Abstract
Anaplastic large cell lymphomas are peripheral T-cell lymphomas that are characterized by a proliferation of large anaplastic blasts expressing CD30. In children, systemic anaplastic large cell lymphomas often present at advanced clinical stage and harbor translocations involving the anaplastic lymphoma kinase (ALK) gene leading to the expression of chimeric anaplastic lymphoma kinase (ALK)-fusion proteins. Primary cutaneous anaplastic large cell lymphoma is regarded as an ALK-negative variant confined to the skin and is part of the spectrum of primary cutaneous CD30-positive T-cell lymphoproliferative disorders. Thirty-three of 487 pediatric patients registered within the Anaplastic Large Cell Lymphoma-99 trial (1999 to 2006) presented with a skin limited CD30-positive lympho-proliferative disorder. In 23 of the 33 patients, material for international histopathological review was available, and the cases were studied for histopathological, immunophenotypical and clinical features as well as for breaks within the ALK gene. Five of 23 cases and one additional case (identified after closure of the trial) expressed ALK-protein. Complete staging excluded any other organ involvement in all children. Expression of ALK proteins was demonstrated by immunohistochemistry in all cases and the presence of breaks of the ALK gene was genetically confirmed in 5 evaluable cases. The histopathological and clinical picture of these skin-restricted ALK-positive lymphomas was indistinguishable from that of cutaneous anaplastic large cell lymphoma. Five children presented with a single skin lesion that was completely resected in 4 and incompletely resected in one. Three of these patients received no further therapy, 2 additional local radiotherapy, and one chemotherapy. All children remain in complete remission with a median follow up of seven years (range 1–8 years). We present 6 pediatric cases of ALK-positive primary cutaneous anaplastic large cell lymphomas. After thorough exclusion of systemic involvement, therapy confined to local measures seems to be sufficient to induce cure.
Introduction
Anaplastic lymphoma kinase-positive (ALK-positive) anaplastic large cell lymphoma (ALCL) is characterized by a neoplastic proliferation of large pleomorphic (anaplastic) CD30-positive (CD30+) T cells with typical translocations involving the ALK gene and subsequent expression of chimeric ALK protein.1 This lymphoma accounts for approximately 15% of childhood non-Hodgkin's lymphomas, but is rare in adult-hood.2,3 ALK-positive (ALK+) ALCL is usually a systemic disease that frequently involves extranodal sites. In children, 18–25% of systemic ALCLs develop skin manifestations during the course of the disease and this is a poor prognostic factor.4–9 Systemic ALK-negative (ALK-) ALCL is included in the updated WHO classification as a separate preliminary entity.1 ALK-negative ALCL accounts for less than 5% of pediatric systemic ALCLs.5,10 However, both ALK-positive and ALK-negative ALCLs are considered potentially disseminated diseases.1
Primary cutaneous ALCL (cALCL) is regarded by the WHO as a separate disease entity and belongs to the spectrum of primary cutaneous CD30-positive lymphoproliferations (CD30+LPD), a group that also includes lymphomatoid papulosis (LyP).1 CD30+ LPDs share with systemic ALCL the presence of neoplastic CD30+ large T cells, but lack ALK translocations and protein expression. cALCLs remain confined to the skin, virtually never disseminate beyond local lymph nodes, and show an excellent prognosis after surgical resection without systemic therapy. Most cases of cALCL present as solitary skin lesions, but multiple skin nodules are also found. In contrast to systemic ALCL, cALCL is only rarely found in children and young adults.11–13 Recently published recommendations for the diagnosis of CD30+LPD state that immunohistochemical detection of ALK expression should be considered highly suspicious of a cutaneous manifestation of underlying systemic ALCL.13 In contrast, IRF4 translocations have been reported in cALCL and in ALK- ALCLs but not in ALK+ ALCL.14,15 In the international multicenter trial ALCL99, children included with localized skin disease were not to receive systemic chemotherapy based on the assumption that their disease would be CD30+LPD. We describe a series of 6 pediatric ALCLs that clinically and histologically resembled cALCL but expressed ALK fusion proteins. These localized cutaneous ALK+ ALCL followed the typical benign clinical course of a CD30+LPD.
