Abstract
Although there are National Institutes of Health consensus criteria for the global assessment of chronic graft-versus-host disease, no validated biomarkers have been established for this disease. Furthermore, whereas the role of T cells, B cells, and dendritic cells in chronic graft-versus-host disease has been established, the contribution of monocytes has not been clearly addressed. Using an enzyme-linked immunospot assay, we measured the spot-forming cells for interferon-γ, interleukin-4, interleukin-10, and interleukin-17 in unstimulated peripheral blood of patients following allogeneic hematopoietic stem cell transplantation. Other immunological examinations, including skin biopsy, were also done. Fifty-seven patients were enrolled. Interleukin-10 spot-forming cells were evaluable for therapeutic monitoring in 16 patients with chronic graft-versus-host disease. The number of interleukin-10 spot-forming cells in patients with active chronic graft-versus-host disease was significantly higher than the number in those with no or inactive chronic graft-versus-host disease. Interleukin-10 was predominantly produced by monocytes. CD29 expression on monocytes in patients with active chronic graft-versus-host disease was elevated. The level of plasma fibronectin, a ligand of CD29, correlated with the number of interleukin-10 spot-forming cells. Immunohistochemical analysis of the skin in active chronic graft-versus-host disease showed that infiltrating CD29+ monocytes might produce interleukin-10. A novel biomarker, interleukin-10 spot-forming cells, shows promise as both a diagnostic and prognostic indicator for chronic graft-versus-host disease, and may allow for early intervention prior to the onset of the disease. Measurement of interleukin-10 spot-forming cells would be helpful in clinical trials and in patients' management.
Introduction
The development of chronic graft-versus-host disease (GVHD) is a major cause of morbidity and mortality in patients who have undergone after allogeneic hematopoietic stem cell transplantation (HSCT).1-3 In most studies, between 30% and 75% of patients who survive after day 100 develop some clinical manifestations of chronic GVHD.4 The treatment of chronic GVHD is limited by a number of factors, including late diagnosis, an inability to predict outcome or response to the therapy, and a poor understanding of the immune targets important for optimal therapy. Although there are National Institutes of Health (NIH) consensus criteria for the global assessment of chronic GVHD,5 no validated biomarkers have been established.6,7
Experimental models and clinical studies have described the pathophysiology of chronic GVHD in various immunological abnormalities, including alloreactivity, autoreactivity, and lymphopenia associated with cellular and humoral immune deficiency.8,9 Several cell types contribute to the biology of chronic GVHD. Regulatory T (Treg) cells, CD4+ effector memory cells, and T-bet+ cytotoxic effector cells have been reported to be involved in the pathogenesis of chronic GVHD.10-14 While donor T cells are known to play a major role, B cells are also important.15-19 A positive response to treatment with rituximab was reported in 50-70% of patients who failed to respond to first-line treatment with glucocorticoids and calcineurin inhibitors.20 Extracorporeal photopheresis has been used with variable efficacy to treat chronic GVHD.21,22 While the immunomodulatory effect of extracorporeal photopheresis is unclear, it is possible that monocytes play a role in reducing chronic GVHD.23
We hypothesized that circulating monocytes could be involved in the biology of chronic GVHD. Circulating monocytes are multifunctional, participating in homeostasis, immune defense, and tissue repair.24,25 In previous studies, we demonstrated the usefulness of enumerating cytokine spot-forming cells (SFC) found in patients with acute GVHD using an enzyme-linked immunospot (ELISPOT) assay.26,27 The numbers of cytokine SFC, determined by ELISPOT assay, reflect the ongoing in vivo immunological status of patients after transplantation.27 In this study we used ELISPOT assays to measure cytokine SFC in patients following HSCT and correlated the results with clinical findings. Furthermore, expression of CD29 (b1 integrin) on monocytes was correlated with plasma levels of fibronectin in patients with active chronic GVHD.
