Abstract
Background
Structural brain abnormalities associated with delusions in Alzheimer’s disease are poorly understood. In addition, whether the neural substrate underlying the delusions develops before the onset of the delusions is unclear. In this study, we used a voxel-based morphometry approach to examine the existence of regional structural abnormalities at baseline in patients with Alzheimer’s disease who did and who did not develop delusions.
Methods
Using the Neuropsychiatric Inventory, we identified patients with Alzheimer’s disease who exhibited delusions during a 2-year period. All the patients had undergone a magnetic resonance imaging examination at the start of the study period (baseline). We conducted a voxel-based morphometry analysis using statistical parametric mapping (SPM5) software and compared the results of patients with Alzheimer’s disease who did and did not develop delusions.
Results
Compared with the patients who did not develop delusions (n = 35), the patients who did develop delusions (n = 18) had significantly smaller gray matter volumes on both sides of the parahippocampal gyrus, the right posterior cingulate gyrus, the right orbitofrontal cortex, both sides of the inferior frontal cortex, the right anterior cingulate, and the left insula.
Conclusion
Structural brain abnormalities involving both the frontal and medial temporal lobes may be crucial to the expression of delusions in patients with Alzheimer’s disease.
Keywords: Alzheimer’s disease, delusions, structural brain abnormalities, voxel-based morphometry
Introduction
Although not all neuropsychiatric diseases manifest psychotic symptoms, some types of brain disorders may be associated with secondary psychosis during the course of illness. Psychotic symptoms often arise because of a general medical illness, such as metabolic disorders without psychotic features. Not all patients with Alzheimer’s disease develop psychotic symptoms during the course of the disorder. However, among a wide variety of neuropsychiatric symptoms in patients with Alzheimer’s disease, psychotic symptoms, such as delusions, are particularly associated with increased caregiver burden,1 a decreased quality of life for both the patient and the caregiver,2 and a relatively poor prognosis for the course of the disease.3 A 2-year, prospective, longitudinal study of patients with Alzheimer’s disease reported that the cumulative incidence of psychotic symptoms (delusions, hallucinations) was 36.9%.4
The examination of possible predictors of the development of psychotic symptoms in patients with Alzheimer’s disease is clinically relevant. Several studies have suggested that greater cognitive impairment predicts an increased risk of the onset of psychosis in patients with Alzheimer’s disease.5.6 Several neuroimaging studies have implicated the frontal lobe, parietal lobe, striatal regions, and insula in the manifestation of delusions in patients with the disease.7–11 In a previous study12 examining structural brain abnormalities, significant findings indicated an association between low volumes of regional gray matter in several areas and delusions in patients with Alzheimer’s disease. However, whether the neural substrate underlying the delusional symptoms develops before the onset of delusions remains unclear.
Thus, we hypothesized that neuroanatomical abnormalities that predate the onset of delusions may exist in patients with Alzheimer’s disease who develop delusions. Therefore, we examined structural brain abnormalities using baseline data obtained for patients with Alzheimer’s disease without delusions at the time of image acquisition who were enrolled in a 2-year, prospective, longitudinal study. After the 2-year follow-up period, we compared the baseline findings of patients who developed delusions with those who did not using a voxel-based morphometry approach.
Materials and methods
Participants
The baseline sample consisted of Japanese patients with Alzheimer’s disease and mild functional severity who attended the outpatient clinic of Nagoya City University Hospital between June 2009 and December 2010. The diagnostic evaluation included a complete history and physical examination, routine blood tests (including an evaluation of serum vitamin B12 level and thyroid function), either a magnetic resonance imaging (MRI) or a computed tomography scan of the brain, and neuropsychological testing. Study inclusion criteria were a diagnosis of probable Alzheimer’s disease according to the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer Disease and Related Disorders Association criteria,13 no history of treatment with antipsychotic medications, and absence of psychotic symptoms as assessed using the Japanese version of the Neuropsychiatric Inventory (NPI).14,15 The NPI is a semiquantitative assessment based on information provided by the caregiver. The questionnaire consists of ten behavior-related questions concerning delusions, hallucinations, depression, anxiety, agitation, disinhibition, euphoria, irritability, apathy, and aberrant motor activity. The composite score for each item is obtained by multiplying the severity by the frequency, with composite scores ranging between 0 and 12. The maximum total NPI score for the 10 manifestations is 120. In accordance with our previous study,2 psychotic symptoms were defined as delusions, hallucinations, agitation, disinhibition, irritability, and aberrant motor activity.