Design and Methods
Identification of cases and histopathological review
In the ALCL99 multicenter study, 487 children and young adults with the diagnosis of ALCL were registered from 1999 to 2006, including 33 patients with a CD30+ lymphoproliferative disorder limited to the skin. Patients with isolated skin lesions diagnosed by complete staging procedures were to be followed after resection by ‘watchful waiting’ without further systemic therapy regardless of the Alk status. For 23 of these, skin limited lymphoma material was available for an international histopathological review. One additional case reported here was identified after completion of registration in 2006. The histological review of the cases was performed by members of the international pediatric lymphoma pathology panel (IO, LL, AN, EdA, UH, KH, ISK, JM, LM, MT) using hematoxylin and eosin (H&E) stained slides as well as slides stained immunohistochemically in various laboratories (see below). The registered clinical data from the study center were reviewed and additional details were obtained by contacting the attending pediatric oncologist. The study was part of the scientific projects accompanying the ALCL99 study, for which informed consent was obtained. The study was carried out according to the local ethical guidelines and in accordance with the ethical guidelines of the studies in which the patients were treated.
Immunohistochemistry and fluorescence in situ hybridization
All immunohistochemical stainings were performed on whole tissue sections. The stainings were scored semiquantitatively as negative, weak (<30% positive tumor cells or all tumor cells weakly positive), positive (>30% positive tumor cells) or not interpretable. The minimal staining panel for each lymphoma included CD20, CD3, CD30, and ALK. Additional stainings for granzyme B, perforin, TIA1, EMA, CD2, and CD5 were available for individual cases. Due to the retrospective nature of the study, the staining procedures and antibody sources for these markers varied between the participating countries but had been previously established within the group as part of the ALCL99 study.16 Fluorescence in situ hybridization (FISH) for chromosomal breaks in the ALK gene or at the IRF4/DUSP22 locus was performed as previously described.17,18
Results
Identification of the 6 ALK-positive cases limited to the skin
Among the 23 cases with ALCL or CD30+ lymphoproliferations confined to the skin registered into the ALCL99 study and available for international histopathological review, 5 patients with expression of ALK protein were identified. During the preparation of the manuscript, another case of ALK+ ALCL limited to the skin was identified by the NHL-BFM study center and included in this series.
Histological and immunohistochemical features
The main histological and immunohistochemical features of the 6 cases are summarized in Table 1. In most cases, a superficial and deep cutaneous infiltration extending into the subcutis was observed (3 of 4 cases in which all skin layers were included in the biopsy specimen). The lesions were rather poorly demarcated. In one case, an isolated subcutaneous nodule without dermal involvement was seen. In 5 cases, the epidermis was included in the specimen and was either normal in appearance (n=2), showed hyperplastic changes (n=2), or hyperplastic changes with additional focal superficial erosion (n=1). A large number of CD30+ neoplastic blasts forming cohesive sheets were detectable in 5 of 6 cases. However, one case displayed only scattered blasts. In 3 of 6 lesions, the growth pattern of the blasts was perivascular. Reactive inflammatory bystander cells were composed of a moderate number of neutrophils (1 of 6) or lymphohistiocytic cells (2 of 6). No inflammatory bystander cells were detectable in 3 of 6 lymphomas. Figure 1 shows one representative example of an ALK+ ALCL confined to the skin.
Table 1.
Histopathological and immunohistochemical features of 6 pediatric cases of ALK-positive primary cutaneous anaplastic large cell lymphoma.
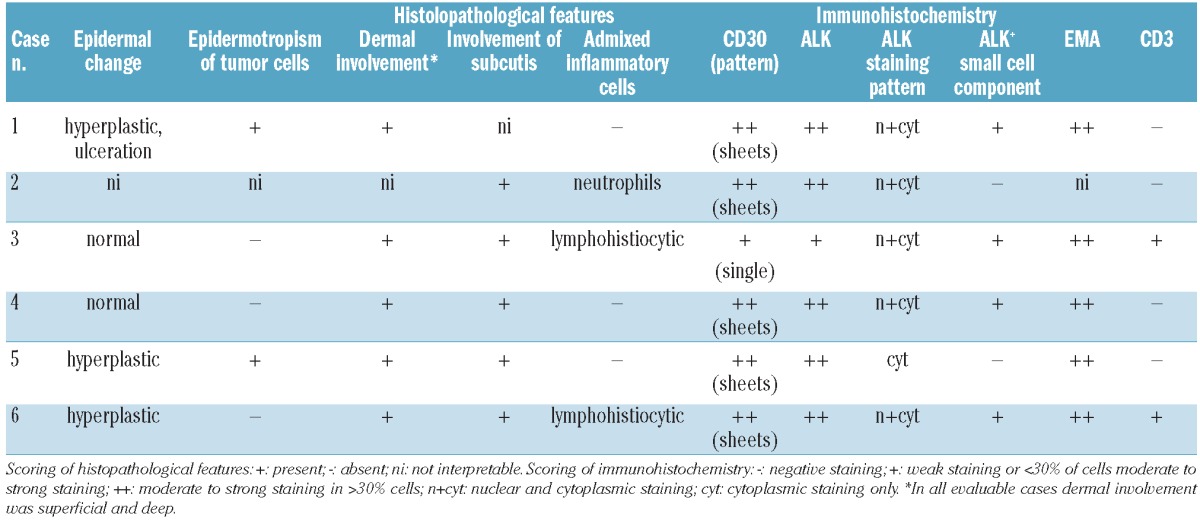
Figure 1.