Design and Methods
Patients
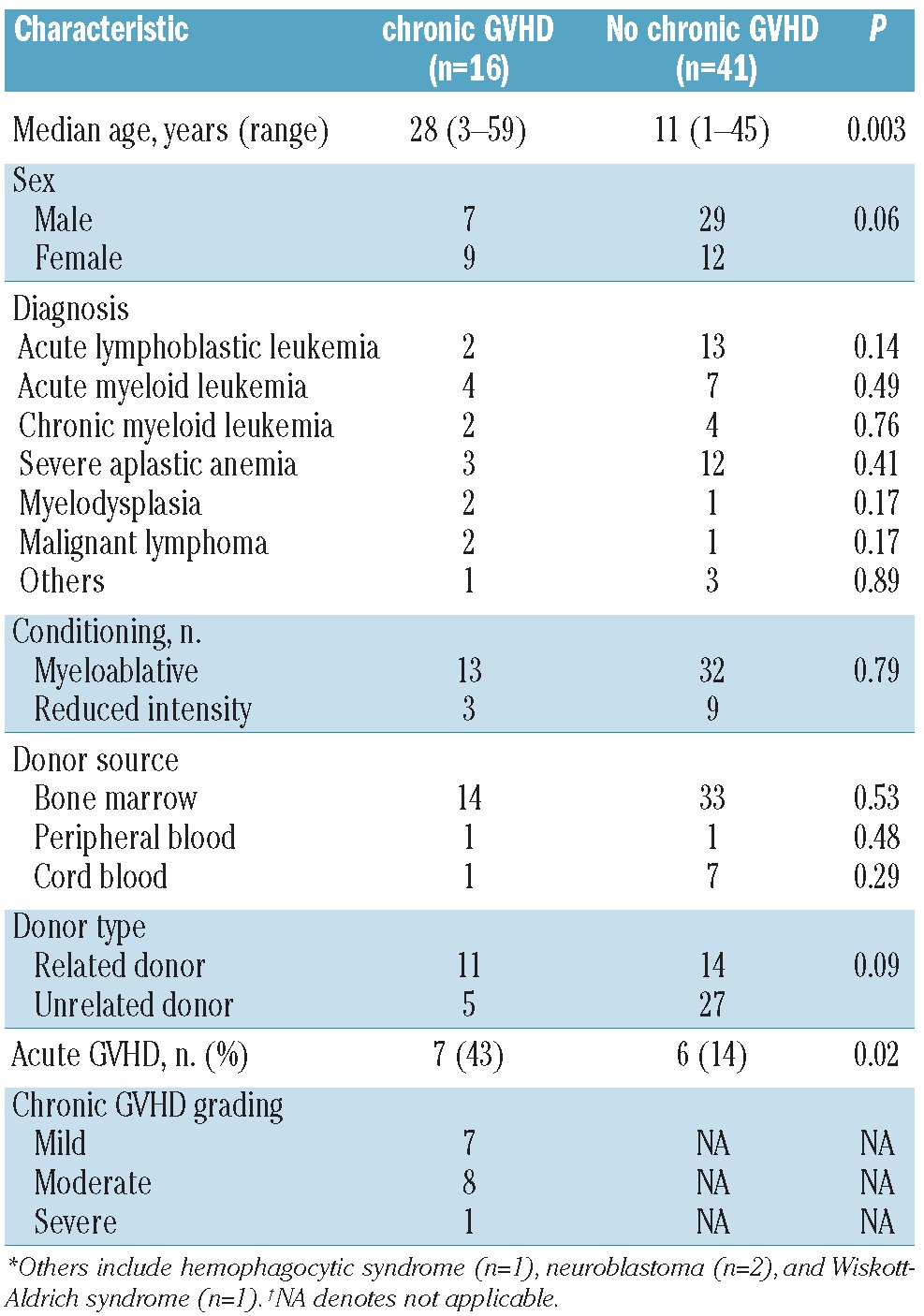
Fifty-seven patients who underwent allogeneic HSCT between 1995 and 2009 were included in this study. The patients' demographic data are shown in Table 1. Sixteen patients with chronic GVHD were grouped into mild (n=7), moderate (n=8), and severe (n=1) categories based on the NIH chronic GVHD consensus criteria.5 According to Sarantopoulos's definition of chronic GVHD activity, patients were subclassified into those with no, active, and inactive chronic GVHD.28 Patients who never developed chronic GVHD (n=41) were designated as “no chronic GVHD”. Patients with active chronic GVHD (n=14) were more likely to be receiving immunosuppressive therapy. Inactive chronic GVHD (n=10) was determined by clinical assessment and included patients who had achieved a complete response to immunosuppressive therapy at the time of analysis. The number of patients with active and inactive chronic GVHD overlapped during the clinical course. Disease activity was assessed without knowledge of the laboratory results. The median time of the analysis was 48 (range, 4-204), 52 (5-142), and 55 (4-151) months after transplantation for patients with no, active, and inactive chronic GVHD, respectively.
Table 1.
Patients' characteristics.
This study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Institutional Ethics Committee Review Board at Mie University Hospital. The study was registered with the national regulatory authority (UMIN-Clinical Trials Registry). All participants or their guardians provided written informed consent for the collection of samples and subsequent analyses.
Diagnosis of chronic graft-versus-host disease and processing of samples
The diagnosis of chronic GVHD requires the presence of at least one diagnostic manifestation of the disease or at least one distinctive manifestation, with the diagnosis confirmed by pertinent biopsy, laboratory tests, or radiology in the same or another organ.5,29 Diagnostic manifestations of chronic GVHD were found in the skin, nails, mouth, eyes, lungs, gastrointestinal tract, and liver. The grade of chronic GVHD was determined according to NIH consensus criteria.5,30 Peripheral blood mononuclear cells (PBMC) were obtained from patients with no evidence of infection, and the diagnoses were confirmed by laboratory testing or radiology, at least 100 days after allogeneic HSCT. The total numbers of white blood cells, lymphocytes, and monocytes were analyzed by an automatic blood cell counter (Sysmex K4500, Toa Medical Electronics, Tokyo, Japan).
Flow cytometry and cell separation
Cells were stained with fluorescein-conjugated monoclonal antibodies to human anti-CD3, CD4, CD8, CD11c, CD16, CD19, CD25, CD29, CD33, CD56, CD123 (BD Biosciences, San Jose, CA, USA), CD14 (Beckman Coulter, CA, USA), CD68, interleukin (IL)-10 (R&D systems, MN, USA), and an isotypematched control monoclonal antibody. Fluorescence staining was analyzed with a FACSCalibur flow cytometer and the CELLQuest Software program (both from BD Immunocytometry Systems, San Jose, CA, USA).
In some experiments, CD4+ T cells, CD8+ T cells, B cells, natural killer (NK) cells, monocytes, and dendritic cells (DC) were negatively separated from PBMC using magnetic beads according to the manufacturer's recommended procedures (BD IMag cell separation kit).
Enzyme-linked immunosorbent assay
Plasma laminin, procollagen type I, and fibronectin were measured using enzyme-linked immunosorbent assay (ELISA) kits (TaKaRa, Otsu, Japan) according to the manufacturer's instructions. The fibronectin kit can detect fragments including the cellular domain of human fibronectin. The detectable levels of laminin, procollagen type I, and fibronectin were 8 ng/mL, 8 ng/mL, and 4 μg/mL, respectively. All samples were stored at -80 °C prior to use. It was confirmed that these measurements were not influenced by the single freezing and thawing of the plasma.
