHC class IIint/hiUEA-1+; Fibroblasts: CD45-PDGFR1 (CD140b)+(*P<0.05). (E) TCR Vβ usage of splenic T cells on day 20 after transplantation in BALB/c recipients is shown for the respective groups (untreated, BM only, +Tc/vehicle, +Tc/FTI, +Tc/GGTI and Tc/ZA). Spectratyping analysis of CDR3 length distribution was performed using C57BL/6-specific Vβ and common Cβ primers from purified splenic T cells. Representative spectratypes of Vβ1, 4, 5.2, 6, 8.2, 8.3, 10, 12, 13 and 16 gene families are shown. Histograms (blue peaks) depict CDR3 sequence lengths (x-axis) and frequency of occurrence (y axis). Red peaks within each histogram represent molecular weight markers added to the run-off reactions. Data are representative of at least three animals studied per group, from one of two experiments.
The next question was to determine whether or not T-cell immune reconstitution was polyclonal. To this end TCR-Vß spectratyping sequence analyses were carried out on the CDR3 region within each Vß region from cDNA derived from RNA extracted from spleens gathered on day 25 after bone marrow transplantation. A Gaussian distribution of the CDR3 region lengths in practically all of the 23 Vβ gene families studied was observed when either untreated animals or mice that only received bone marrow were assessed (Figure 5E). In contrast, bone marrow recipients treated with T cells and vehicle displayed a more restricted distribution of peaks, resulting in an oligoclonal (Vβ1, 5.2, 6.2, 8.3, 12, 16) or virtually clonal (Vβ8.2, 8.3) TCR-Vβ repertoire (Figure 5E). Conversely, the TCR-Vβ repertoire of animals that received T cells and FTI or GGTI appeared to be more diverse, with a polyclonal distribution of the CDR3 lengths of most Vβ regions (Figure 5E). By using congenic markers we determined that bone marrow-derived T cells (Thy1.2+) and transferred T cells (Th1.1+) on day 20 were equally represented in the spleens of the allogeneic HCT recipients. Analysis of the TCR Vβ repertoire by flow cytometry demonstrated a polyclonal repertoire in the groups given bone marrow only, FTI or GGTI, while the groups given ZA or vehicle displayed a clonal repertoire with expansion of Vβ 8.3 TCR+ T cells (Online Supplementary Figure S5).
The data indicate polyclonal development of the TCR-Vβ repertoire when FTI or GGTI treatment was given, which is consistent with the protective effects on the thymus seen histologically.
Farnesyl-transferase inhibitor treatment is permissive for graft-versus-leukemia activity
The intact effector function of cytotoxic T lymphocytes is critical for the elimination of the underlying malignant disease after allogeneic HCT. Our previous observation that CD8+ T-cell expansion was not diminished by FTI or GGTI treatment (Online Supplementary Figure S3) led us to hypothesize that CD8+ cytolytic function could be intact. T cells isolated on day 10 from transplanted BALB/c mice that had been treated with FTI, GGTI or vehicle displayed comparable cytolytic activity against L1210 cells as targets (Online Supplementary Figure S6).