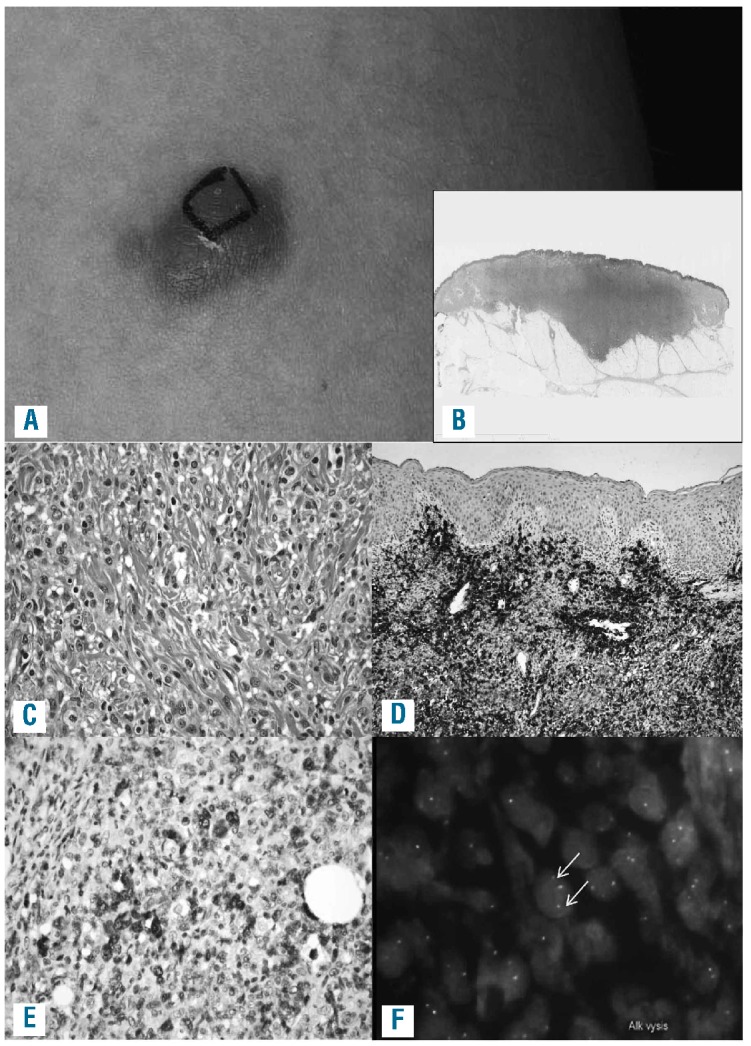
Clinical and histological features of one representative example of ALK-positive ALCL confined to the skin (case 6, see Tables 1 and 2). This is a nodular lesion on the thigh, approximately 2 cm in largest diameter (case 6). The black ink marks the area that had initially been planned for resection; it was later decided to resect the lesion completely (A). At low magnification deep extension of the lesion with a dense dermal infiltration as well as reactive epidermal hyperplasia is observed (B) (Hematoxylin & Eosin staining). Cytologically, histiocytes, a few lymphocytes and intermingled atypical large cells are seen (C) (Hematoxylin & Eosin staining). Large cells in clusters with a perivascular pattern are observed. There is no epidermotropism (D, CD30). Large cells show nuclear and cytoplasmic ALK staining; intermingled with some smaller cells with nuclear ALK (E, ALK). Fluorescence in situ hybridization using the LSI ALK BAP probe (Abbott) indicates a chromosomal breakpoint in the ALK gene (arrows)(F).
ALK expression was immunohistochemically detectable in all cases with nuclear and cytoplasmic staining in 5 of 6 cases, indicating an underlying NPM-ALK fusion due to a t(2;5) translocation. In one lymphoma, diffuse cytoplasmic ALK staining without nuclear positivity was noted. Interestingly, in 4 of 6 lymphomas, a small cell component was detectable, as indicated by predominately nuclear staining of small lymphoma cells (Figure 2). All 5 cases tested for epithelial membrane antigen (EMA) were strongly positive. CD3 was negative (4 of 6) or weakly expressed (2 of 6). All lymphomas expressed at least one cytotoxic protein, such as granzyme B, TIA1 or perforin with the characteristic granular staining pattern (data not shown).