Enzyme-linked immunospot assay
The enzyme-linked immunospot (ELISPOT) assay was undertaken as described previously.26 Briefly, ELISPOT plates (Millipore Corp., Bedford, MA, USA) were coated with anti human interferon (IFN)-γ, IL-4 (Mabtech AB, Stockholm, Sweden), IL-10, or IL-17 (BD Biosciences) monoclonal antibodies. The plate was washed and incubated for 2 h with RPMI-1640 containing 10% fetal bovine serum. Freshly isolated PBMC were added at the concentration of 50,000 cells per well. Unstimulated PBMC were used because they might reflect the in vivo immune status better. As a positive control, PBMC were stimulated with phorbol 12-myristate 13-acetate (100 ng/mL) and ionomycin (1 μg/mL) (Sigma-Aldrich, Tokyo, Japan). The plates were incubated for approximately 40 h at 37 °C with 5% CO2 in a humid atmosphere. The cells were removed and the plates were incubated with secondary biotinylated monoclonal antibodies to human IFN-γ, IL-4 (Mabtech AB), IL-10, or IL-17 (BD Biosciences). The plates were then developed with streptavidin-alkaline phosphatase (Mabtech AB) and a colorimetric substrate (Bio-Rad, Berkeley, CA, USA). The number of resulting spots was counted with an ImmunoSpot Analyzer (Carl Zeiss, Tokyo, Japan). Data were obtained from triplicate samples, and the standard error was less than 10%.
Immunohistochemistry
Immunohistochemical staining was performed as previously described.31 Briefly, 4-μm thick sections of formalin-fixed, paraffin-embedded tissues were stained with hematoxylin & eosin, and the adjacent sections were immunostained using the Bench-Mark XT immunostainer (Ventana Medical Systems, Tucson, AZ, USA). The primary antibodies were anti-human IL-10 (E10, Santa Cruz Biotechnology, CA, USA), CD29 (4B7R, Axxora. com., San Diego, USA), CD68 (KP-1, DAKO, Carpinteria, CA, USA), and fibronectin (Santa Cruz Biotechnology). Anti- CD68 antibody was used for monocyte/macrophage detection. The KP-1 clone of the anti- CD68 antibody is a sensitive monocytic marker.32 Diaminobenzidine was used for visualization. Appropriate positive and negative control staining was also performed in parallel for each experiment. For each section, the number of positively stained mononuclear cells in ten random x400 fields of view was counted, and the average number of positive cells was determined.
Statistical analysis
Data are presented as means ± standard deviation. Unpaired two-tailed Student's t-tests were used to compare differences between groups. Fisher's exact test, the Mann-Whitney U-test, and the Kruskal-Wallis test were used for contingency table analysis. Pearson's correlation coefficient was used to determine correlations between variables. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using the Statview Version 5.0 software program (SAS Institute Inc., Cary, NC, USA).
Results
Interleukin-10 spot-forming cells during the clinical course of chronic graft-versus-host disease
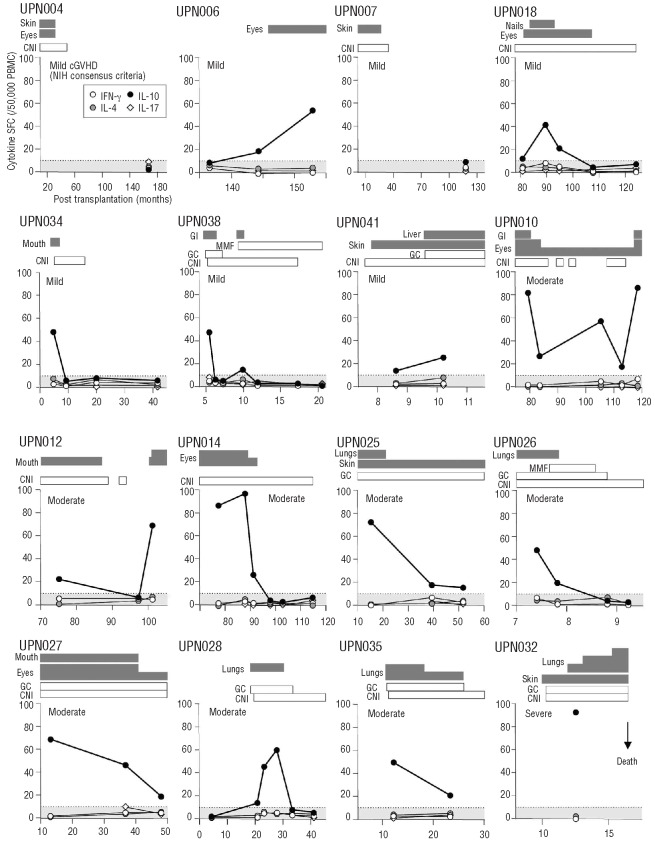
We evaluated the numbers of cytokine SFC in unstimulated PBMC from 57 patients at least 100 days after HSCT (Table 1). The cytokine SFC included were IFN-γ for type 1 helper T cells (Th1 type), IL-4 (Th2 type), IL-10 (regulatorycell type), and IL-17 (Th17 type). The clinical course of chronic GVHD in 16 patients is shown in Figure 1. The increase in the number of IL-10 SFC, but not that of IFN-γ, IL-4, or IL-17 SFC, was consistent with the active form of chronic GVHD. Three patients (UPN014, UPN018, and UPN038), who had no symptoms of chronic GVHD and normal amounts of IL-10 SFC for more than 12 months due to immunosuppressive therapy, successfully discontinued the immunosuppressants without recrudescence. In contrast, two patients (UPN010 and UPN012) who either discontinued immunosuppressive therapy early or showed poor drug adherence, developed symptomatic recurrence of chronic GVHD concomitant with increased numbers of IL-10 SFC. Moreover, the number of IL-10 SFC was found to be elevated 20 and 6 days prior to the onset of chronic GVHD in patients UPN006 and UPN018, respectively. It is tempting to speculate that the number of IL-10 SFC after HSCT may be useful for the prediction and therapeutic monitoring of chronic GVHD.
