Abstract
Treatment with melphalan-prednisone-thalidomide improves the outcome of patients with multiple myeloma and is now considered a standard of care for patients not eligible for transplantation. However, this treatment is a major source of morbidity. A meta-analysis of data from individual patients (n=1680) in six randomized trials was performed, comparing the effects of melphalan-prednisone-thalidomide versus melphalan-prednisone. The main objective was to estimate the risk of serious adverse events and their impact on outcome. The primary endpoints were the 2-year cumulative incidence of grade 3-4 hematologic and non-hematologic toxicities. At least 75% of the grade 3-4 toxicities occurred during the first 6 months of treatment in both treatment groups. The cumulative incidence of grade 3-4 hematologic toxicities was higher in the melphalan-prednisone-thalidomide group than in the melphalan-prednisone group (28% versus 22%; HR 1.32, 95% CI 1.05-1.66) as was the cumulative incidence of non-hematologic toxicities (39% versus 17%, HR 2.78, 95% CI 2.21-3.50). Grade 3-4 non-hematologic toxicities were significantly increased in patients with poor Performance Status. Occurrence of grade 3-4 non-hematologic toxicities had a negative impact on both progression-free survival (HR 1.24, 95% CI 1.07-1.45) and overall survival, (HR 1.23, 95% CI 1.03-1.47). Besides toxicities, progression-free and overall survival were also negatively affected by advanced International Staging System stage, high creatinine levels and poor Performance Status. Age had a negative impact on survival as well. Although melphalan-prednisone-thalidomide improved outcome, it increased toxicities, especially non-hematologic ones. Serious non-hematologic toxicities, older age, poor Performance Status, and high creatinine levels negatively affected survival.
Introduction
The introduction of thalidomide in the treatment of multiple myeloma (MM) has improved patients' outcome.1 Six randomized, phase 3 studies have compared the association of melphalan-prednisone-thalidomide (MPT) with standard melphalan-prednisone (MP) in patients with newly diagnosed MM.2-8 A recent meta-analysis of data from individual patients in these six trials found that the addition of thalidomide to MP increases the median overall survival by approximately 6.6 months (HR 0.83, 95% CI 0.73-0.94), and the median progression-free survival by 5.4 months (HR 0.68, 95% CI 0.61-0.76).9 Based on the results of all these trials, MPT is now considered a standard first-line treatment for elderly MM patients, or those ineligible for high-dose therapy. However, MPT is also a less safe treatment than MP and the lack of reliable estimates of the risk and the severity of hematologic and non-hematologic adverse events and of their impact on clinical outcomes represents an area of uncertainty in clinical practice. Frequencies of grade 3-4 adverse events varied significantly among studies because of the heterogeneity of treatment policies, study designs, the inclusion criteria for patients, data collection and reporting. The most frequent non-hematologic adverse events in MPT-treated patients in the six trials were infections (10-28%), peripheral neuropathy (2-23%), venous thromboembolism (3-12%) and constipation (3-10%).2-8
In elderly patients, treatment-related adverse events are a major cause of drug discontinuation and reduced dose-intensity, thus limiting treatment efficacy. Several studies have reported a negative impact of treatment-related adverse events on survival for elderly patients treated with more aggressive therapies.10-12 In a randomized study, the overall survival of elderly patients receiving thalidomide-dexamethasone was significantly shorter than that of those receiving MP (41.5 versus 49.4 months; P=0.024). The number of deaths from non-myeloma-related causes was twice as high in the group treated with thalidomide-dexamethasone group than in the group treated with MP.10 In another randomized study, overall survival was decreased in patients receiving lenalidomide plus high-dose dexamethasone in comparison with those receiving lenalidomide plus low-dose dexamethasone. Higher rates of toxicities, treatment discontinuation and early mortality were reported in patients receiving high-dose dexamethasone.11 In a recent phase 3 trial, non-hematologic grade 3-4 adverse events were reported in 51% of patients who received bortezomib twice-weekly and 35% of patients who received bortezomib once-weekly (P=0.003); 15% of patients in the group treated with twice weekly bortezomib and 5% in the group treated with once weekly bortezomib discontinued therapy (P<0.001). Long-term outcomes were similar in the two groups. These results suggest that preventing severe toxicities is essential to reduce treatment discontinuation and consequently maximize efficacy.13
We performed a meta-analysis of data from individual patients in six randomized phase III trials that compared MPT and MP in elderly patients with MM. We aimed to provide a reliable estimate of the cumulative incidence of severe toxicities and of their impact on clinical outcomes. This safety analysis is part of a project agreed upon by the principal investigators of the six trials; an efficacy meta-analysis with the same studies and criteria has been published recently.9
Design and Methods
Objectives
The primary objective was to compare the risk of developing grade 3-4 hematologic and non-hematologic adverse events during the first 2 years of treatment with MPT or MP.