Figure 2.
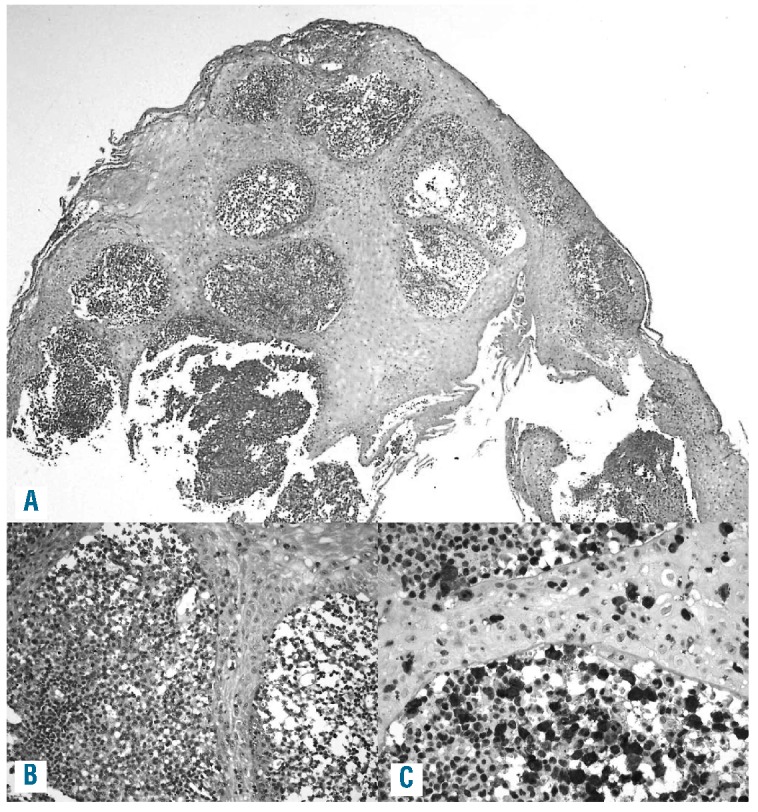
An example of ALK-positive ALCL (case 1, see Table 1) with epidermotropism of lymphoma cells and a subepithelial small cell tumor component. (A and B) Hematoxylin & Eosin staining. (C) ALK1).
Fluorescence in situ hybridization
Material for fluorescence in situ hybridization was available for 4 lymphomas. Breaks in the ALK gene were detectable in all 4 analyzed cases (Figure 1). In the additional patient with multilocular skin disease NPM-ALK-transcripts were detected in the bone marrow and blood by polymerase chain reaction (data not shown) so that the ALK-translocation was confirmed molecularely in 5 of 6 patients. In contrast, breaks affecting the IRF4/DUSP22 locus in 6p25 recurrently involved in cALCL were not detectable in the 3 cases studied.
Clinical characteristics, therapy and outcome
Table 2 summarizes the clinical characteristics of the patients reported in this series. Median age was 10.8 years (range 7.5–13.8 years). Three patients were male and 3 female. None of the children had a clinically documented history of lymphomatoid papulosis (LyP) or mycosis fungoides. The lymphomas presented clinically as papulonodular skin lesions (5 of 6) and/or subcutaneous nodules (3 of 6). One patient displayed multiple skin lesions (case 4) which were described as multiple pink nodules on the trunk, arms and neck. The isolated lesions in the other 5 patients involved the thigh (n=3), neck (n=1) or knee (n=1). Figure 1 shows the clinical presentation of one case with a solitary lesion on the thigh (case 6). None of the children suffered from B symptoms. All patients underwent a complete initial staging procedure to exclude systemic disease according to the ALCL99 protocol, including imaging of the abdomen and thorax, full blood cell count and bone marrow cytology. Lumbar puncture was performed in 5 of the 6 patients. In one patient, minimal disseminated disease (MDD) was detectable, measured by polymerase chain reaction for NPM-ALK transcripts19 in the bone marrow and blood (case 4, Table 2 and data not shown). The single skin lesion was surgically completely resected in 4 of the 5 patients. One patient received additional local radiotherapy after complete excision and in one case an incomplete resection of the skin lesion was followed by local radiotherapy. The patient with multiple skin nodules and MDD in the bone marrow and peripheral blood (case 4, Table 1) received 6 courses of chemotherapy according to the protocol ALCL99 in the high-risk arm.20 None of the other patients received chemotherapy. All patients reached a complete remission and remained disease-free with a median follow up of seven years (range 1–8 years).