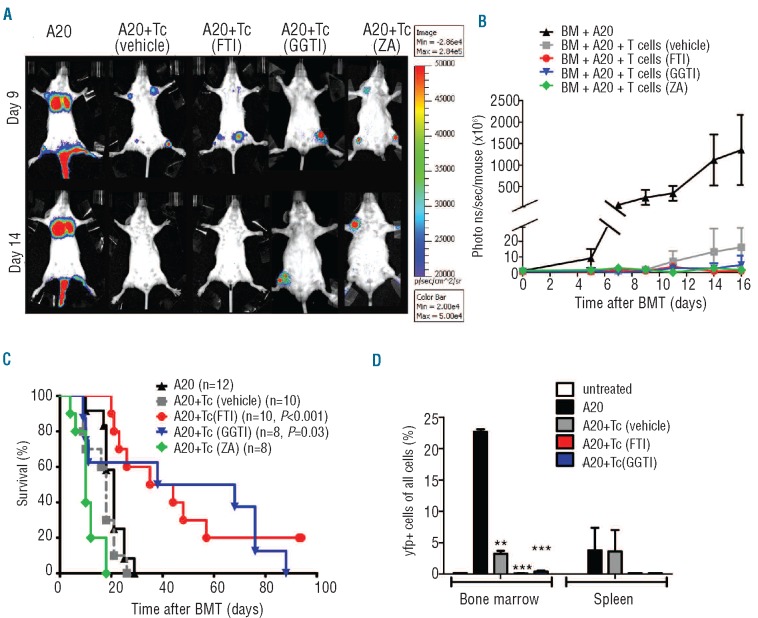
To study whether in vivo anti-tumor activity was maintained an A20luc leukemia rejection model17 was used. When indicated, vehicle, FTI, GGTI or ZA treatment was given. Serial bioluminescence images demonstrated leukemic bone marrow and organ infiltration at different time points after transplantation in all animals (Figure 6A). Animals which received bone marrow and T cells plus vehicle achieved tumor control (Figure 6B), yet died from acute GvHD (Figure 6C). FTI-, GGTI- or ZA-treated recipients rejected the A20 cells, as determined by loss of tumor signal (Figure 6A,B) and elimination of yfp+ A20 cells (Figure 6D), which is indicative of an intact in vivo effector function of CD8 T cells. Survival was significantly improved in the group that received T cells/FTI or T cells/GGTI as compared to the group given T cells/vehicle (Figure 6C). The viability of A20 or L1210 cell lines was not affected by FTI or GGTI exposure in vitro (Online Supplementary Figure S7A, B).
Figure 6.
FTI allows for graft-versus-leukemia activity in vivo (A) Bone marrow transplantation (BMT) was performed as described in the Design and Methods section. On day 0 following irradiation 2×105 A20 luc+ were given (i.v.) while T cells (Tc) (3×105) were given on day 2. Representative bioluminescence images on days 9 and 14 demonstrate intact rejection of A20 cells in the groups receiving T cells [A20+Tc (vehicle), A20+Tc (FTI), A20+Tc (GGTI) and A20+Tc (ZA)], while progressive tumor growth is seen in the absence of T cells (A20). (B) Expansion of luc+ A20 tumor cells as measured in photons over total body area (photons/second/mouse). Animals rejecting the A20 leukemia cells demonstrate a lasting loss in signal. Data from three independent experiments are combined. Signal intensity derived from luc+ A20 cells was measured in photons over total body area at different time points. BALB/c mice receiving bone marrow (BM) plus A20 leukemia cells (A20, n=5) BM plus A20 leukemia cells plus T cells (Tc) after treatment with vehicle [A20+Tc (vehicle, n=5) or FTI (A20+Tc (FTI), n=3) or GGTI (A20+Tc (GGTI), n=3) or ZA (A20+Tc (ZA), n=3]. (C) Survival of the groups described in (B). Survival: group A20+Tc (vehicle) versus A20+Tc (FTI), P<0.001, group A20+Tc (vehicle) versus A20+Tc (GGTI), P=0.03. Data from three independent experiments are combined, numbers of animals are indicated next to each group. (D) Bone marrow transplantation was performed as described in (B) with luc+yfp+A20 cells for three mice in each group. On day 16 mice were sacrificed and bone marrow and spleens were isolated and analyzed for yfp+ A20 cells by flow cytometry. The P-values are shown when a significant difference compared to the A20 only group was found (**P<0.01, ***P<0.001).
Farnesyl-transferase inhibitor treatment allows the T-cell immune reconstitution required for immunity to murine cytomegalovirus
Since reactivation of CMV in the immunodeficient patient after allogeneic HCT is a significant clinical problem, we next studied whether FTI- or GGTI-mediated immune modulation would interfere with the generation of MCMV-specific T-cell clones.