Secondary objectives were: (i) to analyze the role of patients' characteristics on the risk of grade 3-4 adverse events; (ii) to assess the impact of the occurrence of grade 3-4 adverse events on progression-free and overall survival; (iii) to explore the safety profile of MPT versus MP in subgroups of patients stratified by age, gender, Durie and Salmon (DS) stage, International Staging System (ISS) stage, World Health Organization Performance Status (WHO-PS) and serum creatinine.
Data source and search
At the beginning of 2011, the principal investigators of the six MPT trials completed within the European Myeloma Network agreed to perform an individual patient data meta-analysis and provided the safety data of their respective trials. An efficacy analysis, based on the same criteria, has already been performed and published under the coordination of the Nordic Myeloma Study Group.9 To verify the completeness of the studies included in this project, we carried out a MEDLINE search using the terms melphalan, prednisone, thalidomide, multiple myeloma, elderly patients. In total, we found 73 original full papers in English, published between January 2000 to January 2011. There were seven articles providing a direct comparison between the old standard MP and the new standard MPT in randomized phase 3 studies, one of which was an update of a previously published analysis, confirming the completeness of our data. To assess the likelihood of bias of each study, two authors independently reviewed the original articles using the Jadad scale.14 The score associated with each study is reported in Table 1.
Table 1.
Characteristics of the six randomized trials.
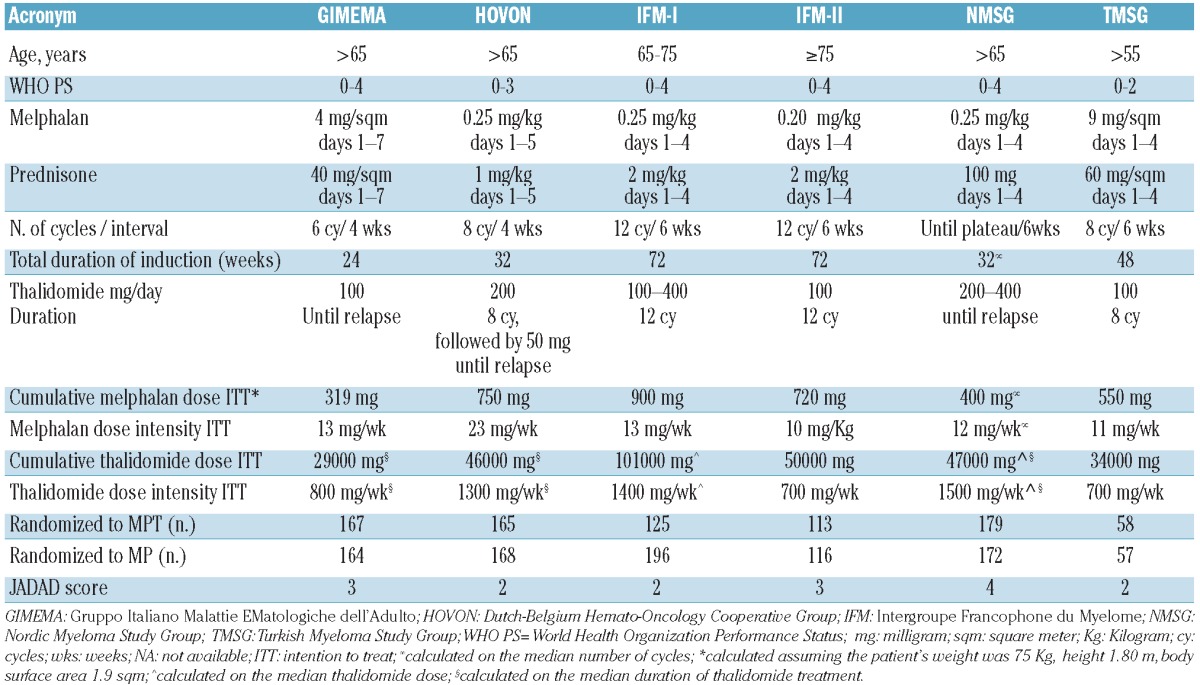
Data extraction
From 2000 onwards, six randomized phase 3 trials comparing MPT versus MP in newly diagnosed MM patients were completed by five groups in Europe and Turkey [Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA), Dutch-Belgium Hemato-Oncology Cooperative Group (HOVON), Intergroupe Francophone du Myelome (IFM), Nordic Myeloma Study Group (NMSG), and Turkish Myeloma Study Group (TMSG)]. These studies were approved by the respective institutional review boards at each of the participating centers. Individual patients' data were collected by investigators for each patient enrolled at each coordinating center. The main inclusion criteria and planned treatment schedule of the six randomized controlled trials, described in detail elsewhere,2-8 are summarized in Table 1. The characteristics of patients selected (WHO-PS, stage of disease and age range), study design and treatment policy (dose and the number of cycles/duration of thalidomide and MP) were different among the studies. The following data available across all studies were collected for each patient: baseline data, including age, creatinine levels, WHO-PS, ISS stage,15 DS stage;16 date of progression/last known remission, date of death/date last known to be alive; grade, type and date of adverse events. All adverse event data were defined and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 3.0).17 Toxic deaths were defined as deaths related to treatment and were assessed by investigators. Adverse events were systematically reviewed for consistency and completeness by the central coordinating center and any problem was discussed and resolved by contacting the study investigators.
Statistical analysis