Table 2.
Clinical features of 6 pediatric cases of ALK-positive primary cutaneous anaplastic large cell lymphoma.
Discussion
We report here 6 cases of ALK+ ALCL limited to the skin. These lymphomas mimicked primary cutaneous CD30+LPD in their histopathology, clinical presentation and response to therapy.
CD30+LPD comprise a spectrum of diseases confined to the skin, including LyP and cALCL, which show overlapping histological features. Both diseases are characterized by a neoplastic infiltrate of anaplastic CD30+ T cells with a variable admixture of reactive inflammatory cells. Single nodular skin lesion or, less frequently, multiple nodules that do not undergo spontaneous regression are the typical presentation of cALCL.1,13 Distinguishing a primary cutaneous CD30+LPD, such as LyP and cALCL, from secondary involvement of the skin by systemic ALCL is clinically relevant. Treatment of systemic ALCL consists of risk-adapted polychemotherapy. Secondary skin involvement is regarded as a clinical risk factor, often utilized to stratify patients to a more aggressive treatment regimen.21,22 In contrast, primary cutaneous CD30+LPD, which is limited to the skin and rarely disseminates, usually either resolves spontaneously or is treated locally, e.g. by surgical excision.13
All of our cases fulfilled the clinical and histological criteria of a primary cutaneous anaplastic large cell lymphoma with predominantly solitary skin lesions, no history of LyP, no extracutaneous dissemination and response to local therapy,13 but all cases were ALK+. Given the higher incidence of cALCL in adults, most published series analyzing ALK expression have included predominately adult patients.23 There have been only single case reports and small series of pediatric cALCL, and in these ALK staining was inconsistently performed.24–28 We assume that our series is not population-based as cutaneous CD30+LPD are diagnosed and treated either by dermatologists or pediatric oncologists. Nevertheless, our data suggest that ALK+ cALCL might be more frequent than anticipated within the pediatric population, and recommend that all CD30+LPD of the skin in children should be carefully analyzed for ALK expression.
Lamant et al.29 recently reported 5 children with systemic ALK+ ALCL that presented as skin lesions at the site of preceding insect bites, often with involvement of the draining local lymphnode. Thus, the skin might not only present a preferred microenvironment for ALK+ ALCL but might even be the primary site of lymphomagenesis. At the moment, no reliable histopathological features are known to distinguish secondary skin involvement by a systemic ALCL from primary cutaneous CD30+LPD. EMA has been reported to be positive in most systemic ALK+ and ALK− ALCLs30 but negative in cALCL.13,31 ALK protein expression, as well as the underlying ALK-gene translocation, are considered indicative of systemic ALK+ ALCL and are seen in nearly all pediatric systemic ALCL cases.10,20 In contrast, cALCL is considered ALK- both at the molecular and the protein level.32–35 Our cases were ALK and EMA positive on the one hand but localized and limited to the skin on the other. They, therefore, presented as and thus could be named as primary cutaneous ALK-positive ALCL. One could discuss whether the child with multiple skin lesions and positive MDD should have been classified as child with systemic type ALCL. Nevertheless, for the moment, staging is determined by clinical imaging as well as by the evaluation of bone marrow cytology, and all these investigations were negative in this child, indicating isolated skin disease. In practical terms, the child was treated with systemic chemotherapy despite the isolated skin involvement, and we would support this treatment decision, especially since positive MDD has been shown to be an adverse prognostic factor in systemic ALCL.19 To the best of our knowledge, skin-confined variants of ALK+ ALCL have previously been published in 5 cases only. Table 3 shows a summary of the literature33,36-40 and the cases presented here. However, the published cases differ from our series in two main points. First, all previously published cases were adult patients (Table 2). Second, 2 of 5 previously published cases developed systemic disease years after the initial primary skin disease; a feature that was absent in our cohort (Table 2). Just recently, at the joint workshop of the Society for Hematopathology and the European Association for Hematopathology (SH/EAHP) on cutaneous lymphomas held in Los Angeles in October 2011,41 5 new cases of ALK+ ALCLs confined to the skin were presented as case reports. Four of these occurred in adults with variable clinical scenarios, ALK-staining-patterns and histomorphological features, and only one ALK+ ALCL confined to the skin was described in a child with a very unusual mycosis fungoides as clinical and histological presentation. Therefore, more attention to ALK-staining in cutaneous T-cell lymphoproliferations seems justified.