Patients were excluded if other neurological diseases were present, the patient had a previous history of mental illness or substance abuse before the onset of dementia, either an MRI or a computed tomography scan had revealed focal brain lesions, the patient’s Mini-Mental State Examination (MMSE)16 score was <11, or reliable informed consent could not be obtained from the patient and/or his/her relatives. The study protocol was approved by the ethics committee of Nagoya City University Medical School. Both the subjects and their caregivers were informed of the purpose and procedures of this study and were asked to sign a consent form.
Follow-up assessment
The follow-up assessment was conducted within 2 years of the baseline assessment. The presence of delusions was diagnosed based on the delusion score on the NPI. In agreement with a previous study,4 a score > 3 was regarded as indicating the presence of delusions. An independent psychiatrist who was unaware of the MRI results conducted the NPI evaluation. Both the MMSE and the NPI were assessed at 3-month intervals from the baseline examination.
MRI image acquisition
All the brain images were acquired at baseline using a 1.5-T MRI system (Gyoro Scan Intera; Philips Medical Systems, Best, The Netherlands). The scanning parameters for the three-dimensional T1-weighted turbo field echo sequences were as follows: echo time 3.90 msec; repetition time 8400 msec; flip angle 15 degrees; 256 × 256 matrices; field of view 25 cm; voxel size 1.0 × 1.0 × 1.0 mm; and slice thickness 1.0 mm. The MRI studies were performed in the Department of Radiology at Nagoya City University Hospital.
Voxel-based morphometry protocol
Voxel-based morphometry was performed using the VBM5.1 toolbox (http://dbm.neuro.uni-jena.de/vbm) with Statistical Parametric Mapping 5 (SPM5) running on Matlab 7.5 (Mathworks Inv, Sherborn, MA). Each 3D-MIRAGE image was normalized and segmented into gray matter, white matter, and cerebrospinal fluid components using the unified segmentation model,17 which combines both normalization and segmentation parameters into a single generative model. The Volex values were modulated using Jacobian determinants for affine and nonlinear warping and smoothed using a Gaussian kernel with a full width at half maximum of 12 mm.
Statistical analysis
Comparisons between patients with Alzheimer’s disease who developed delusions and those who did not were conducted for the regional gray matter and white matter volumes using a two-samples t-test as an implement in SPM5. For all the analyses, the total brain volume, patient age, gender, education, duration (in years), and MMSE score were used as covariates of no interest. In addition, a multiple regression analysis was performed to test the relationship between the regional gray matter or white matter volumes at baseline and the change in the delusion score on the NPI between baseline and the time of appearance of delusions in patients with Alzheimer’s disease who developed them. For these analyses, the total brain volume and time between baseline and appearance of delusions (in months) and degree of change in MMSE score over this period were used as nuisance covariates. An absolute gray matter and white matter threshold of 0.1 was used to avoid possible edge effects between different tissue types. In this analysis, the voxel-wise statistical threshold of significance was set at P < 0.05, corrected for multiple comparisons using the false discovery rate approach.18 The Montreal Neurologic Institute coordinates were transformed into Talairach coordinates and were then identified using the Talairach Daemon Client. Differences in the demographic and clinical variables between the two groups were examined using the t-test. The male to female ratio was compared using the Chi-square test.
