Abstract
Fludarabine-cyclophosphamide-rituximab is the most efficient first-line treatment for chronic lymphocytic leukemia patients. Many dose adjustments of the original MD Anderson Cancer Center regimen have been proposed. However, whether fludarabine-cyclophosphamide-rituximab relative dose intensity may have an impact on outcome has not yet been investigated. We retrospectively assessed relative dose intensity in 106 community-based patients included in our regional healthcare network from 2004-11, all receiving fludarabine-cyclophosphamide-rituximab as first-line treatment outside clinical trials. Dose reductions were observed in 51.4% of patients, mainly decided by the individual physician and not based on recommendations (52.7%), while there were fewer reports of toxicity or dose reduction because of impaired renal function. Progression-free survival was significantly reduced in patients who had a reduction in dose intensity of more than 20% in fludarabine-cyclophosphamide and/or rituximab. Multivariate analysis showed dose of rituximab had a significant impact on minimal residual disease and progression-free survival. Although prophylactic granulocyte-colony stimulating factor significantly reduced the rate of grade 3-4 neutropenia and febrile neutropenia, it had no impact on relative dose intensity and outcome. This study shows that, in routine clinical practice, there is low adherence to the original MD Anderson Cancer Center fludarabine-cyclophosphamide-rituximab schedule, and that the decision to modify dosage was mostly taken by the individual physician and was based on anticipated toxicity. This study shows that reduction of fludarabine-cyclophosphamide and, more importantly, of rituximab doses seriously interferes with progression-free survival.
Introduction
Fludarabine-cyclophosphamide-rituximab (FCR) is now considered to be the most efficient drug combination in chronic lymphocytic leukemia (CLL) patients for overall response rate (ORR), and progression-free and overall survivals (PFS and OS). Historically, FCR was developed by the Houston group according to specification recommendations as follows: fludarabine (F) 25 mg/m2 Days 1-3 + cyclophosphamide (C) 250 mg/m2 Days 1-3 + rituximab (R) 375 mg/m2 Day 1 Cycle 1 then 500 mg/m2 Day 1 Cycles 2-6, all drugs being administered intravenously (iv).1 These treatment modalities have also been applied for the CLL8 trial performed by the German CLL Study Group. This trial demonstrated the superiority of FCR over FC for ORR, PFS and OS despite increased hematologic toxicities.2 However, the introduction in 2001 of F oral formulation resulted in new modalities of FC or FCR administration based on bioequivalence of a 40 mg/m2 oral dose with a 25 mg/m2 iv dose. For example, in two clinical trials in the UK and France, F has been used orally at 24 and 25 mg/m2 Days 1-5 (respectively) + C 150-200 mg/m2 Days 1-5.3,4 In elderly patients, an Italian group has used oral F 15 mg/m2 Days 1-4 + C 200 mg/m2 Days 1-4, whereas another group has preferred oral F 25 mg/m2 Days 1-4 + C 120 mg/m2 Days 1-4.5,6 More recently, Foon and co-workers have developed the FCR-Lite schedule using low doses of FC in combination with high-dose rituximab.7,8 Finally, the French CLL study group is currently promoting FCR combination in young or fit elderly patients with oral F 40 mg/m2 Days 1-3 + C 250 mg/m2 Days 1-3 + R 375-500 mg/m2. From all these studies, it seems that toxicity is more related to the dose rather than to the route of administration.9 However, the influence of the dose on clinical benefit remains uncertain.
The impact of drug dosage on outcome has been investigated in aggressive lymphomas by measuring the influence of relative dose intensity (RDI), calculated as the ratio of the dose actually delivered over time to the standard dose intensity, on PFS and OS.10 Through this methodology, it has been demonstrated that, in diffuse large B-cell lymphomas treated with CHOP, a reduction equal to or over 30% of RDI for cyclophosphamide or anthracyclines seriously influenced both PFS and OS.10 Since the introduction of rituximab, four independent studies, including our own,11 have described similar findings with 10-30% of reduced RDI resulting in decreased PFS and OS in DLBCL patients.11-14 It is interesting to note that all these studies are retrospective and focused on patients treated outside the setting of clinical trials.
However, very little is known about the impact of RDI in indolent lymphomas, including CLL. Therefore, it still remains to be determined whether RDI for F and C, or even rituximab plays a role in PFS or OS. This is an important question because of the number of reported FC protocols, and also because FC dosage is frequently modified as part of planned or unplanned decision making because of the frequency of FC-induced neutropenia and the interference between F and renal insufficiency.15,16 In our regional care network, CLL has been treated for more than a decade with FCR according to standard French administration guidelines (oral F 40 mg/m2 Days 1-3 + C 250 mg/m2 Days 1-3). However, occasionally the individual physician modified the dosages of the three drugs according to age, renal function, anticipated or documented myelosuppression, and evolving recommendation for rituximab dose in France (375 mg/m2 6 cycles, or 375 mg/m2 Cycle 1 and then 500 mg/m2 Cycles 2-6).