Table 3.
Literature review of reported ALK-positive cutaneous anaplastic large cell lymphomas and findings in this series.
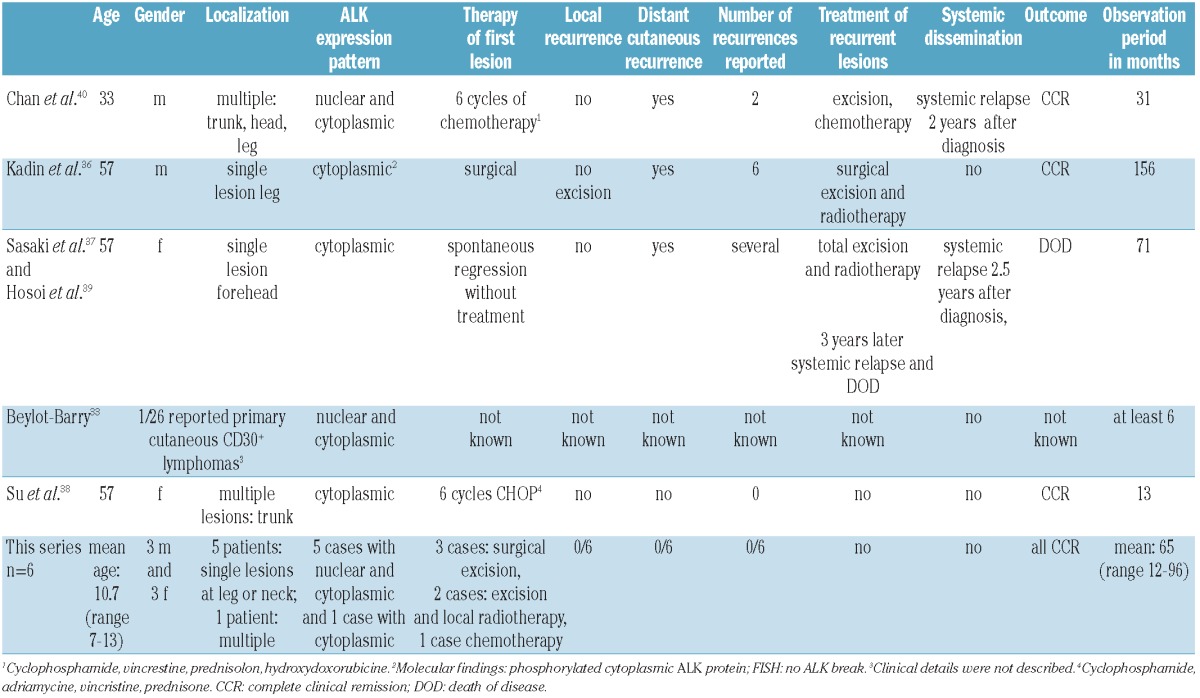
Interesting histological features of the lymphomas reported here were the presence of a small cell component in 4 of the 6 cases, and a perivascular growth pattern in 3. The presence of a small cell component and a perivascular growth pattern have recently been reported to be associated with a poorer outcome in systemic ALK+ ALCL.16 However, there was no relapse among the 5 patients with exclusive local therapy reported in our series. This emphasizes again that ALK-positive ALCL limited to the skin may represent a specific subgroup of ALK+ ALCL for which prognostic parameters established in systemic ALK+ ALCL do not apply.
In summary, our cases illustrate that ALK+ ALCL can present as a localized skin-limited disease. Localized treatment with careful follow up seems justified after thorough exclusion of systemic disease in this rare variant. Understanding the biology of ALK+ ALCLs that are confined to the skin might influence therapy strategies for ALK+ ALCL also in other locations.
Acknowledgments
The authors are indebted to all the children and parents who participated in this study, to Nathalie Bouvet, Institut Gustave-Roussy, Villejuif, France, for database management, and Oliviera Batic, Dimitry Abramov and Reina Zühlke-Jenisch for their technical assistance.
Funding: This work was supported by the José-Carreras-Foundation (DJCLS R08/09). RS and WK are supported by the Kinderkrebs Initiative Buchholz, Holm-Seppensen, Germany. The ALCL99 study was supported by the Forschungshilfe Peiper and the Association Cent pour Sang la Vie, France. None of the authors reported any other potential conflicts of interest.