Figure 1.
The clinical course of chronic GVHD and the cytokine SFC. The numbers of IFN-γ, IL-4, IL-10, and IL-17 SFC are shown, along with the clinical course of chronic GVHD in 16 patients. The sites and grading of chronic GVHD are depicted as gray bars according to the NIH chronic GVHD scoring system. Immunosuppressants, including calcineurin inhibitors (CNI), glucocorticoids (GC), and m y c o p h e n o l a t e mofetil (MMF), are shown as white bars. The shaded area shows the number of IL-10 SFC in PBMC obtained from healthy volunteers (n=35), which was used as the cut-off value for IL-10 SFC (mean+2SD). The number of cytokine SFC is shown per 50,000 PBMC.
Interleukin-10 spot-forming cells were mostly monocyte-derived in patients with active chronic graft-versus-host disease
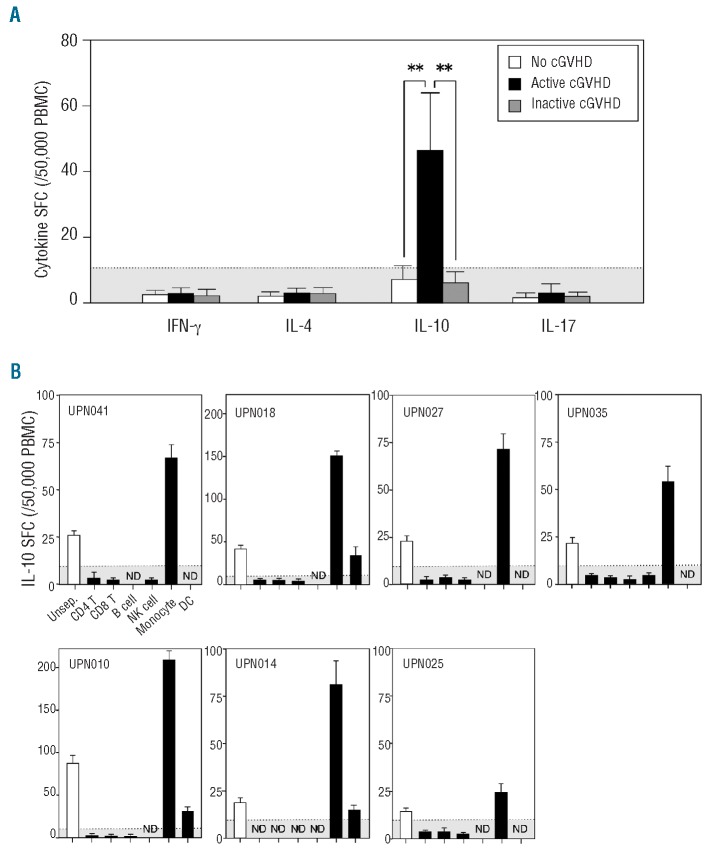
The numbers of cytokine SFC after HSCT were compared in patients with no, active, and inactive chronic GVHD. Samples were obtained from patients between 4 and 204 months (mean, 50.6 months) after HSCT. The number of IL-10 SFC, but not that of other cytokine SFC, was significantly higher in patients with active chronic GVHD than in patients with no or inactive chronic GVHD (Figure 2A). The number of IL-10 SFC in patients with inactive chronic GVHD was within the normal range. The number of IL-10 SFC could be used as a therapeutic index for evaluating the severity of active chronic GVHD. With regards to the diagnostic sensitivity and specificity of the assay, the cut-off value for the number of IL-10 SFC determined from healthy volunteers was 10.8 spots/50,000 PBMC (mean+2SD). Based on this cut-off value, the true positive rate was 97% (32/33 samples) in patients with active chronic GVHD, and the false positive rate was 11% (7/64 samples) in patients with no or inactive chronic GVHD. The amounts of IL-10 SFC measured were proportional to the severity of the active chronic GVHD (mild; sample n=17 versus moderate; n=16, P<0.001).
Figure 2.
The value of IL-10 SFC derived from monocytes is a versatile biomarker for active chronic GVHD (cGVHD). (A) The numbers of IFN-γ, IL-4, IL-10, and IL-17 SFC are shown in patients with no cGVHD (white column; sample n=41), active cGVHD (black column; n=33), and inactive cGVHD (gray column; n=23). The number of cytokine SFC is shown per 50,000 PBMC. (B) PBMC obtained from seven patients with active cGVHD were separated with magnetic beads using negative selection into six subpopulations of cells before the ELISPOT assay. The data are expressed as mean±SD. The shaded area shows the cut-off value for IL-10 SFC (mean+2SD). **P<0.01.
We investigated which cells produced IL-10. When the amounts of white blood cells, lymphocytes, monocytes, and lymphocyte subsets were measured, no significant differences were found among patients with no, active, and inactive chronic GVHD (Online Supplementary Table S1). The number of CD4+ CD25high T cells was not found to be associated with the presence of active chronic GVHD although some authors reported that FoxP3+ CD4+ CD25high Treg cells contributed to chronic GVHD.10,13,33 Next, we analyzed the origins of the IL-10 SFC using immunomagnetic selection before the ELISPOT assay. PBMC obtained from seven evaluable patients with active chronic GVHD were sorted into CD4+ T cells, CD8+ T cells, NK cells, B cells, DC, and monocytes. The cell purity ranged from 87% to 99%. Surprisingly, the number of IL-10 SFC after selection was elevated predominantly by the monocytes, partly by DC, but not by the other cell populations (Figure 2B). These results indicate that IL-10 SFC are primarily derived from monocytes.