All 1680 (out of 1685) patients who could be evaluated for toxicity were included in the analysis. Descriptive statistics were used to summarize the frequency of adverse events, deaths and treatment discontinuation. In the case of multiple grade 3-4 hematologic or grade 3-4 non-hematologic adverse events we considered only the first event for each type of toxicity. Patients who experienced the same type of toxicity more than once were counted only once. We described baseline patients' characteristics and study endpoints according to treatment (pooled analysis of all patients treated with MPT versus MP) and according to study.
Progression-free survival was calculated from the time of randomization until the date of progression, relapse, death from any cause, or the date the patient was last known to be in remission. Overall survival was calculated from the time of randomization until the date of death or the date the patient was last known to be alive.
We performed a competing risk analysis on the incidence of adverse events, either grade 3-4 hematologic or grade 3-4 non-hematologic, with the competing events defined as progression or death. We used a proportional hazard frailty model for the subdistribution to analyze the effect of baseline factors (age, sex, WHO-PS, ISS and DS stages, creatinine level) on the cumulative incidence function of toxicity, incorporating heterogeneity across studies.18
The effects of grade 3-4 hematologic or grade 3-4 non-hematologic adverse events on progression-free survival and overall survival were estimated with Cox models, with frailty shared within study and assumed to be Gamma distributed across studies,19 adjusting for baseline factors and treatment, and treating grade 3-4 hematologic and grade 3-4 non-hematologic adverse events as time-dependent variables.
In these analyses, the time of adverse event occurrence was censored at 2 years after the start of treatment. Data from the IFM-I study were excluded from all time-to-event analyses since the time of toxicity onset was not recorded for the majority of patients. All analyses were performed using the 'cmprsk' and ‘coxme’ packages of R software.
Results
Patients' characteristics
A total of 1680 patients were analyzed: 807 received MPT and 873 received MP. Overall, baseline demographic and disease characteristics were equally distributed in the two treatment groups (Table 2). The median age was 71-74 years in all studies, with the exception of the IFM-I study (69 years) in which patients older than 75 years were excluded, and the IFM-II study (78 years) in which patients younger than 75 years were excluded. The proportion of patients with WHO-PS 3-4 was 4-8% in all studies, except the NMSG trial in which it was 33%. The frequency of ISS stage III at diagnosis was higher in the NMSG and TMSG trials.
Table 2.
Baseline characteristics of patients included in the safety meta-analysis, according to treatments (pooled data) and according to trial (MPT+MP).

Grade 3-4 hematologic and non-hematologic adverse events
The frequency of toxic deaths was 6% among patients treated with MPT and 7% in those treated with MP. The most common causes of toxic death were infections (3% for MPT and 4% for MP) and cardiovascular adverse events, including thromboembolic events, pulmonary edema, cardiac events and stroke (2% in both the MPT and MP groups). The raw frequency of toxic deaths varied between trials: it was 1% in the IFM-II and HOVON trials but 10-11% in the GIMEMA and IFM-I studies, which had less stringent inclusion criteria.