Markers reported to be down-regulated on T cells upon viral infection (CD62L, CD27, CD127) were lower after MCMV infection than in non-infected controls (Online Supplementary Figure S8A). The levels of KLRG1, which have been reported to increase after viral infection, were higher in the MCMV treated groups than in the group receiving only bone marrow, independently of inhibitor treatment (Online Supplementary Figure S8A).21-23 In a further step we aimed to assess the amount of virus-specific T cells in the spleen. The frequency of CD8+ cells in the spleen was comparable in all groups on day 58, when animals were sacrificed (Online Supplementary Figure S8B), indicating that FTI, GGTI or ZA treatment did not induce lymphopenia. We assessed the frequency of CD8 T cells specific for the MCMV peptides pp89 or M164 by tetramer staining and found that the frequency of MCMV-specific T cells in the spleen did not differ significantly regardless of FTI, GGTI or ZA treatment with the exception of the T cells specific for M164 peptide which were slightly less frequent in the GGTI-treated group (P=0.049) (Online Supplementary Figure S8C). Almost all tetramer-positive cells also expressed KLRG1, a marker of proliferating cells.23 The frequency of pp89 or M164 assessed by tetramer-positive CD8+ T cells was 1% in uninfected bone marrow controls indicating that this level represents the non-specific staining of the tetramers (data not shown). We detected a comparable MCMV viral load in the salivary glands of all groups (Online Supplementary Figure S8D). There was no MCMV detectable in the liver of the infected animals (Online Supplementary Figure S9) indicating that effective viral clearance had taken place. These data show that FTI and GGTI treatment did not affect the reconstitution of the T cells that were subsequently responsible for antiviral immunity.
Discussion
Despite the identification of risk factors, acute GvHD remains a frequent complication of allogeneic HCT, having a significant impact on early morbidity and mortality.24 Our previous studies have shown that extracellular adenosine triphosphate, which is released from dying or stressed cells that are damaged by the conditioning regimen, affects the CD4 T-cell phenotype.15,25 These and other studies in models of inflammation26-28 demonstrated the importance of metabolites for T-cell responses. In this study we used specific inhibitors that targeted key branches of the L-mevalonate pathway which lead to the depletion of its active downstream products. Our data demonstrate the protective effects of inhibitors of farnesylation and geranylgeranylation against GvHD-related mortality and histologically confirmed GvHD severity. We observed that interfering with protein-geranylgeranylation or farnesylation reduced pro-inflammatory cytokines. In vitro studies using murine Th1 and Th2 clones, stimulated in the presence of FTI, showed a dose-dependent reduction of lineage-specific cytokine secretion.29 The cytokines analyzed in this study included IFN-γ, IL-2, IL-4 and IL-5. However, the cells used in these studies29 were clonal cell lines committed to a Th1 or Th2 cytokine profile. Conversely, in our in vitro experiments we used primary T cells which were stimulated by either allogeneic antigen-presenting cells or CD3/CD28 beads. Our observation that FTI reduced alloantigen-driven CD4 T-cell proliferation in vitro are, therefore, novel. Furthermore, we extended previous findings29 by studying the in vivo effects of FTI and GGTI in a GvHD model. Thus the effects of an FTI and a GGTI on alloreactive immune responses that we observed are beyond the previously reported in vitro findings using cell lines.29 A possible explanation for the potent reduction of GvHD that we observed was the strong effect of both the FTI and the GGTI on TNF-α and IFN-γ cytokine levels in mice, an effect which could involve other types of cells besides T cells. In light of the observed reduction of T-cell expansion in vivo in FTI-treated recipients and the previously reported central role of ERK1/2 phosphorylation during T-cell activation in acute GvHD,30 the inhibitory effect of FTI and GGTI in vivo could be via this pathway. The reduction in ERK activation observed with FTI/GGTI is likely to be secondary to reduced cytokine production and autocrine signaling by cytokine receptors. This may also explain the potent effects in GvHD since multiple pro-inflammatory cytokines are released following irradiation and allogeneic HCT. Compatible with the importance of ERK1/2 phosphorylation for T-cell alloreactivity in vivo, the ERK1/2 inhibitor PD98059 was reported to attenuate murine cardiac allograft rejection, with decreased IFN-γ and increased IL-4 secretion.31
When using prenylation inhibitors it is important to consider the relative specificity of the inhibitors. The specificity of FTI-276 for farnesyltransferase is 100-fold higher than for geranylgeranyltransferase,32 whereas the GGTI that we used (GGTI-2133) has been shown to have a 140-fold higher specificity for geranylgeranyltransferase than for farnesyltransferase.33 We next studied the effects of FTI/GGTI treatment on immune reconstitution and observed a more intact thymic structure as well as polyclonal development of the TCR-Vβ repertoire.