Increased CD29+ monocytes and plasma fibronectin in active chronic graft-versus-host disease
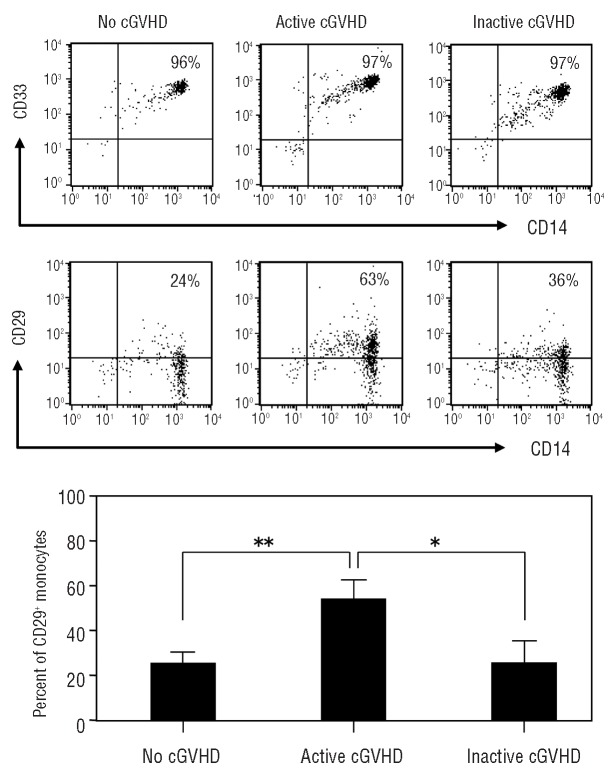
We addressed whether there was an index for activated monocytes in patients with chronic GVHD. Bergijk et al. reported that several extracellular matrix components, especially fibronectin, were markedly increased in sclerotic glomeruli after the induction of chronic GVHD in a murine model.34 Fibronectin, a major component of the extracellular matrix, is an important ligand for CD29 on monocytes entering the skin.35 CD29 is a b1 integrin subunit, forming the very late activation antigen (VLA) receptor (VLA-4, CD49d/CD29) that reacts with extracellular matrix molecules including collagen, laminin, and fibronectin. We, therefore, analyzed the surface expression level of CD29 on the gated monocyte population (Figure 3A). The gated cells were CD33+ CD14+ populations present in circulating monocytes,36 with 96-97% purity of monocytes in each group. The expression of CD29 on monocytes in patients with active chronic GVHD was significantly greater than that in patients with no or inactive chronic GVHD (Figure 3B).
Figure 3.
The levels of CD29 expression on monocytes in patients with active chronic GVHD (cGVHD). (A) PBMC gated with monocytoid light-scatter characteristics expressed CD14 and CD33 to more than 96% purity in all groups. The levels of CD29 expression on CD14+ monocyte-gated cells were compared in patients with no, active, and inactive cGVHD as representative data. (B) CD29+CD14+ cell populations were identified in patients with no cGVHD (n=9), active cGVHD (n=8), and inactive cGVHD (n=8). Data are expressed as mean±SD. *P<0.05.**P<0.01.
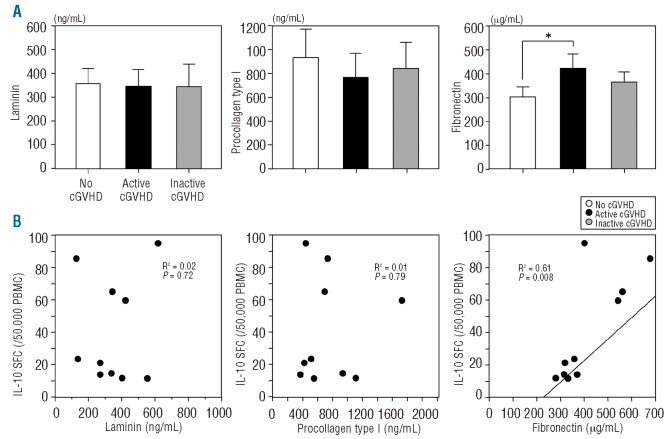
We also measured plasma levels of laminin, procollagen type I, and fibronectin in patients with chronic GVHD. Plasma fibronectin level was significantly higher in patients with active chronic GVHD than in those with no chronic GVHD (P<0.05) (Figure 4A). However, plasma levels of laminin and procollagen type I were not significantly different among patients with no, active and inactive chronic GVHD. Furthermore, the number of IL-10 SFC correlated with the plasma level of fibronectin (R2=0.61, P=0.008), but not laminin (R2=0.02, P=0.72) or procollagen type I (R2=0.01, P=0.79) (Figure 4B). These results suggest that fibronectin may play a role in active chronic GVHD.
Figure 4.
The levels of plasma fibronectin in patients with active chronic GVHD (cGVHD). (A) Plasma levels of laminin, procollagen type I, and fibronectin were compared in patients with no (n=10), active (n=10), and inactive cGVHD (n=10). (B) The correlation of the number of IL-10 SFC with plasma laminin, procollagen type I, or fibronectin levels was analyzed in patients with active cGVHD. Data are expressed as mean±SD. *P<0.05.