The proportions of patients who developed at least one grade 3-4 hematologic adverse event were 32% and 29% in the MPT and MP groups, respectively. Grade 3-4 non-hematologic adverse events were more frequent in MPT patients (40%) than in MP patients (18%) (Table 3). The most common grade 3-4 non-hematologic adverse event was infection (13% for MPT versus 9% for MP). Other adverse events, more common among MPT patients than MP patients, were peripheral/central neuropathy (15% versus 3%), deep vein thrombosis (6% versus 2%), and dermatological toxicity (3% versus 1%). Gastrointestinal events, pulmonary embolism and cardiac toxicities were less frequent and similarly distributed between the two groups. Overall, toxicity-related discontinuation of thalidomide was reported in 35% of MPT patients: neuropathy was the main reason (15%), followed by infections (3%), venous thromboembolism (3%), dermatological toxicity (3%) and cardiac toxicities (3%). Melphalan discontinuation was reported in 5% of MPT and MP patients and was mainly caused by hematologic toxicities.
Table 3.
Frequency of adverse events, either grade 3-4 hematologic or grade 3-4 non-hematologic, according to treatment (pooled data) and according to trial (MPT+MP).
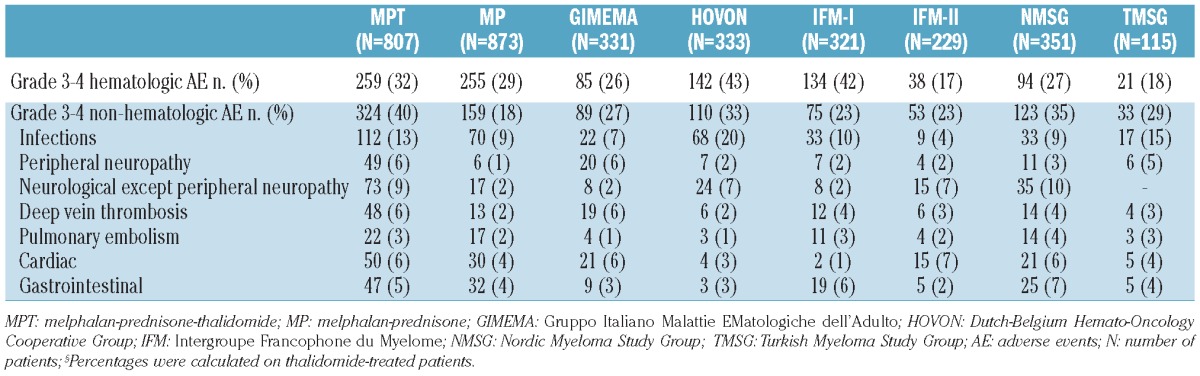
Toxicities differed between trials (Table 3). Patients enrolled in the HOVON and IFM-I studies, who received the highest dose of melphalan, reported a higher proportion of grade 3-4 hematologic toxicity compared with patients in the four other trials. The highest incidences of non-hematologic adverse events were reported in the GIMEMA, HOVON, NSMG and TMSG trials probably because of the higher number of patients with creatinine levels ≥176 μmol/L and/or higher thalidomide doses. Infections were more frequent in the HOVON trial, in which there was also a higher incidence of hematologic toxicity. The incidence of neurological toxicity was higher in the GIMEMA, HOVON and NMSG trials with thalidomide maintenance after induction and in the IFM-II trial, which included older patients. In the GIMEMA study, the incidence of thrombotic events decreased significantly after the introduction of prophylactic anticoagulation.2,3 Cardiac toxicity was less frequent in the IFM-I trial, which only included patients younger than 75 years.
The cumulative incidences of grade 3-4 hematologic (Figure 1A) and non-hematologic adverse events (Figure 1B) in the patients treated with MPT or MP have been plotted along with the cumulative incidences of the competing events (disease progression or deaths without grade 3-4 adverse events). Eighty percent of grade 3-4 hematologic adverse events occurred within the first 6 months of treatment in both groups. The cumulative incidence of grade 3-4 hematologic adverse events slowly increased with time in both groups and at 2 years was slightly higher in MPT patients than in MP patients (28% versus 22%; Gray test P=0.023).
Figure 1.