The observed reduction in thymic damage in FTI- and GGTI-treated animals could be an indirect effect due to a reduction in CD4 T-cell expansion and dendritic cell migration, affecting the alloreactivity of the donor T cells in general. Previous studies have shown that alloreactive T cells play a central role in thymic GvHD.34 Besides alloreactive Tc, the conditioning-induced epithelial injury is a central event that affects thymic function.35 However, there is no evidence in the literature that FTI or GGTI could protect tissue against irradiation or chemotherapy. We observed reduced levels of TNF-α and IFN-γ under FTI and GGTI treatment. TNF-α was shown to cause epithelial damage36 which could participate in damage to thymic epithelial cells and its reduction by FTI and GGTI would support thymic protection. IFN-γ was shown to cause thymic damage37 and the reduced serum levels in animals treated with FTI and GGTI could contribute to thymic protection. In the light of these observation the described protection of thymic structure and improved TCR repertoire are due to reduced expansion and activation of the donor T-cell compartment and reduced TNF-α and IFN-γ levels.
Previous studies had addressed the immunosuppressive effects of statins on GvHD17,38 and in this study we observed that interfering with certain steps of the L-mevalonate pathway is not purely immunosuppressive but rather immunomodulatory. In line with intact T-cell immunity we found intact graft-versus-leukemia effects. CMV reactivation is a frequent complication after allogeneic HCT in humans and causes significant morbidity and mortality.39 We observed that FTI treatment allowed for reconstitution of T cells that were subsequently responsible for anti-MCMV immunity indicating that the treatment did not cause general immune paralysis.
While our study shows the effectiveness of FTI on GvHD, other preclinical studies demonstrated that FTI can reduce the severity of collagen-induced arthritis,3 delay the rejection of cardiac allografts,40 and prolong the survival of skin allografts in mice.4 Mechanistically it was shown in vitro that FTI can modulate T-cell differentiation and proliferation.6-8 In particular the study by Gaylo et al.,4 showing that FTI can significantly delay the rejection of skin allografts in mice, provides strong evidence that FTI have potent immunmodulary properties. In their studies the authors showed that FTI affected both CD4+ and CD8+ T cells.4 We found that the FTI had a major effect on the expansion of CD4 T cells and only modest effects on CD8 T cells; this difference between the findings of the two studies may be a consequence of differences in the models used. However, both, our study and the study by Gaylo et al.4 demonstrate that CD4+ Th cytokine production is inhibited by FTI.
Different molecular mechanisms have been proposed for the immunomodulatory effects of FTI. The FTI manumycin A has been shown to inhibit IκB kinase activity41 and IKK catalytic subunits play a key role in cytokine-mediated NFκB signaling which is a central factor in multiple pro-inflammatory pathways.9,42 FTI have been studied in clinical trials for the treatment of leukemia and shown a moderate toxicity profile,43,44 suggesting their potential applicability in patients with residual leukemia burden undergoing allogeneic HCT.
In summary, we demonstrate that GGTI and FTI exert differential immunomodulatory effects that result from inhibition of the L-mevalonate pathway. Both inhibitors interfered with CD4 but not CD8 T-cell activation and proliferation while the graft-versus-leukemia effect, thymic function and anti-MCMV effects were preserved. Based on these results the use of FTI and GGTI as prophylaxis could help to reduce GvHD in patients without having a negative impact on immune reconstitution.
Acknowledgments
The authors are grateful to C. Faller, S. Krüger, and V. Schmidt for excellent technical assistance.