Results
Demographic and clinical characteristics
Sixty-two patients with Alzheimer’s disease were enrolled in this study. Nine patients had been lost to follow-up at the end of the 2-year period, including six patients who had been transferred to another hospital and three patients who had been institutionalized. As a result, the remaining 53 patients participated in this study throughout the 2-year study period. Of these patients, 18 developed delusions and the remaining 35 did not develop delusions during the follow-up period. The types of delusions experienced by the patients, as identified by the NPI, were persecutory delusions (n = 13), misidentification delusions (n = 4), and both types (n = 1). Other types of delusions were not identified in this study. The mean time until development of delusions from the baseline assessment was 15.6 months (minimum of 6 months, maximum of 21 months).
Baseline demographic data for the patients are summarized in Table 1. No significant differences in demographic variables were found between the patients who developed delusions and those who did not. Regarding the NPI subscale scores, with the exception of depression/dysphoria and apathy, the two groups of patients both scored 0. Regarding the depression/dysphoria and apathy subscale, no significant differences were found between the two groups of patients (Table 1).
Table 1.
Demographic data for patients with Alzheimer’s disease and delusions and those without delusions
| Patients with Alzheimer’s disease | ||
|---|---|---|
|
|
||
| With delusions (A) (n = 18, 95% CI) | Without delusions (B) (n = 35, 95% CI) | |
| Male/female | 4/12 | 8/27 |
| Age, years | 78.2 ± 5.7 (75.2–81.2) | 76.3 ± 7.7 (74.5–80.1) |
| Education, years | 9.7 ± 2.7 (8.3–11.1) | 9.6 ± 3.2 (8.4–10.8) |
| Duration of illness, years | 1.1 ± 0.8 (0.8–1.8) | 1.3 ± 0.9 (0.7–1.4) |
| MMSE score | 21.1 ± 3.6 (18.9–20.7) | 20.2 ± 3.2 (19.9–21.3) |
| NPI | ||
| Depression/dysphoria | 0.2 ± 0.4 (0.1–0.4) | 0.1 ± 0.3 (0.1–0.3) |
| Apathy | 2.6 ± 1.2 (1.9–3.1) | 3.1 ± 1.1 (2.8–3.5) |
Notes: No significant differences in any of the items were observed between patients with Alzheimer’s disease and delusions and those without delusions. Data are presented as the mean ± standard deviation.
Abbreviations: CI, confidence interval; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory.
Results of voxel-based morphometric analysis
Table 2 and Figure 1 show the gray matter volume differences between the patients with Alzheimer’s disease who developed delusions and those who did not. At the time of the baseline assessments, the patients who developed delusions had significantly smaller gray matter volumes on both sides of the parahippocampal gyrus (Brodmann 19, 30), the right posterior cingulate gyrus (Brodmann 30), the right orbitofrontal cortex (Brodmann 11), both sides of the inferior frontal cortex (Brodmann 44, 47), the right anterior cingulate (Brodmann 24), the left claustrum, and the left insula (Brodmann 13), compared with the patients who did not develop delusions. No significantly smaller white matter volumes were observed in any brain region between the patients who developed delusions and those who did not. In addition, no significant correlation between regional gray matter or white matter volumes at baseline and change in the NPI delusions score at the time of appearance of delusions was observed among the patients who developed them.
Table 2.