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial and other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO Classification of Tumors of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008 [Google Scholar]
- 2.Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R, et al. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131(1):39-49 [DOI] [PubMed] [Google Scholar]
- 3.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-30 [DOI] [PubMed] [Google Scholar]
- 4.Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97(12):3699-706 [DOI] [PubMed] [Google Scholar]
- 5.Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27(6):897-903 [DOI] [PubMed] [Google Scholar]
- 6.Le Deley MC, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008;111(3):1560-6 [DOI] [PubMed] [Google Scholar]
- 7.Reiter A, Schrappe M, Parwaresch R, Henze G, Muller-Weihrich S, Sauter S, et al. Non-Hodgkin's lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage--a report of the Berlin-Frankfurt-Munster Group. J Clin Oncol. 1995;13(2):359-72 [DOI] [PubMed] [Google Scholar]
- 8.Williams DM, Hobson R, Imeson J, Gerrard M, McCarthy K, Pinkerton CR. Anaplastic large cell lymphoma in childhood: analysis of 72 patients treated on The United Kingdom Children's Cancer Study Group chemotherapy regimens. Br J Haematol. 2002;117(4):812-20 [DOI] [PubMed] [Google Scholar]
- 9.Brugieres L, Deley MC, Pacquement H, Meguerian-Bedoyan Z, Terrier-Lacombe MJ, Robert A, et al. CD30(+) anaplastic large-cell lymphoma in children: analysis of 82 patients enrolled in two consecutive studies of the French Society of Pediatric Oncology. Blood. 1998;92(10):3591-8 [PubMed] [Google Scholar]
- 10.Damm-Welk C, Klapper W, Oschlies I, Gesk S, Rottgers S, Bradtke J, et al. Distribution of NPM1-ALK and X-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: a molecular-histological correlation. Br J Haematol. 2009;146(3):306-9 [DOI] [PubMed] [Google Scholar]
- 11.Bekkenk MW, Geelen FA, van Voorst Vader PC, Heule F, Geerts ML, van Vloten WA, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95(12):3653-61 [PubMed] [Google Scholar]
- 12.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768-85 [DOI] [PubMed] [Google Scholar]
- 13.Kempf W, Pfaltz K, Vermeer MH, Cozzio A, Ortiz-Romero PL, Bagot M, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118(15):4024-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada DA, Law ME, Hsi ED, Dicaudo DJ, Ma L, Lim MS, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24(4):596-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman AL, Dogan A, Smith DI, Law ME, Ansell SM, Johnson SH, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117(3):915-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamant L, McCarthy K, d'Amore E, Klapper W, Nakagawa A, Fraga M, et al. Prognostic Impact of Morphologic and Phenotypic Features of Childhood ALK-Positive Anaplastic Large-Cell Lymphoma: Results of the ALCL99 Study. J Clin Oncol. 201;29(35):4669-76 [DOI] [PubMed] [Google Scholar]
- 17.Salaverria I, Philipp C, Oschlies I, Kohler CW, Kreuz M, Szczepanowski M, et al. Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood. 2011;118(1):139-47 [DOI] [PubMed] [Google Scholar]
- 18.Ventura RA, Martin-Subero JI, Jones M, McParland J, Gesk S, Mason DY, et al. FISH analysis for the detection of lymphoma-associated chromosomal abnormalities in routine paraffin-embedded tissue. J Mol Diagn. 2006;8(2):141-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damm-Welk C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK positive anaplastic large cell lymphoma. Blood. 2007;110(2):670-7 [DOI] [PubMed] [Google Scholar]
- 20.Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27(6):897-903 [DOI] [PubMed] [Google Scholar]
- 21.Le Deley MC, Rosolen A, Williams DM, Horibe K, Wrobel G, Attarbaschi A, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28(25):3987-93 [DOI] [PubMed] [Google Scholar]
- 22.Reiter A, Schrappe M, Tiemann M, Parwaresch R, Zimmermann M, Yakisan E, et al. Successful treatment strategy for Ki-1 anaplastic large-cell lymphoma of childhood: a prospective analysis of 62 patients enrolled in three consecutive Berlin-Frankfurt-Munster group studies. J Clin Oncol. 1994;12(5):899-908 [DOI] [PubMed] [Google Scholar]