In this study, we took advantage of these disparities to measure the influence of RDI for F, C, and rituximab on PFS in a cohort of 106 CLL patients treated with FCR as front-line therapy.
Design and Methods
Patients' and CLL characteristics
This was a retrospective cohort study of medical records from 106 previously untreated patients participating in the Oncomip (Oncologie Midi-Pyrénées) healthcare network from 2005-2011. All patients signed informed consent before inclusion in this network, and phenotype, fluorescence in situ hybridization (FISH) positive karyotype, IgVH mutational analysis, and minimal residual disease (MRD) by 4-color flow cytometry analyses and assessment of response to FCR were centrally reviewed or performed (University Hospital of Purpan, Toulouse, France). All patients received treatment in their community hospitals. Creatinine clearance and body weight/surface were also verified. Fresh and thawed samples from CLL patients have been obtained after obtaining informed consent and stored in the HIMIP collection (collection d'hémopathies malignes de l'INSERM Midi-Pyrénées). According to French law, the HIMIP collection was declared to the Ministry of Higher Education and Research (DC 2008-307 collection 1) and a transfer agreement was obtained (AC 2008-129) after approval by the “Comité de Protection des Personnes Sud-Ouest et Outremer II” ethical committee. Clinical and biological annotations of the samples have been declared to the CNIL (Comité National Informatique et Libertés, i.e. Data Processing and Liberties National Committee).
Dose intensity and relative dose intensity
In each center, doses of FCR were calculated from either the pharmacist's and/or the oncologist's records. Dose calculations were compared to the standard FCR doses (planned dose intensity, DI): oral F 40 mg/m2 3 days, C 250 mg/m2 3 days and R 375 mg/m2 Day 1 Cycle 1, then 500 mg/m2 Day 1 for Cycles 2-6. At the end of the planned therapeutic period (24 weeks for 6 cycles), relative dose intensity (RDI) (mg/m2/week) was calculated as the ratio of the amount of F, C or R (375 mg/m2 6 cycles instead of 500 mg/m2 for 5 cycles is equal to a dose reduction of over 20%) actually delivered (i.e. actual DI for each drug) to the planned DI for the fixed time period of 24 weeks.
Measurement of outcomes
Three months after the last FCR cycle, overall response rate was assessed as clinical complete response (clinical CR), CR with incomplete bone marrow recovery (CRi), partial response (PR), or failure. The method of response assessment differed from the NCI2008 criteria in that in France bone marrow biopsy is not required outside the setting of clinical trials. This explains why we used the term “clinical CR” instead of CR. This difference may explain the discrepancy between the CR rates in our study and those from other clinical trials such as the CLL8. For example, in the MDACC experience, FCR resulted in 70% “CR” without bone marrow biopsy. Furthermore, patients with deletion 17p were excluded because FCR is not the standard treatment for this population in our healthcare network.
Creatinine clearance was also calculated. Patients were followed up for one year in order to detect late toxicities such as prolonged cytopenia (<50×109/L platelets or <1×109/L neutrophils). Progression-free survival (PFS) was calculated from the first day of the last FCR cycle until relapse (>5×109/L lymphocytes and/or palpable spleen, liver or lymph nodes), treatment-free survival (TFS) and overall survival (OS) were calculated after any second-line therapy and death from any cause, respectively. Survival status was confirmed by phone to the referring physicians, or was based on the database of our hospital outpatients' clinic.
Analysis of toxicity
Patients with at least one complete inter-cycle blood count (CBC, 1-4 CBCs every FCR cycle) were included (n=76) to monitor hematologic toxicity between cycles. Grade 3-4 neutropenia was recorded according to common toxicity criteria for adverse events (CTC-AE) classification. Fever episodes (with or without neutropenia), and hospitalizations for sepsis were monitored at every cycle (i.e. monthly). For 20 of 76 (26.3%) patients, prospective evaluation of AEs was managed by a clinical nurse who planned weekly phone calls for six months (“Assistance aux Malades Ambulatoires” protocol, AMA11). Use of primary prophylactic G-CSF (pegfilgrastim or filgrastim) or nadir-driven secondary prophylactic G-CSF (prescribed for grade 4 febrile/non-febrile neutropenia for at least one cycle) was also obtained for all 106 patients.
Statistical analysis
Survival curves were plotted according to Kaplan-Meier analysis and compared using the log rank test. Medians were compared using the non-parametric Mann-Whitney analysis. Variables linked to prolonged survival in univariate analysis (P<0.1) were included in a Cox's model for multivariate comparisons (P<0.05 was considered significant).