Smaller gray matter volumes in patients with Alzheimer’s disease and delusions (n = 18) than in those without delusions (n = 35)
| Region | Brodmann’s area | Talairach coordinates | Voxels in cluster | Z value | ||
|---|---|---|---|---|---|---|
|
|
||||||
| x | y | z | ||||
| Left parahippocampal gyrus | 19 | −22 | −42 | −1 | 34054 | 6.31 |
| Right posterior cingulate gyrus | 30 | 19 | −57 | 16 | 34054 | 6.26 |
| Right parahippocampal gyrus | 30 | 10 | −37 | −4 | 34054 | 5.77 |
| Right orbitofrontal cortex | 11 | 37 | 44 | −18 | 2918 | 5.59 |
| Right inferior frontal gyrus | 47 | 39 | 29 | −9 | 2918 | 5.54 |
| Right orbitofrontal cortex | 11 | 26 | 38 | −17 | 2918 | 5.49 |
| Right medial frontal gyrus | 11 | 2 | 34 | −16 | 2275 | 5.68 |
| Right anterior cingulate | 24 | 7 | 24 | −2 | 2275 | 5.09 |
| Left medial frontal gyrus | 11 | −8 | 24 | −19 | 2275 | 4.83 |
| Left inferior frontal gyrus | 47 | −31 | 27 | −11 | 1384 | 5.98 |
| Left claustrum | −32 | 8 | 5 | 1266 | 5.85 | |
| Left inferior frontal gyrus | 44 | −48 | 9 | 7 | 1266 | 4.97 |
| Left insula | 13 | −38 | 19 | 5 | 1266 | 4.91 |
Notes: False discovery rate set at P < 0.05. Results of a voxel-wise statistical parametric map analysis.
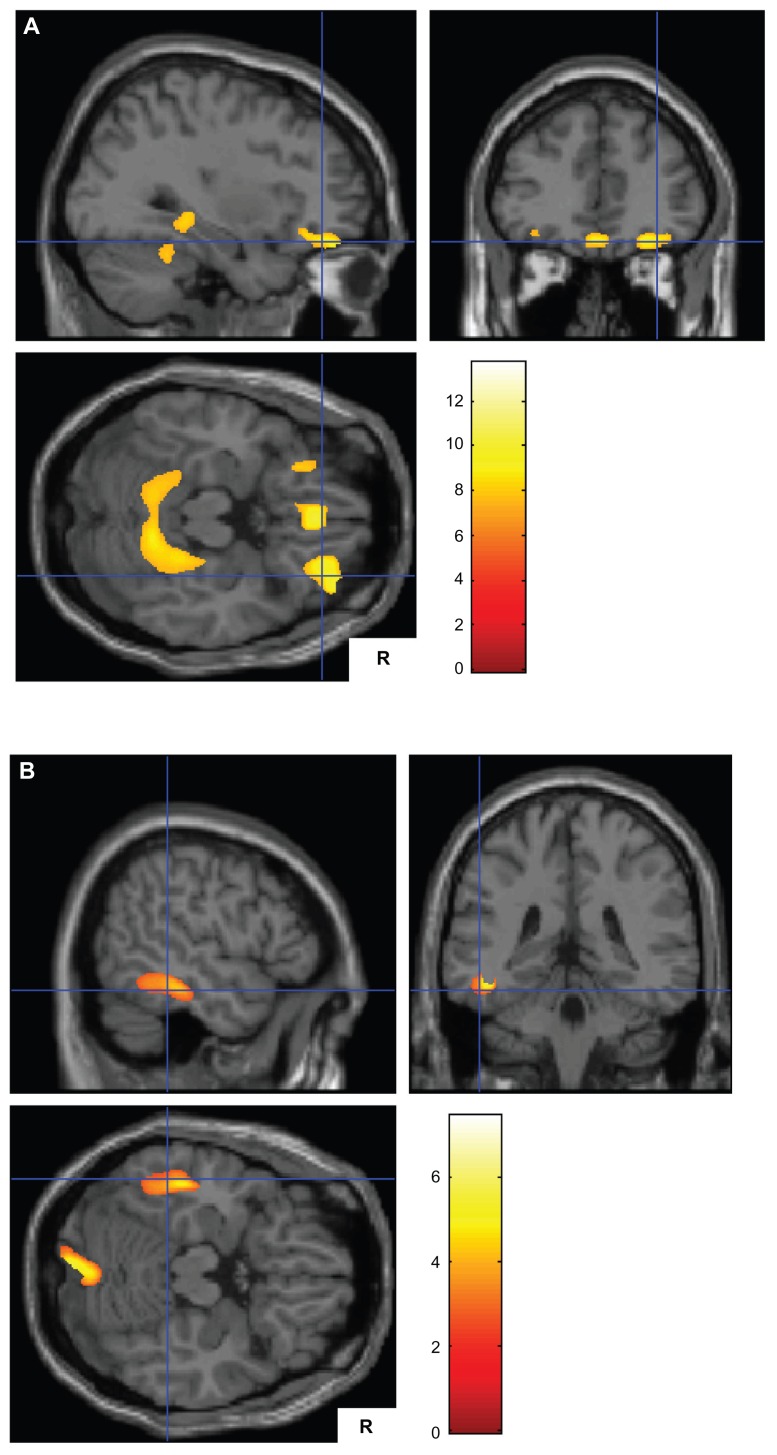
Figure 1.