(A) Cumulative incidences of grade 3-4 hematologic adverse events and cumulative incidences of progression or death without adverse events in melphalan-prednisone-thalidomide (MPT) and melphalan-prednisone (MP) treated patients. (B) Cumulative incidences of grade 3-4 non-hematologic adverse events and cumulative incidences of progression or death without adverse events in MPT and MP treated patients.
Seventy-five percent of grade 3-4 non-hematologic adverse events occurred in the first 6 months of therapy in both groups. The cumulative incidence of grade 3-4 non-hematologic adverse events was significantly higher in the MPT group (39% at 2 years) than in the MP group (17% at 2 years), (Gray test P<0.0001).
In a multivariable model, including several baseline characteristics, the risk of developing a grade 3-4 hematologic adverse event was higher among females (HR 1.48, 95% CI 1.18-1.85; P=0.001) and patients randomized to MPT treatment (HR 1.32, 95% CI 1.05-1.66; P=0.016) (Table 4). The risk of grade 3-4 non-hematologic adverse events was strongly increased by MPT treatment (HR 2.78, 95%CI 2.21-3.50; P=0.000) and by WHO-PS score (HR 1.18 per point, 95% CI 1.06-1.32, P=0.003).
Table 4.
Risk factors for grade 3-4 hematologic and non-hematologic adverse events. Hazard ratios (HR) and 95% confidence intervals (95% CI) from proportional hazard frailty models for competing risks.

An exploratory subgroup analysis, considering age, gender, ISS stage, WHO-PS and serum creatinine, did not find any important effect modifier of the increased risk of MPT for grade 3-4 hematologic and non-hematologic adverse events.
Impact of treatment-related adverse events on clinical outcomes
Progression-free survival was negatively influenced by the occurrence of a non-hematologic adverse event (HR 1.24, 95% CI 1.07-1.45; P=0.006) but not by hematologic adverse events. Other risk factors for progression-free survival were poor WHO-PS, high creatinine and more advanced ISS myeloma stage (Table 5).
Table 5.
Influence of baseline patients' characteristics, MPT treatment and grade 3-4 hematologic and non-hematologic adverse events on progression-free survival and overall survival. Hazard ratios (HR) and 95% confidence interval (95% CI) from Cox frailty models.

The occurrence of non-hematologic adverse events was also associated with a worse overall survival (HR 1.23, 95% CI 1.03-1.47; P=0.024). Other baseline factors associated with shorter overall survival were age ≥75 years (HR 1.18, 95% CI 1.01-1.38), WHO-PS (HR 1.29 per point, 95% CI 1.20-1.39), creatinine levels ≥ 176 μmol/L (HR 1.66, 95% CI 1.31-2.10) and advanced ISS stage (ISS 3 versus 1: HR 2.05, 95% CI 1.57-2.67; ISS 2 versus 1: HR 1.59, 95% CI 1.24-2.03) (Table 5).
After accounting for baseline characteristics and despite the increased occurrence of adverse events, patients randomized to MPT showed a better progression-free survival (HR 0.68, 95% CI 0.60- 0.78; P<0.0001) and overall survival (HR 0.82, 95% CI 0.70-0.86; P=0.014).
In all the fitted models the point estimate of the frailty variance was not statistically significant, indicating a low heterogeneity between studies when most important patients' characteristics were included in the models.
Discussion
Six randomized, phase 3 studies compared the association of MPT with standard MP as first-line treatment in elderly MM patients. A meta-analysis of efficacy data of these trials showed a clear overall survival and progression-free survival benefit for patients receiving thalidomide.9 In the present meta-analysis, data from the individual patients enrolled in the same six trials were pooled and safety data were analyzed. Although the results showed an increased incidence of toxic events, especially non-hematologic, in patients treated with MPT, the benefit of adding thalidomide to MP was clearly confirmed.
In the pooled analysis, the rate of toxic deaths was similar in both MPT and MP patients. In a previous meta-analysis of 27 randomized trials comparing MP with combination chemotherapies, overall survival was unchanged but the incidence of toxic deaths was higher among the patients treated with combination chemotherapy.20 Similarly, for the same reason, dexamethasone-based regimens did not improve survival compared with MP.21
The cumulative incidence of hematologic grade 3-4 adverse events at 2 years was marginally higher in MPT patients than in MP patients (28% versus 22%, adjusted HR 1.32, 95% CI 1.05-1.66), confirming the low risk of hematologic toxicity caused by adding thalidomide to MP.22 As regards baseline patients' characteristics, the risk of hematologic toxicity was higher for females.