Results
Patients' and disease characteristics
Main characteristics of the cohort are summarized in Table 1. Median age was 60 years (range 21-83 years); 21.7% of patients were aged 65 years or over. The median comorbidity score by the Cumulative Illness Rating Scale - Geriatric (CIRS-G) was 1 (range 0-8); only 2 of 106 had a CIRS over 6. Median creatinine clearance levels before treatment calculated according to Cockroft-Gault or MDRD equations were 79 mL/mn (range 47-169 mL/mn) and 75 mL/mn (range 47-122 mL/mn), respectively. Baseline prognostic parameters included stage B or C in 75% of patients, unmutated IgVH in 67%, del(11q23) in 23%, CD38 over 20% in 46%. No patient with del(17p) was included in this study because in our healthcare network front-line treatment for these patients consists of alemtuzumab-based therapy. Treatment decisions were made according to the 2008 IWCLL criteria.
Table 1.
Main characteristics of the 106 patients.
Outcomes of FCR in the entire cohort
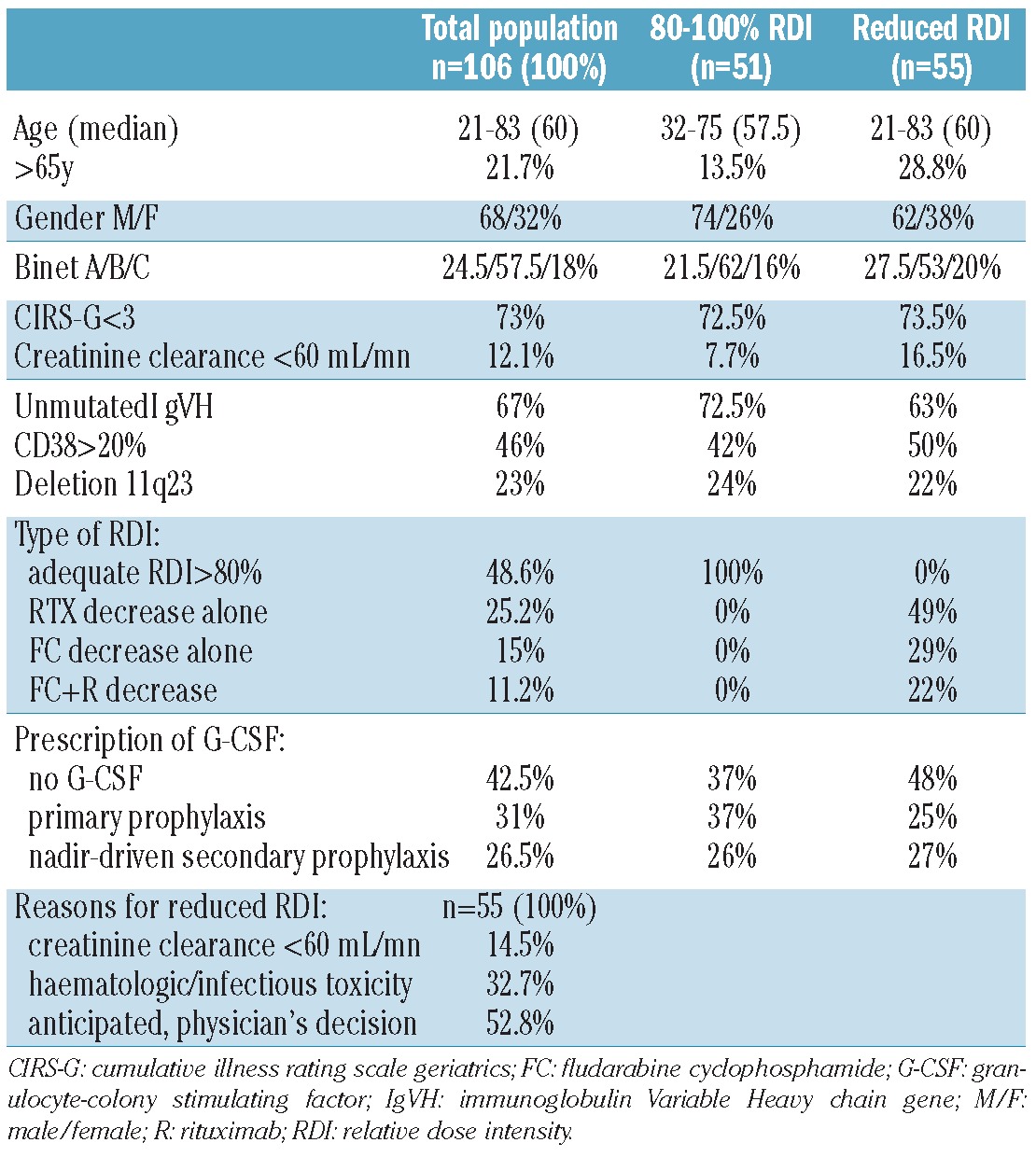
At the time of evaluation, overall response rate (ORR) was 100%, with 72.9% of clinical CR, and 26% of CRi. Among 67 of 106 patients in whom phenotypic response was assessed, 56.7% reached undetectable MRD, as measured by 4-color flow cytometry analysis of peripheral blood. Prolonged cytopenia for at least six months was observed in 11.6% of patients. Median follow up was 5.5 years. PFS (median 56 months), TFS (median 70 months) and OS (median not reached) curves according to Kaplan-Meier analysis are shown in Figure 1A-C. In univariate analysis of our cohort, PFS was not linked to age over 65 years, creatinine clearance of less than 60 mL/mn, IgVH status, CD38, Binet stage, or del(11q). Finally, post-treatment MRD level was highly predictive for PFS, as previously described in the CLL-8 trial (Figure 2A). The kinetics of MRD re-growth (or recovery) was then measured between the first MRD result (assessed three months after completion of FCR) and a second MRD assessment (performed 12 months after the first). A rapid MRD recovery has been defined in our study as a gain of at least 1 log of MRD. Interestingly, MRD re-growth kinetics was predictive not only for PFS but also for OS, as described by ourselves and others (Figures 2B and C).17,18 Altogether, these results were very similar to those described in CLL patients treated with FCR iv formulation as front-line therapy.