(A) Smaller gray matter volumes in patients with Alzheimer’s disease and delusions (n = 18) than in those without delusions (n = 35) on both sides of the parahippocampal gyrus, both sides of the orbitofrontal cortex, and both sides of the medial frontal gyrus ventromedial prefrontal cortex. (B) Smaller gray matter volumes in patients with Alzheimer’s disease without delusions (n = 35) than in those with delusions (n = 18) in the left inferior temporal gyrus and the right cerebellum.
Note: The Talairach coordinates are shown in Tables 2 and 3.
At the time of the baseline assessments, the patients with Alzheimer’s disease who did not develop delusions showed significantly smaller gray matter volumes, as shown in Table 3 (Figure 1), in the right cerebellum, the left lingual gyrus (Brodmann 20), the left inferior temporal cortex (Brodmann 20, 37), and the left occipital cortex (Brodmann 18), compared with the patients who developed delusions. No significantly smaller white matter volumes were observed in any brain region among the patients who did not develop delusions compared with those who did develop delusions. In addition, we could not observe any significant correlations between regional gray matter or white matter volumes at baseline and reduction in delusion score on the NPI between baseline and the time of appearance of the delusions among the patients who developed delusions.
Table 3.
Smaller gray matter volumes in patients with Alzheimer’s disease and without delusions (n = 35) than in those with delusions (n = 18)
| Region | Brodmann’s area | Talairach coordinates | Voxels in cluster | Z value | ||
|---|---|---|---|---|---|---|
|
|
||||||
| x | y | z | ||||
| Right cerebellum | 8 | −60 | −18 | 7757 | 4.82 | |
| Left lingual gyrus | 18 | −4 | −90 | −9 | 7757 | 4.07 |
| Right cerebellum | 35 | −75 | −20 | 7757 | 4.00 | |
| Left inferior temporal gyrus | 20 | −46 | −32 | −10 | 6274 | 4.27 |
| Left occipital cortex | 18 | −29 | −81 | 1 | 6274 | 4.14 |
| Left inferior temporal gyrus | 37 | −40 | −58 | −3 | 6274 | 4.08 |
Notes: False discovery rate set at P < 0.05. Results of a voxel-wise statistical parametric map analysis.
Discussion
To our knowledge, this is the first MRI study to examine baseline structural differences using a voxel-based morphometry approach in patients with Alzheimer’s disease who developed delusions and in those who did not. Compared with the patients who did not develop delusions, the patients who did develop delusions showed significantly smaller gray matter volumes in clinically important areas, including both sides of the parahippocampal gyrus (Brodmann 19, 30), the right orbitofrontal cortex (Brodmann 11), both sides of the inferior frontal gyrus (Brodmann 44, 47), the right anterior cingulate (Brodmann 11), and the left insula (Brodmann 13). These areas may be associated with the pathogenesis of delusion in patients with Alzheimer’s disease.