The 2-year incidence of grade 3-4 non-hematologic adverse events in MPT patients was more than double that in MP patients (39% versus 17%, with an adjusted HR of 2.78, 95% CI 2.21-3.50). Infections were the most frequent non-hematologic adverse events in both groups; neuropathy, deep-vein thrombosis, and dermatological toxicity were the most frequent thalidomide-related adverse events. The most important risk factor for non-hematologic toxicity was the WHO-PS, suggesting that frail patients are at increased risk of severe adverse events, independently of the specific treatment.
An analysis of the incidence of hematologic toxicities by trial may suggest a correlation between hematologic toxicity and melphalan doses. The toxicity analyses in each trial suggest that neuropathy was probably associated with the duration of thalidomide treatment, whereas cardiac toxicities were likely to be age-related. However, after adjusting for differences among study protocols and patients' characteristics, the difference in cumulative incidences of adverse events between studies was negligible.
Eighty percent of grade 3-4 hematologic adverse events and 75% of grade 3-4 non-hematologic adverse events occurred during the first 6 months of treatment in both the MPT and MP groups, supporting the need for careful monitoring of treatment-related adverse events during this period. At least for some avoidable complications, appropriate prophylactic measures, such as antibiotic prophylaxis to reduce infections, should be considered to improve safety.
One limitation of our analysis lies in the fact that we used data from randomized trials only. Patients enrolled in randomized trials are usually selected subjects, and treatment feasibility may be different when the same schema is administered to a wider population of patients. Results from studies other than randomized trials are, therefore, particularly important. However, the criteria for including patients in the studies considered were not strict: the Nordic trial included patients with WHO-PS 0-4, with 30% of patients having WHO-PS 3-4; the Italian study also included patients with abnormal cardiac, respiratory, liver and renal function, and the IFM II trial enrolled patients over the age of 75 years.
The occurrence of severe complications, especially non-hematologic toxicities, is detrimental to these patients, not only because of their direct consequences, but also because the adverse events often lead to dose reductions or a premature interruption of treatment. This meta-analysis found that the occurrence of grade 3-4 non-hematologic adverse events was associated, irrespectively of treatment, with a poor prognosis, in terms of both progression-free survival (adjusted HR 1.24, 95% CI 1.07-1.45) and overall survival (adjusted HR 1.23, 95% CI 1.03-1.46). Other baseline factors were confirmed to be strong predictors of a worse progression-free survival and overall survival, such as advanced ISS stage, high creatinine levels and poor WHO-PS. As expected, age had a negative impact on overall survival.
The adverse prognostic impact of advanced age is probably multifactorial. Myeloma biology may differ by age at presentation.23 Moreover, the aging process is associated with reductions in renal and gastric function, hepatic mass and blood flow, bone marrow status, and cardiovascular function.24-26 Poor WHO-PS was one of the most relevant factors, and it increased the risk of death by 1.29-fold for each point (95% CI 1.20-1.39). It may represent an indirect measure of multiple organ dysfunction, frequently present in elderly patients. Unfortunately, no data on baseline comorbidities, other than renal failure, were available. Creatinine ≥ 176 μmol/L did not increase the risk of severe adverse events, but significantly increased the risk of death by 1.66-fold (95% CI 1.31-2.10). Thalidomide pharmacokinetics is not affected by renal impairment and no dose reduction is required.22 The increased risk of death seems to be mainly related to the poor prognosis of renal disease by itself rather than to a toxic effect of thalidomide on renal function.
Our analysis clearly showed that the occurrence of grade 3-4 non-hematologic adverse events is associated with a worse prognosis, independently of treatment. At least in part, these adverse events may be related to preexisting sub-clinical organ dysfunction that emerges clinically after MM treatment. The occurrence of serious adverse events increased the risk of death in both the MPT and MP groups, suggesting that comorbidity and reduced functional reserves in patients with cancer have a negative impact on survival, independently of treatment. Thus, it is important to search for subclinical comorbidities in all elderly patients before starting treatment. The recent efficacy meta-analysis showed that the benefit of MPT over MP is limited in patients with high creatinine levels and poor WHO-PS.9 Our analysis suggests that this lack of benefit might be related to increased toxicity and to the reduced treatment efficacy caused by the occurrence of adverse events. Patients with poor WHO-PS, generally presenting with co-morbidities, are likely to be at higher risk of developing adverse events.