Figure 1.
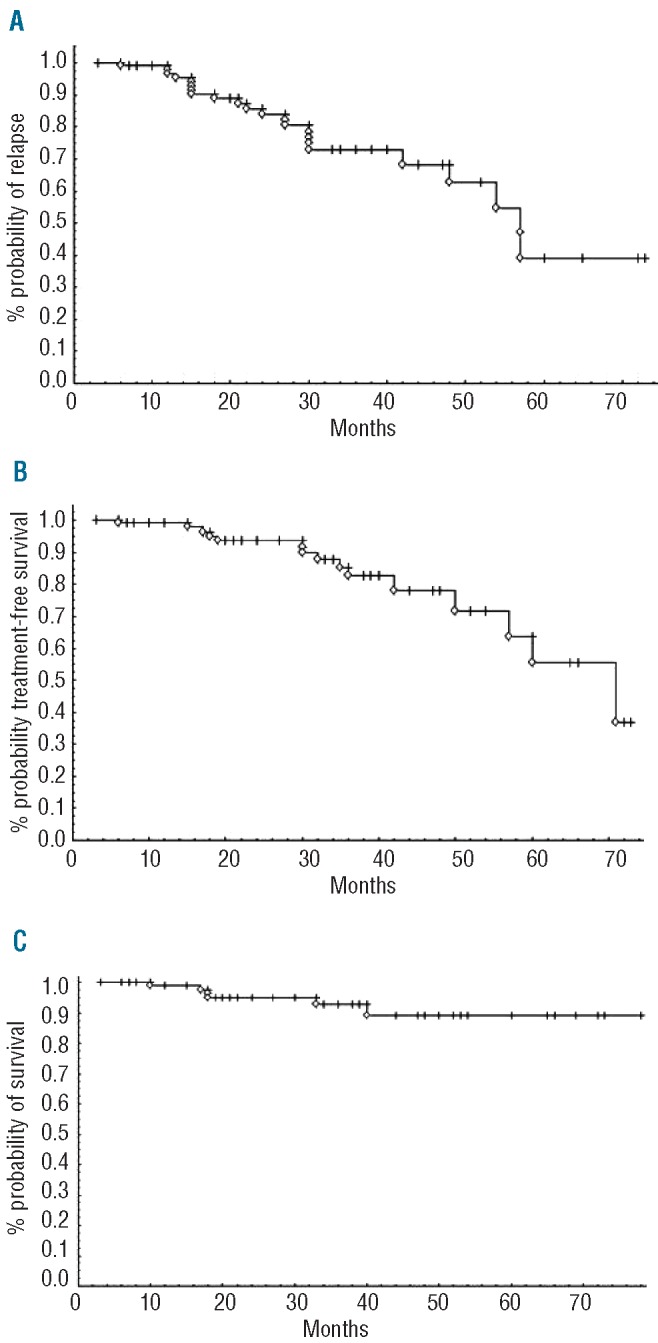
(A) Progression-free (B) treatment-free and (C) overall survivals for the entire cohort of 106 patients receiving FCR front line for CLL in the healthcare network.
Figure 2.