Increased infiltrating CD29+ monocytes and interleukin-10-producing cells in the skin of patients with active chronic graft-versus-host disease
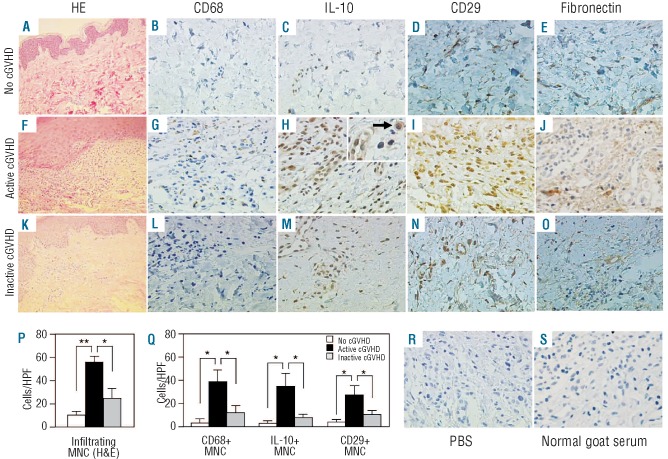
We prepared paraffin sections of skin biopsy samples from patients with no, active and inactive chronic GVHD, and examined the relationship between monocytes and fibronectin in target organs of chronic GVHD (Figure 5). Hematoxylin & eosin staining revealed more mononuclear cells infiltrating the superficial layer of the dermis in patients with active chronic GVHD than in patients with no or inactive chronic GVHD (Figure 5P, P<0.05). Immunohistochemical staining demonstrated that the number of mononuclear cells positive for CD68, IL-10, or CD29 was significantly higher in patients with active chronic GVHD than in those with no or inactive chronic GVHD (Figure 5Q, P<0.05). Thus, IL-10-producing mononuclear cells increased in active chronic GVHD, as shown in the inset of Figure 5H. In addition, fibronectin was present more abundantly in the superficial layer of the dermis in patients with active chronic GVHD than in patients with no or inactive chronic GVHD (Figure 5E, J, O).
Figure 5.
Immunohistochemical analysis of the skin of patients with chronic GVHD (cGVHD). Paraffin-embedded skin biopsy sections obtained from patients with no, active, and inactive cGVHD were stained with hematoxylin and eosin (H&E) (original magnifications ×100; A, F, and K) and immunostained for anti-human CD68 (×400; B, G, and L), IL-10 (×400; C, H, and M; ×1000 inset in H), CD29 (×400; D, I, and N), and fibronectin (×400;E, J, and O). Inset: the arrow indicates IL-10-producing mononuclear cells (MNC) (×1000). For the negative controls, either phosphate-buffered saline (×400; R) or normal goat serum (×400; S) was used as the primary antibody. Representative results from the indicated groups are shown (A-O). The mean numbers of infiltrating MNC per high power field (HPF) (×400) in patients with no (n=3), active (n=4), and inactive cGVHD (n=4) are shown (P). The mean numbers of CD68-positive, IL-10-positive, and CD29-positive MNC, per HPF (×400), are shown (Q). The data are expressed as mean±SD. *P<0.05. **P<0.01.
Discussion
We demonstrated that patients with active chronic GVHD have markedly elevated numbers of IL-10 SFC compared to patients with no or inactive chronic GVHD. The number of IL-10 SFC does, therefore, appear to be a useful and versatile biomarker for monitoring the clinical course of active chronic GVHD although the number of patients studied was small. Most of the IL-10 SFC were derived from CD29+ monocytes which may react with fibronectin, a ligand of CD29, in patients with active chronic GVHD. These results suggest that monocytes may participate in the biology of chronic GVHD.
The NIH-supported chronic GVHD biomarker consensus group made several recommendations regarding areas of focus needed to identify markers in chronic GVHD.6 Biomarkers were defined as any biological product that could be used to predict the development of chronic GVHD, measure disease activity, and aid in establishing the diagnosis, classification or prognosis of chronic GVHD or its response to treatment. To date, validated biomarkers have not been well characterized, and a poor understanding of the pathophysiology of chronic GVHD has hampered progress in the area. In the current observational study, we have shown that IL-10 SFC could be a candidate biomarker for clinical use (Figure 1).
IL-10, encoded by the IL10 gene, is a known anti-inflammatory cytokine. Although Lin et al. found no significant association between recipients' IL-10 promoter polymorphisms and the risk of chronic GVHD,37 there have been no reports describing the significance of the donors' IL-10, as was investigated in this study. IL-10 is produced by various hematologic cells, such as CD4 T cells, CD8 T cells, NK cells, B cells, DC, and monocytes. In the current study, IL-10 was produced mainly by monocytes, and to a lesser extent by DC, in patients with active chronic GVHD (Figure 2B). A DC separation procedure enriched populations of plasmacytoid DC and myeloid DC that could produce IL-10.38 In the current study, most of the IL-10 was produced by monocytes, with some also produced by plasmacytoid or myeloid DC.
As regards the diagnostic value of cytokines, including IL-10, we demonstrated the usefulness of enumeration of cytokine SFC in acute GVHD by ELISPOT assay.26,27 The number of IL-10 SFC in patients with acute GVHD was variable, irrespective of the grade of the disease. We also reported that both type 1 (IFN-γ) and type 2 (IL-4) cytokines contributed to the development of acute GVHD.26 On the basis of these previous results and the current data, the cytokine SFC in acute GVHD showed a distinct pattern from those in chronic GVHD (Online Supplementary Table S2). Therefore, acute GVHD should not interfere with the diagnostic modality for chronic GVHD in terms of the patterns of type 1, type 2, and regulatory type cytokine SFC although there were no cases with simultaneous acute and chronic GVHD in the current study.