In conclusion, the incidence of severe adverse events, especially non-hematologic ones, was higher in patients treated with MPT than in those treated with MP, and their occurrence had a negative impact on prognosis. However, patients randomized to MPT showed a better outcome. Older patients and those with high creatinine levels or poor WHO-PS status should be carefully considered for more appropriate treatment choices. In these patients, lower doses of melphalan (from 0.25 to 0.18 to 0.13 mg/kg) and thalidomide (from 100 to 50 mg/day, to 50 mg every other day) have been suggested.27 Personalized therapy, with appropriate dose adjustments or modified treatment schema, are needed to improve the balance between benefits and harm of available treatments. Better tolerated regimens are likely to reduce the need for treatment interruptions and thus optimize treatment efficacy. Timely prophylactic interventions and adequate and prompt management of adverse events are also necessary. Future clinical trials, with few exclusion criteria, comparing different individualized therapeutic strategies on clinically relevant endpoints, should be considered a high research priority.28 The results from such trials will eventually lead to tailored treatment schema for elderly MM patients, with further improvements in survival, quality of life and cost-effectiveness.
Acknowledgments
We thank the patients who agreed to participate in the studies analyzed, the nurses, the data manager Federica Leotta and the editorial assistant Giorgio Schirripa.
Footnotes
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565-71 [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825-31 [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107-14 [DOI] [PubMed] [Google Scholar]
- 4.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209-18 [DOI] [PubMed] [Google Scholar]
- 5.Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664-70 [DOI] [PubMed] [Google Scholar]
- 6.Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: The HOVON 49 Study. J Clin Oncol. 2010;28(19):3160-6 [DOI] [PubMed] [Google Scholar]
- 7.Waage A, Gimsing P, Fayers P, Abildgaard N, Ahlberg L, Björkstrand B, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405-12 [DOI] [PubMed] [Google Scholar]
- 8.Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2010;86(1):16-22 [DOI] [PubMed] [Google Scholar]
- 9.Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksaç M, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118(5):1239-47 [DOI] [PubMed] [Google Scholar]
- 10.Ludwig H, Hajek R, Tóthová E, Drach J, Adam Z, Labar B, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113(15):3435-42 [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zonder JA, Crowley J, Hussein MA, Bolejack V, Moore DF, Sr, Whittenberger BF, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood. 2010;116(26):5838-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745-53 [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin Trials 1996;17(1):1-12 [DOI] [PubMed] [Google Scholar]
- 15.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412-20 [DOI] [PubMed] [Google Scholar]
- 16.Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842-54 [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, Version 3.0. 9 August 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30
- 18.Katsahian S, Resche-Rigon M, Chevret S, Porcher R. Analysing multicentre competing risk data with mixed proportional hazards model for the subdistribution. Stat Med. 2006;25(24):4267-78 [DOI] [PubMed] [Google Scholar]
- 19.Clayton D, Cuzick J. Multivariate generalisations of the proportional hazards model (with discussion). Journal of the Royal Statistical Society, Series A 1985;148:82-117 [Google Scholar]
- 20.Myeloma Trialists' Collaborative Group Combination chemotherapy versus melphalan and prednisone as treatment for multiple myeloma: an overview of 6633 patients from 27 randomized trials. J Clin Oncol. 1998;16(12):3832-42 [DOI] [PubMed] [Google Scholar]
- 21.Facon T, Mary JY, Pégourie B, Attal M, Renaud M, Sadoun A, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006;107(4):1292-8 [DOI] [PubMed] [Google Scholar]
- 22.Palumbo A, Facon T, Sonneveld P, Bladè J, Offidani M, Gay F, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111(8):3968-77 [DOI] [PubMed] [Google Scholar]
- 23.Ludwig H, Durie BG, Bolejack V, Turesson I, Kyle RA, Blade J, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vestal RE. Aging and pharmacology. Cancer. 1997;80(7):1302-10 [DOI] [PubMed] [Google Scholar]
- 25.Yuen GJ. Altered pharmacokinetics in the elderly. Clin Geriatr Med. 1990;6(2):257-67 [PubMed] [Google Scholar]
- 26.Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61(3):331-9 [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-60 [DOI] [PubMed] [Google Scholar]
- 28.Liberati A. Need to realign patient-oriented and commercial and academic research. Lancet. 2011;378(9805):1777-8 [DOI] [PubMed] [Google Scholar]