Funding: This study was supported by the Deutsche Forschungsgemeinschaft, Germany, SFB 620, TP15 (to RZ), Heisenberg Fellowship to RZ (DFG ZE 872/2-1), TP2 (to HP) and partly by the BIOSS Centre for Biological Signalling Studies (to RZ).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295-322 [DOI] [PubMed] [Google Scholar]
- 2.Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241-69 [DOI] [PubMed] [Google Scholar]
- 3.Na HJ, Lee SJ, Kang YC, Cho YL, Nam WD, Kim PK, et al. Inhibition of farnesyltransferase prevents collagen-induced arthritis by down-regulation of inflammatory gene expression through suppression of p21(ras)-dependent NF-kappaB activation. J Immunol. 2004;173(2):1276-83 [DOI] [PubMed] [Google Scholar]
- 4.Gaylo AE, Laux KS, Batzel EJ, Berg ME, Field KA. Delayed rejection of MHC class II-disparate skin allografts in mice treated with farnesyltransferase inhibitors. Transpl Immunol. 2009;20(3):163-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung ND, Cho YK, Kwon BM, Hyun KH, Kim CK. 3D QSAR studies on cinnamaldehyde analogues as farnesyl protein transferase inhibitors. Arch Pharm Res. 2004;27(10):1001-8 [DOI] [PubMed] [Google Scholar]
- 6.Koh WS, Yoon SY, Kwon BM, Jeong TC, Nam KS, Han MY. Cinnamaldehyde inhibits lymphocyte proliferation and modulates T-cell differentiation. Int J Immunopharmacol. 1998;20(11):643-60 [DOI] [PubMed] [Google Scholar]
- 7.Si MS, Ji P, Tromberg BJ, Lee M, Kwok J, Ng SC, Imagawa DK. Farnesyltransferase inhibition: a novel method of immunomodulation. Int Immunopharmacol. 2003;3(4):475-83 [DOI] [PubMed] [Google Scholar]
- 8.Xue X, Lai KT, Huang JF, Gu Y, Karlsson L, Fourie A. Anti-inflammatory activity in vitro and in vivo of the protein farnesyltransferase inhibitor tipifarnib. J Pharmacol Exp Ther. 2006;317(1):53-60 [DOI] [PubMed] [Google Scholar]
- 9.Degeorge KC, Degeorge BR, Jr, Testa JS, Rothstein JL. Inhibition of oncogene-induced inflammatory chemokines using a farnesyltransferase inhibitor. J Inflamm (Lond). 2008;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mor A, Keren G, Kloog Y, George J. N-Ras or K-Ras inhibition increases the number and enhances the function of Foxp3 regulatory T cells. Eur J Immunol. 2008;38(6):1493-502 [DOI] [PubMed] [Google Scholar]
- 11.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T cell function by calcineurin dependent interleukin-2 production. Blood. 2006;108(1):390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333(10):621-7 [DOI] [PubMed] [Google Scholar]
- 13.Leonhardt F, Zirlik K, Buchner M, Prinz G, Hechinger AK, Gerlach UV, et al. Spleen tyrosine kinase (Syk) is a potent target for GvHD prevention at different cellular levels. Leukemia. 2012;26(7):1617-29 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173(9):5467-75 [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, et al. Graft-versus-host disease enhanced by extracellular adenosine triphosphate activating P2X7R. Nat Med. 2010;16(12):1434-8 [DOI] [PubMed] [Google Scholar]
- 16.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, et al. Differential impact of mTOR inhibition on CD4+CD25+Foxp3+ regulatory T cells as compared to conventional CD4+ T cells. Blood. 2008;111(1):453-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeiser R, Youssef S, Baker J, Kambham N, Steinman L, Negrin RS. HMG-CoA reductase inhibitors (statins) provide acute-graft-versus-host disease protection by Th-2 cytokine induction while sparing graft-versus-leukemia activity. Blood. 2007;110(13):4588-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dürr C, Pfeifer D, Claus R, Schmitt-Graeff A, Gerlach UV, Graeser R, et al. CXCL12 mediates immunosuppression in the lymphoma microenvironment after allogeneic transplantation of hematopoietic cells. Cancer Res. 2010;70(24):10170-81 [DOI] [PubMed] [Google Scholar]
- 19.Shachaf CM, Perez OD, Youssef S, Fan AC, Elchuri S, Goldstein MJ, et al. Inhibition of HMGcoA reductase by atorvastatin prevents and reverses MYC-induced lymphomagenesis. Blood. 2007;110(7):2674-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 2005;280(35):31101-8 [DOI] [PubMed] [Google Scholar]
- 21.Bachmann MF, Wolint P, Schwarz K, Jäger P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175(7):4686-96 [DOI] [PubMed] [Google Scholar]