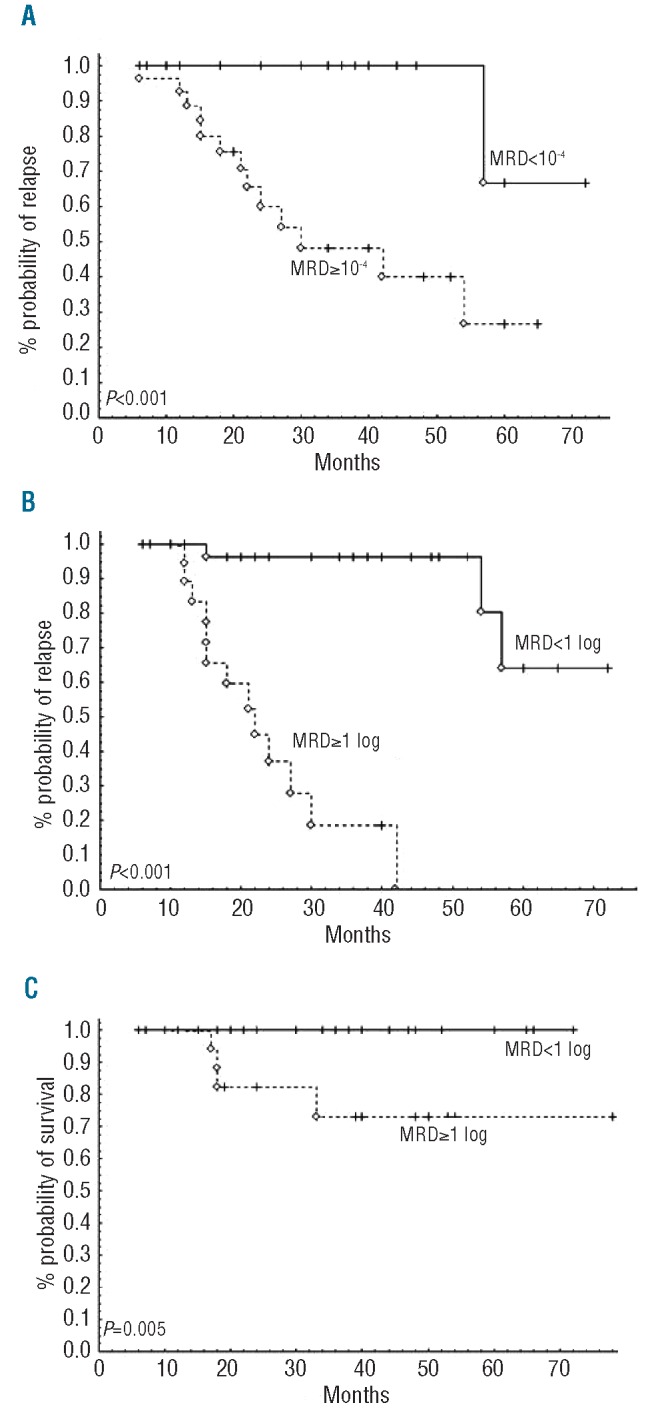
Impact of minimal residual disease (MRD) eradication on progression-free and overall survivals after FCR. (A) Eradication of MRD, as assessed by 4-color flow cytometry at the end of treatment (3 months post-FCR), is strongly predictive of PFS. Monitoring of MRD re-growth kinetics (<1 vs. ≥1 log of MRD increase between end of treatment and one year) predicts both PFS (B) and OS (C) the first 12 months after FCR.
Incidence of low FCR relative dose intensity
Nearly half of the patients received the standard FCR doses. However, a decrease of more than 20% of RDI in FC alone, rituximab alone, or both drugs, was reported in the other patients (Table 1). For FC, predictors of dose reduction were age over 65 years (P=0.02) and female gender (41% of females and 17% of males had reduced FC doses; P=0.01), but not Binet stage or renal function. A similar fraction of males and females were over 65 years of age (22% and 21%, respectively). For rituximab, no relation was found with Binet stage, gender, age, leukocyte count, or renal function.
The reasons for dose reduction of FC and/or R were: impaired renal function (18.5%), hematologic and/or infectious toxicity (inducing dose delays and, therefore, reduction in RDI, 32.7%), and anticipation of toxicity or reduced rituximab dose at 375 mg/m2 (decided by the individual physician, 52.8%) (Table 1). These results show that in most cases a reduction in RDI was not related to disease status or comorbidity, but rather to the decision of the individual physician.
Impact of toxicity on RDI
Neutropenia and infections
In spite of the possible underestimation of AEs in a retrospective analysis, we found grade 3-4 neutropenia, febrile neutropenia, and hospitalizations due to sepsis in 58.6%, 15.8% and 19% of patients, respectively, whereas G-CSF was used in 57.5% of patients as primary prophylaxis or nadir-driven secondary prophylaxis (upon occurrence of grade 3 neutropenia). However, primary prophylaxis with G-CSF significantly reduced the rate of FN and grade 3-4 neutropenia at any cycle (37.9% vs. 65.9% of patients without prophylaxis, P=0.02). Reduction of FC doses was observed in 33 of 106 (27.3%) patients who had received primary G-CSF (pegfilgrastim or lenograstim) compared to 27.2% of patients who had not. Therefore, whereas the use of G-CSF resulted in effective prevention of FN, it played no role in maintaining RDI, as reported in meta-analyses.19 This could explain why G-CSF had no impact on PFS (Figure 3).
Figure 3.