The pathophysiology of chronic GVHD in humans remains poorly understood, although T cells have been identified as a key player. Matsuoka et al. reported that patients with chronic GVHD had a relative deficiency of Treg cells.13 Recently, Koreth et al. demonstrated that daily low dose IL-2 was associated with preferential, sustained Treg-cell expansion in vivo and improved the manifestations of chronic GVHD in 12 out of the evaluable 23 patients.14 B cells were also recently recognized to be an important part of the immune response in chronic GVHD.6,15,16,19,20 The Boston group described that patients with chronic GVHD had increased plasma levels of B-cell activation factor of the TNF family (BAFF).18,28 Thus, T cells and B cells play major roles in the pathogenesis of chronic GVHD. However, it has been suggested that monocytes may also be important in chronic GVHD. Arpinati et al. reported that patients with chronic GVHD had increased numbers of donor monocytes in their bone marrow and possibly in their peripheral blood. Monocytes in patients with chronic GVHD showed increased expression of a costimulatory molecule.39 Predominant infiltration of monocytes, recruited from the circulation to target organs, was present in the epidermis, as well as in the conjunctiva, in patients with chronic GVHD.40,41 However, these reports did not show how monocytes contribute to the pathogenesis of chronic GVHD. In the current study, we found that IL-10 SFC derived from monocytes were significantly increased, and could provide a powerful tool for the diagnosis and therapeutic monitoring of chronic GVHD. Moreover, plasma fibronectin level was elevated in subjects with active chronic GVHD, and there was a positive correlation between IL-10-producing monocytes and cellular fibronectin (Figure 4). Based on these results, it is tempting to speculate that CD29+ monocytes became IL-10-producing cells after reacting with fibronectin on target organs in patients with active chronic GVHD.
We speculate that circulating monocytes are stimulated by cleaved cellular fibronectin and come out of target organs in active chronic GVHD. Fibronectin was described to be strongly deposited under the basal layer of the skin in cutaneous chronic GVHD.42 Fibronectin is an adhesive extracellular matrix glycoprotein that plays crucial roles in various cellular functions, including cell adhesion, migration, proliferation, and differentiation.43 The CD29 expression of monocytes increases following fibronectin fragmentation.44 These fibronectin fragments, but not intact fibronectin, are potent chemoattractants for circulating monocytes, and selectively increase the recruitment of monocytes into sites of tissue inflammation.35,43 CD29+ monocytes produce IL-10 by interacting with cellular fibronectin in the target organs.45 Ogawa et al. described that it is likely that stromal fibroblasts are actively involved in the pathogenic process of chronic GVHD in the lacrimal gland by producing excessive extracellular matrix components.46 Thus, our hypothesis is that IL-10-producing monocytes might play an important role in the remodeling of damaged tissues in chronic GVHD.
In summary, the findings described in this report highlight an alternative mechanism for the biology of chronic GVHD by focusing on IL-10-producing CD29+ monocytes, and suggest that therapy to improve monocyte function may control active chronic GVHD. This is an intriguing hypothesis and further identification of informative biomarkers would be helpful for clinical trials and patients' management.47
Acknowledgments
Funding: This study was funded in part by a grant from the New Research Project of Mie University Graduate School of Medicine and by a Research Grant for Allergic Diseases and Immunology (H20-015) from the Japanese Ministry of Health, Labor, and Welfare.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Pidala J, Kim J, Anasetti C, Nishihori T, Betts B, Field T, et al. The global severity of chronic graft-versus-host disease, determined by National Institutes of Health consensus criteria, is associated with overall survival and non-relapse mortality. Haematologica. 2011;96(11):1678-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsohn DA, Arora M, Klein JP, Hassebroek A, Flowers ME, Cutler CS, et al. Risk factors associated with increased nonrelapse mortality and with poor overall survival in children with chronic graft-versus-host disease. Blood. 2011;118(16):4472-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101(9):3373-85 [DOI] [PubMed] [Google Scholar]
- 5.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-56 [DOI] [PubMed] [Google Scholar]
- 6.Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12(2):126-37 [DOI] [PubMed] [Google Scholar]
- 7.Levine JE, Logan BR, Wu J, Alousi AM, Bolanos-Meade J, Ferrara JL, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119(16)3854-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation's greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(1 Suppl 1):2-10 [DOI] [PubMed] [Google Scholar]
- 9.Banovic T, MacDonald KP, Morris ES, Rowe V, Kuns R, Don A, et al. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 2005;106(6):2206-14 [DOI] [PubMed] [Google Scholar]
- 10.Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood. 2004;103(6):2410-6 [DOI] [PubMed] [Google Scholar]
- 11.Yamashita K, Choi U, Woltz PC, Foster SF, Sneller MC, Hakim FT, et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4(+) effector memory cells relative to central memory cells. Blood. 2004;103(10):3986-8 [DOI] [PubMed] [Google Scholar]
- 12.Imanguli MM, Swaim WD, League SC, Gress RE, Pavletic SZ, Hakim FT. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood. 2009;113(15):3620-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She K, Gilman AL, Aslanian S, Shimizu H, Krailo M, Chen Z, et al. Altered Toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(4):386-97 [DOI] [PubMed] [Google Scholar]