- 22.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, et al. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35(11):3131-41 [DOI] [PubMed] [Google Scholar]
- 23.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood. 2002;100(10):3698-702 [DOI] [PubMed] [Google Scholar]
- 24.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeiser R, Penack O, Holler E, Idzko M. Danger signals activating innate immunity in graft-versus-host disease. J Mol Med. 2011;89(9):833-45 [DOI] [PubMed] [Google Scholar]
- 26.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13(8):913-9 [DOI] [PubMed] [Google Scholar]
- 27.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1(39):ra6. [DOI] [PubMed] [Google Scholar]
- 28.Turner CM, Tam FW, Lai PC, Tarzi RM, Burnstock G, Pusey CD, et al. Increased expression of the pro-apoptotic ATP-sensitive P2X7 receptor in experimental and human glomerulonephritis. Nephrol Dial Transplant. 2007;22(2):386-95 [DOI] [PubMed] [Google Scholar]
- 29.Marks RE, Ho AW, Robbel C, Kuna T, Berk S, Gajewski TF. Farnesyltransferase inhibitors inhibit T cell cytokine production at the post-transcriptional level. Blood. 2007;110(6):1982-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu SXLu SX, Alpdogan O, Lin J, Balderas R, Campos-Gonzalez R, Wang X, et al. STAT-3 and ERK 1/2 phosphorylation are critical for T-cell alloactivation and graft-versus-host disease. Blood. 2008;112(13):5254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Guan Q, Diao H, Lian D, Zhong R, Jevnikar AM, Du C. Prolongation of cardiac allograft survival by inhibition of ERK1/2 signaling in a mouse model. Transplantation. 2007;83(3):323-32 [DOI] [PubMed] [Google Scholar]
- 32.Lerner EC, Qian Y, Blaskovich MA, Fossum RD, Vogt A, Sun J, et al. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J Biol Chem. 1995;270(45):26802-6 [DOI] [PubMed] [Google Scholar]
- 33.Vasudevan A, Qian Y, Vogt A, Blaskovich MA, Ohkanda J, Sebti SM, Hamilton AD. Potent, highly selective, and non-thiol inhibitors of protein geranylgeranyltransferase-I. J Med Chem. 1999;42(8):1333-40 [DOI] [PubMed] [Google Scholar]
- 34.Na IK, Lu SX, Yim NL, Goldberg GL, Tsai J, Rao U, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. J Clin Invest. 2010;120(1):343-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458-66 [DOI] [PubMed] [Google Scholar]
- 36.Hindryckx P, De Vos M, Jacques P, Ferdinande L, Peeters H, Olievier K, et al. Hydroxylase inhibition abrogates TNF-alpha-induced intestinal epithelial damage by hypoxia-inducible factor-1-dependent repression of FADD. J Immunol. 2010;185(10):6306-16 [DOI] [PubMed] [Google Scholar]
- 37.Hauri-Hohl MM, Keller MP, Gill J, Hafen K, Pachlatko E, Boulay T, et al. Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood. 2007;109(9):4080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Li D, Jones D, Bassett R, Sale GE, Khalili J, et al. Blocking LFA-1 activation with lovastatin prevents graft-versus-host disease in mouse bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15(12):1513-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255-60 [DOI] [PubMed] [Google Scholar]
- 40.Si MSSi MS, Ji P, Lee M, Kwok J, Kusumoto J, Naasz E, et al. Potent farnesyltransferase inhibitor ABT-100 abrogates acute allograft rejection. J Heart Lung Transplant. 2005;24(9):1403-9 [DOI] [PubMed] [Google Scholar]
- 41.Bernier M, Kwon YK, Pandey SK, Zhu TN, Zhao RJ, Maciuk A, et al. Binding of manumycin A inhibits IkappaB kinase beta activity. J Biol Chem. 2006;281(5):2551-61 [DOI] [PubMed] [Google Scholar]
- 42.Takada Y, Khuri FR, Aggarwal BB. Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-kappaB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-kappaB-regulated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279(25):26287-99 [DOI] [PubMed] [Google Scholar]
- 43.Karp JE, Vener TI, Raponi M, Ritchie EK, Smith BD, Gore SD, et al. Multi-institutional phase 2 clinical and pharmacogenomic trial of tipifarnib plus etoposide for elderly adults with newly diagnosed acute myelogenous leukemia. Blood. 2012;119(1):55-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karp JE, Lancet JE, Kaufmann SH, End DW, Wright JJ, Bol K, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97(11):3361-9 [DOI] [PubMed] [Google Scholar]