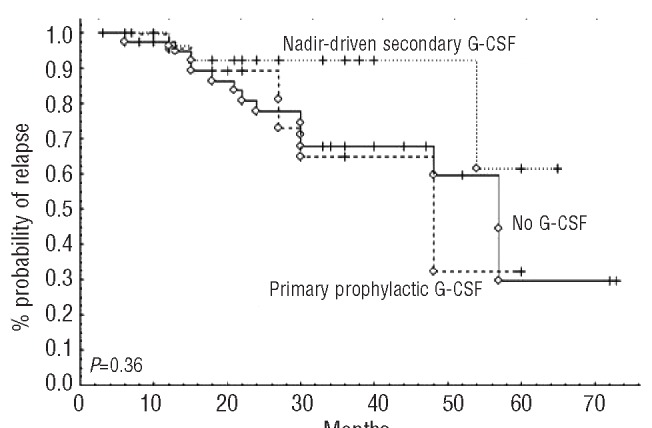
Impact of G-CSF use on progression-free survival after FCR. G-CSF: granulocyte-colony stimulating factor.
Renal function
Thirteen of 106 patients presented an altered renal function with creatinine clearance less than 60 mL/min (range 47-59 mL/min), justifying an over 20% FC dose reduction in 8 of 13 cases. Surprisingly, in these patients, FCR treatment significantly improved renal function (mean pre-FCR 53.6 mL/min vs. mean post FCR 72.7; P<0.05).
Impact of RDI on FCR efficacy
On univariate analysis, dose reductions in both FC (Figure 4A) and rituximab (Figure 4B) had an impact on PFS, together with MRD eradication post-treatment. Reductions in both FC and rituximab doses resulted in a dramatic decrease in PFS (median 24 months vs. 60 months without dose adjustment, P<0.01, Figure 4C). Reduction in RDI for FC or rituximab did not influence either ORR or post-treatment MRD level (data not shown). Nevertheless, rituximab dose (but not FC dose) had a dramatic impact on MRD recovery. In 64 patients tested, 24% displayed rapid re-growth kinetics when treated with 500 mg/m2, as compared to 48% when treated with 375 mg/m2 (P<0.05). In multivariate analysis, rituximab dose (P=0.05) and MRD results (P<0.01) were statistically linked to better PFS, but not FC dose (P=0.67). Altogether, these results suggest that rituximab dose is critical for MRD control and prolonging PFS.
Figure 4.
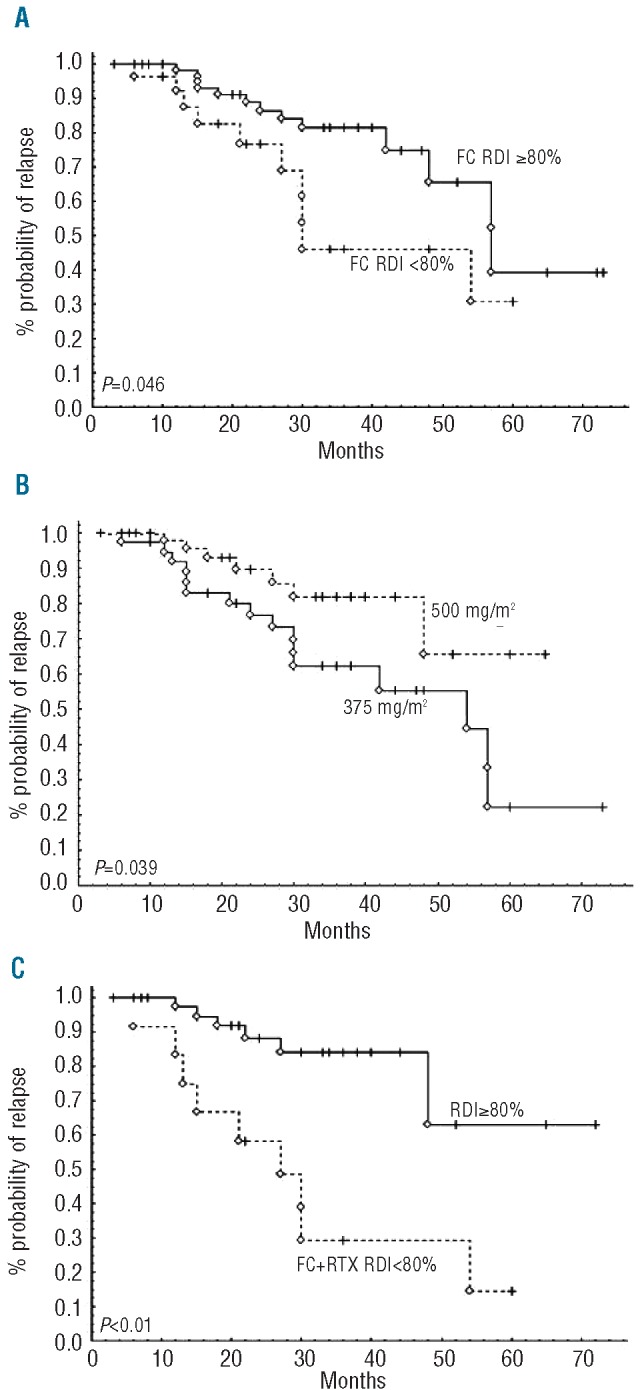
Impact of dose intensity on progression-free survival after FCR. (A) Reduction by more than 20% of initial DI of fludarabine and cyclophosphamide compromises PFS. (B) Higher dose of rituximab (500 mg/m2 from Cycle 2 to 6) yields better PFS than using the 375 mg/m2 dosage across FCR cycles. (C) Reduction by more than 20% of FC and rituximab doses dramatically impairs PFS after FCR, as compared to standard FCR doses (RDI>80%).