- 23.Benner MF, Willemze R. Applicability and prognostic value of the new TNM classification system in 135 patients with primary cutaneous anaplastic large cell lymphoma. Arch Dermatol. 2009;145(12):1399-404 [DOI] [PubMed] [Google Scholar]
- 24.Tomaszewski MM, Moad JC, Lupton GP. Primary cutaneous Ki-1(CD30) positive anaplastic large cell lymphoma in childhood. J Am Acad Dermatol. 1999;40(5 Pt 2):857-61 [DOI] [PubMed] [Google Scholar]
- 25.Fink-Puches R, Chott A, Ardigo M, Simonitsch I, Ferrara G, Kerl H, et al. The spectrum of cutaneous lymphomas in patients less than 20 years of age. Pediatr Dermatol. 2004;21(5):525-33 [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Pittaluga S, Raffeld M, Guerrera M, Seibel NL, Jaffe ES. Primary cutaneous CD30-positive anaplastic large cell lymphoma in childhood: report of 4 cases and review of the literature. Pediatr Dev Pathol. 2005;8(1):52-60 [DOI] [PubMed] [Google Scholar]
- 27.Santiago-et-Sanchez-Mateos D, Hernandez-Martin A, Colmenero I, Mediero IG, Leon A, Torrelo A. Primary cutaneous anaplastic large cell lymphoma of the nasal tip in a child. Pediatr Dermatol. 2011;28(5):570-5 [DOI] [PubMed] [Google Scholar]
- 28.Hung TY, Lin YC, Sun HL, Liu MC. Primary cutaneous anaplastic large cell lymphoma in a young child. Eur J Pediatr. 2008;167(1):111-3 [DOI] [PubMed] [Google Scholar]
- 29.Lamant L, Pileri S, Sabattini E, Brugieres L, Jaffe ES, Delsol G. Cutaneous presentation of ALK-positive anaplastic large cell lymphoma following insect bites: evidence for an association in five cases. Haematologica. 2010;95(3):449-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugieres L, Terrier-Lacombe MJ, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998;91(6):2076-84 [PubMed] [Google Scholar]
- 31.ten Berge RL, Oudejans JJ, Dukers DF, Meijer CJ. Anaplastic large cell lymphoma: what's in a name? J Clin Pathol. 2001;54(6):494-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Pulford K, Willemze R, Falini B, et al. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol. 2000;53(6):445-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beylot-Barry M, Groppi A, Vergier B, Pulford K, Merlio J P. Characterization of t(2;5) reciprocal transcripts and genomic breakpoints in CD30+ cutaneous lymphoproliferations. Blood. 1998;91(12):4668-76 [PubMed] [Google Scholar]
- 34.Herbst H, Sander C, Tronnier M, Kutzner H, Hugel H, Kaudewitz P. Absence of anaplastic lymphoma kinase (ALK) and Epstein-Barr virus gene products in primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis. Br J Dermatol. 1997;137(5):680-6 [PubMed] [Google Scholar]
- 35.Liu HL, Hoppe RT, Kohler S, Harvell JD, Reddy S, Kim YH. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49(6):1049-58 [DOI] [PubMed] [Google Scholar]
- 36.Kadin ME, Pinkus JL, Pinkus GS, Duran IH, Fuller CE, Onciu M, et al. Primary cutaneous ALCL with phosphorylated/activated cytoplasmic ALK and novel phenotype: EMA/MUC1+, cutaneous lymphocyte antigen negative. Am J Surg Pathol. 2008;32(9):1421-6 [DOI] [PubMed] [Google Scholar]
- 37.Sasaki K, Sugaya M, Fujita H, Takeuchi K, Torii H, Asahina A, et al. A case of primary cutaneous anaplastic large cell lymphoma with variant anaplastic lymphoma kinase translocation. Br J Dermatol. 2004;150(6):1202-7 [DOI] [PubMed] [Google Scholar]
- 38.Su LD, Schnitzer B, Ross CW, Vasef M, Mori S, Shiota M, et al. The t(2;5)-associated p80 NPM/ALK fusion protein in nodal and cutaneous CD30+ lymphoproliferative disorders. J Cutan Pathol. 1997;24(10):597-603 [DOI] [PubMed] [Google Scholar]
- 39.Hosoi M, Ichikawa M, Imai Y, Kurokawa M. A case of anaplastic large cell lymphoma, ALK positive, primary presented in the skin and relapsed with systemic involvement and leukocytosis after years of follow-up period. Int J Hematol. 2010;92(4):667-8 [DOI] [PubMed] [Google Scholar]
- 40.Chan DV, Summers P, Tuttle M, Cooper KD, Cooper B, Koon H, et al. Anaplastic lymphoma kinase expression in a recurrent primary cutaneous anaplastic large cell lymphoma with eventual systemic involvement. J Am Acad Dermatol. 2011;65(3):671-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Workshop of the Society for Hematopathology and the European Association for Hematopathology (SH/EAHP) on Cutaneous Lymphomas and Their Mimics. 2011. Proceedings.