- 16.Fujii H, Cuvelier G, She K, Aslanian S, Shimizu H, Kariminia A, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children's Oncology Group. Blood. 2008;111(6):3276-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greinix HT, Pohlreich D, Kouba M, Kormoczi U, Lohmann I, Feldmann K, et al. Elevated numbers of immature/transitional CD21- B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(2):208-19 [DOI] [PubMed] [Google Scholar]
- 18.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzmina Z, Greinix HT, Weigl R, Kormoczi U, Rottal A, Frantal S, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117(7):2265-74 [DOI] [PubMed] [Google Scholar]
- 20.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100(3):941-7 [DOI] [PubMed] [Google Scholar]
- 22.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107(8):3074-80 [DOI] [PubMed] [Google Scholar]
- 23.Craciun LI, Stordeur P, Schandene L, Duvillier H, Bron D, Lambermont M, et al. Increased production of interleukin-10 and interleukin-1 receptor antagonist after extracorporeal photochemotherapy in chronic graft-versus-host disease. Transplantation. 2002;74(7):995-1000 [DOI] [PubMed] [Google Scholar]
- 24.Naito M, Hasegawa G, Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech. 1997;39(4):350-64 [DOI] [PubMed] [Google Scholar]
- 25.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953-64 [DOI] [PubMed] [Google Scholar]
- 26.Hirayama M, Azuma E, Kumamoto T, Iwamoto S, Yamada H, Nashida Y, et al. Prediction of acute graft-versus-host disease and detection of distinct end-organ targets by enumeration of peripheral blood cytokine spot-forming cells. Transplantation. 2005;80(1):58-65 [DOI] [PubMed] [Google Scholar]
- 27.Hirayama M, Azuma E, Kumamoto T, Iwamoto S, Nashida Y, Araki M, et al. Discrimination of acute graft-versus-host disease from infections by enumeration of peripheral blood interferon-gamma spot-forming cells. Transplantation. 2006;81(4):632-5 [DOI] [PubMed] [Google Scholar]
- 28.Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12(1):31-47 [DOI] [PubMed] [Google Scholar]
- 30.Pidala J, Vogelsang G, Martin P, Chai X, Storer B, Pavletic S, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97(3):451-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa A, Nakamura S, Nakamine H, Yoshino T, Takimoto T, Horibe K, et al. Pathology review for paediatric non-Hodgkin's lymphoma patients in Japan; a report from the Japan Association of Childhood Leukaemia Study (JACLS). Eur J Cancer. 2004;40(5):725-33 [DOI] [PubMed] [Google Scholar]
- 32.Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW. Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis. 2004;63(7):774-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergijk EC, Munaut C, Baelde JJ, Prins F, Foidart JM, Hoedemaeker PJ, et al. A histologic study of the extracellular matrix during the development of glomerulosclerosis in murine chronic graft-versus-host disease. Am J Pathol. 1992;140(5):1147-56 [PMC free article] [PubMed] [Google Scholar]
- 35.Norris DA, Clark RA, Swigart LM, Huff JC, Weston WL, Howell SE. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982;129(4):1612-8 [PubMed] [Google Scholar]
- 36.Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209(1-2):199-207 [DOI] [PubMed] [Google Scholar]
- 37.Lin MT, Storer B, Martin PJ, Tseng LH, Gooley T, Chen PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349(23):2201-10 [DOI] [PubMed] [Google Scholar]
- 38.Cox K, North M, Burke M, Singhal H, Renton S, Aqel N, et al. Plasmacytoid dendritic cells (PDC) are the major DC subset innately producing cytokines in human lymph nodes. J Leukoc Biol. 2005;78(5):1142-52 [DOI] [PubMed] [Google Scholar]
- 39.Arpinati M, Chirumbolo G, Marzocchi G, Baccarani M, Rondelli D. Increased donor CD86+CD14+ cells in the bone marrow and peripheral blood of patients with chronic graft-versus-host disease. Transplantation. 2008;85(12):1826-32 [DOI] [PubMed] [Google Scholar]
- 40.Namba N, Shinagawa K, Fujii N, Maeda Y, Ishimaru F, Ikeda K, et al. Predominant infiltration of monocytes in chronic graft-versus-host disease. Transplantation. 2007;83(2):220-4 [DOI] [PubMed] [Google Scholar]
- 41.Rojas B, uhna R, Zafirakis P, Ramirez JM, Lizan-garciia M, Zhao T, et al. ell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81(3):313-25 [DOI] [PubMed] [Google Scholar]
- 42.van der Straaten HM, Canninga-van Dijk MR, Verdonck LF, Castigliego D, Borst HP, Aten J, et al. Extra-domain-A fibronectin: a new marker of fibrosis in cutaneous graft-versus-host disease. J Invest Dermatol. 2004;123(6):1057-62 [DOI] [PubMed] [Google Scholar]
- 43.Valenick LV, Hsia HC, Schwarzbauer JE. Fibronectin fragmentation promotes alpha4beta1 integrin-mediated contraction of a fibrin-fibronectin provisional matrix. Exp Cell Res. 2005;309(1):48-55 [DOI] [PubMed] [Google Scholar]
- 44.Puig-Kroger A, Sanz-Rodriguez F, Longo N, Sanchez-Mateos P, Botella L, Teixido J, et al. Maturation-dependent expression and function of the CD49d integrin on monocyte-derived human dendritic cells. J Immunol. 2000;165(8):4338-45 [DOI] [PubMed] [Google Scholar]
- 45.Yoshida Y, Kang K, Chen G, Gilliam AC, Cooper KD. Cellular fibronectin is induced in ultraviolet-exposed human skin and induces IL-10 production by monocytes/macrophages. J Invest Dermatol. 1999;113(1):49-55 [DOI] [PubMed] [Google Scholar]
- 46.Ogawa Y, Yamazaki K, Kuwana M, Mashima Y, Nakamura Y, Ishida S, et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001;42(1):111-9 [PubMed] [Google Scholar]
- 47.Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease:VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12(5):491-505 [DOI] [PubMed] [Google Scholar]