Discussion
In this retrospective series of 106 medically fit patients, we investigated the role of dose intensity on the outcome of CLL patients receiving first-line treatment with combination of rituximab and oral formulation of F and C (FCR). Overall, our results emphasize that drug doses and, most importantly, the dose of rituximab are critical to maintain prolonged phenotypic response and, ultimately, prolonging PFS.
Our study emphasizes that, in the setting of community hospital practice, FC dose reductions were common (62% reductions were >10% and 24% were >20%). These rates of low intensity chemotherapy were higher than those reported in the CLL-8 study in which only one-third of the patients were treated with FC reduction of over 10%.2 In our study, the reduction of FCR doses was most often related to inadequate dose adjustments mainly leading to decreased rituximab doses (52.8%), hematologic or infectious toxicities (32.7%), whereas altered renal function was not a frequent cause of dose adjustment in 14.5% of cases. In the CLL-8 study dealing with patients with similar age and normal renal function, the incidence of FC dose reduction was also mainly related to cumulative hematologic toxicity (70%). However, 20% of patients received reduced doses the reasons for which were not reported.2 In our study, G-CSF was broadly used (approx. 60% of patients). G-CSF was found to be highly efficient for preventing both grade 3-4 and febrile neutropenia. Nevertheless, in contrast with a previous report,20 the use of G-CSF did not contribute to either maintaining RDI or to improving PFS or OS,
The impact of FC RDI on clinical outcome has not been thoroughly investigated in the CLL8 trial. In our study, with a cut off at 20%, we found that FC RDI was predictive for PFS in univariate but not in multivariate analysis. This does not mean that a greater than 20% FC reduction is not deleterious for patients treated with FCR, but it does suggest that other parameters should be more critical. Among them, based on previous studies which have established a dose effect for rituximab in CLL,21 we speculated that rituximab dose could have influenced clinical outcome. In our study, two-thirds of patients received rituximab therapy as designed by the Houston group (375 mg/m2 Cycle 1 followed by 500 mg/m2 thereafter1) while the others received 375 mg/m2 from Cycles 1-6, i.e. a dose reduction of over 20%. Our study shows for the first time that, in the context of FCR therapy, rituximab dose has a significant impact on the kinetics of MRD recovery and PFS in multivariate analysis. This result supports the design of the historical FCR regimen as promoted by Keating and co-workers. In the CLL8 trial, rituximab dose was marginally modified with less than 10% of patients experiencing dose reduction of over 10%.2 This could explain why the investigators have not identified any correlation between rituximab dose and clinical outcome. Our study supports the notion that FCR is indeed a dose-dense regimen for rituximab. This concept was originally developed by the Pittsburgh group according to the FCR-Lite protocol, with high ORR and an excellent safety profile. However, patient numbers in the authors reports were limited, patients had low Rai stage, and received prophylactic pegfilgrastim despite reduced FC doses (-20% for F, -40% for C). Furthermore, these patients benefited from two years of rituximab maintenance and this makes it difficult to interpret the impact of altered RDI on the recently published long-term PFS results.7,8 Two French randomized studies have now incorporated the concept of high-dose rituximab associated with FC: i) the CLL-2010 FMP study (500 mg Day 0, then 2,000 mg at Days 1, 8 and 15, FCR starting at Day 22 at 500 mg/m2) for patients under the 65 years of age; and ii) the CLL 2007SA (rituximab 500 mg/m2 at Day 15 of Cycles 1 and 2, in association with 4 cycles of standard FCR) for patients over 65 years of age.
To conclude, our study shows that the efficiency of FCR depends on precise modalities of administration. Outside the setting of clinical trials, FCR dose adjustments are frequent because of objective considerations (e.g. pre-existing renal function impairment, documented hematologic toxicities, rituximab infusion-related side effects) but also subjective considerations (e.g. the individual physician's assessment or personal decision). Although all these parameters may ultimately reduce clinical benefit, this study identified rituximab dose as being the most critical factor for disease control.
Acknowledgments
The authors would like to thank their colleagues within the ONCOMIP healthcare network, from the community hospitals and private clinics of Ariège, Aveyron, Gers, Haute-Garonne, Tarn, and Tarn-et-Garonne, France.
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org
References
- 1.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376(9747):1164-74 [DOI] [PubMed] [Google Scholar]
- 3.Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370(9583):230-9 [DOI] [PubMed] [Google Scholar]
- 4.Cazin B, Divine M, Leprêtre S, Travade P, Tournilhac O, Delmer A, et al. High efficacy with five days schedule of oral fludarabine phosphate and cyclophosphamide in patients with previously untreated chronic lymphocytic leukaemia. Br J Haematol. 2008;143(1):54-9 [DOI] [PubMed] [Google Scholar]
- 5.Forconi F, Fabbri A, Lenoci M, Sozzi E, Gozzetti A, Tassi M, et al. Low-dose oral fludarabine plus cyclophosphamide in elderly patients with untreated and relapsed or refractory chronic lymphocytic Leukaemia. Hematol Oncol. 2008;26(4):247-51 [DOI] [PubMed] [Google Scholar]
- 6.Marotta G, Bigazzi C, Lenoci M, Tozzi M, Bocchia M, Lauria F. Low-dose fludarabine and cyclophosphamide in elderly patients with B-cell chronic lymphocytic leukemia refractory to conventional therapy. Haematologica. 2000;85(12):1268-70 [PubMed] [Google Scholar]
- 7.Foon KA, Boyiadzis M, Land SR, Marks S, Raptis A, Pietragallo L, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27(4):498-503 [DOI] [PubMed] [Google Scholar]
- 8.Foon KA, Mehta D, Lentzsch S, Kropf P, Marks S, Lenzner D, et al. Long-term results of chemoimmunotherapy with low-dose fludarabine, cyclophosphamide and high-dose rituximab as initial treatment for patients with chronic lymphocytic leukemia. Blood. 2012;119(13):3184-5 [DOI] [PubMed] [Google Scholar]
- 9.Dearden CE, Richards S, Else M, Catovsky D, Hillmen P. A comparison of the efficacy and safety of oral and intravenous fludara-bine in chronic lymphocytic leukemia in the LRF CLL4 trial. Cancer. 2011;117(11):2452-60 [DOI] [PubMed] [Google Scholar]
- 10.Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77(3):221-40 [DOI] [PubMed] [Google Scholar]
- 11.Compaci G, Ysebaert L, Obéric L, Derumeaux H, Laurent G. Effectiveness of telephone support during chemotherapy in patients with diffuse large B cell lymphoma: the Ambulatory Medical Assistance (AMA) experience. Int J Nurs Stud. 2011;48(8):926-32 [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-cell lym-phoma. J Clin Exp Hematop. 2011;51(1):1-5 [DOI] [PubMed] [Google Scholar]
- 13.Terada Y, Nakamae H, Aimoto R, Kanashima H, Sakamoto E, Aimoto M, et al. Impact of relative dose intensity (RDI) in CHOP combined with rituximab (R-CHOP) on survival in diffuse large B-cell lymphoma. J Exp Clin Cancer Res. 2009;28:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettengell R, Schwenkglenks M, Bacon P, Lawrinson S, Duehrsen U. Pegfilgrastim primary prophylaxis in patients with non-Hodgkin lymphoma: results from an integrated analysis. Hematol Oncol. 2011;29(4):177-84 [DOI] [PubMed] [Google Scholar]
- 15.Lichtman SM, Etcubanas E, Budman DR, Eisenberg P, Zervos G, D'Amico P, et al. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest. 2002;20(7-8):904-13 [DOI] [PubMed] [Google Scholar]
- 16.Martell RE, Peterson BL, Cohen HJ, Petros WP, Rai KR, Morrison VA, et al. Analysis of age, estimated creatinine clearance and pre-treatment hematologic parameters as predictors of fludarabine toxicity in patients treated for chronic lymphocytic leukemia: a CALGB (9011) coordinated intergroup study. Cancer Chemother Pharmacol 2002;50:37-45 [DOI] [PubMed] [Google Scholar]
- 17.Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch R, Fingerle-Rowson G, et al. Minimal Residual Disease (MRD) Re-Growth Kinetics Are An Independent Predictor for Progression Free Survival (PFS) in Chronic Lymphocytic Leukemia (CLL) and Are Related to Biologically Defined CLL-Subgroups – Results From the CLL8 Trial of the German CLL Study Group (GCLLSG). J Clin Oncol. 2012;30(9):980-8 [DOI] [PubMed] [Google Scholar]
- 18.Ysebaert L, Gross E, Kühlein E, Blanc A, Corre J, Fournié JJ, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24(7):1310-6 [DOI] [PubMed] [Google Scholar]
- 19.Bohlius J, Herbst C, Reiser M, Schwarzer G, Engert A. Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database Syst Rev. 2008;(4):CD003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber M, Fleiss K, Porpaczy E, Skrabs C, Hauswirth AW, Gaiger A, et al. Prolonged progression-free survival in patients with chronic lymphocytic leukemia receiving granulocyte colony-stimulating factor during treatment with fludarabine, cyclophosphamide, and rituximab. Ann Hematol. 2011;90(10):1131-6 [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhi J, Wenger M, Valente N, Dmoszynska A, Robak T, et al. Population Pharmacokinetics of Rituximab in Patients With Chronic Lymphocytic Leukemia. J Clin Pharmacol. 2012;52(12):1918-26 [DOI] [PubMed] [Google Scholar]