Previous neuroimaging studies have implicated frontal lobe dysfunction in the manifestation of delusions in patients with Alzheimer’s disease. The most consistent findings among previous functional neuroimaging studies were that delusions are associated with hypoperfusion and hypometabolism, primarily in the frontal lobe. These findings suggest that cortical dysfunction in the frontal lobe may be expressed as delusions during the course of Alzheimer’s disease.19 Consistent with these findings, we observed a smaller gray matter volume in several areas of the frontal lobe in the patients with Alzheimer’s disease who developed delusions, compared with the patients who did not. Bruen et al12 identified an association between reduced gray matter volumes on both sides of the inferior frontal gyrus and the left medial frontal gyrus and the experience of delusions in patients with Alzheimer’s disease. Alterations in other regions, such as the right orbitofrontal region and the anterior cingulate, have been reported in several studies using either single photon emission computed tomography or positron emission tomography.9,10,20 These areas are thought to play an important role in attention and executive function associated with errors of logic, self-monitoring, and regulation of attention.9,10 Supporting this hypothesis, Tsoi et al6 demonstrated that frontal executive impairments predict an increased risk of the onset of psychosis in patients with Alzheimer’s disease. Thus, structural abnormalities in these areas within the frontal lobe may result in dysfunction of attention and executive function, contributing to development of delusions in patients with the disease.
During the earliest stage of Alzheimer’s disease and mild cognitive impairment, apathy, depression, irritability, and anxiety, rather than delusions, are typically the most prominent symptoms among neuropsychiatric symptoms.21–23 Also, a recent study24 based on data from the Alzheimer’s Disease Neuroimaging Initiative database suggested that anxiety at baseline was associated with an increased hazard of progression from mild cognitive impairment to Alzheimer’s disease. Unlike in schizophrenia, delusions are not a core symptom of Alzheimer’s disease. Thus, while a cognitive decline may occur before the onset of delusions during the early stages of Alzheimer’s disease,5,6 symptoms of delusion are rarely a significant predictor of dementia associated with Alzheimer’s disease.
We also observed a reduced gray matter volume on both sides of the parahippocampal gyrus in patients with Alzheimer’s disease who developed delusions compared with patients who did not. Previous neuroimaging studies have suggested that medial temporal involvement is associated with delusions in patients with Alzheimer’s disease.25,26 Recently, Serra et al27 demonstrated that delusions in patients with the disease were associated with the gray matter volume in the tail of the right hippocampus, which is involved in memory retrieval. Considering these findings, structural brain abnormalities in both the frontal and the medial temporal lobes may increase the likelihood that patients with Alzheimer’s disease will experience delusions. In addition, both the claustrum and the insula are reported to be associated with delusions in patients with the disease.11,12 Matsuoka et al11 suggested that insular dysfunction might be responsible for exacerbation of delusions.
With regard to the smaller gray matter volumes observed in patients with Alzheimer’s disease who did not develop delusions compared with those who did, these findings are difficult to interpret. Although the cerebellum is not considered to be a primary area of pathological involvement in Alzheimer’s disease, the cerebellum may play an important role in coordination of motor and cognitive information.28 However, we did not examine tasks associated with cerebellar function. Other areas, such as the lingual gyrus, inferior temporal gyrus, and occipital cortex, are known to be markers of progression of Alzheimer’s disease.29,30 Whether the patients with Alzheimer’s disease with gray matter reductions in these areas who did not develop delusions may experience a faster cognitive decline relative to patients with Alzheimer’s disease who develop delusions remains unclear. Further investigations of a long-term cohort study are needed to clarify the implications of the smaller gray matter volumes in patients who do not develop delusions.
Finally, we must address several limitations of this study. First, we observed a predominance of women among our patients with Alzheimer’s disease, as the prevalence of the disease is reportedly higher in women than in men.31 Ikeda et al32 also reported that a female predominance was evident among patients with delusion. Thus, the patients with Alzheimer’s disease enrolled in this study may have had a predisposition to delusions because of their gender. However, we could not identify any significant difference in gender between the patients with Alzheimer’s disease who developed delusions and those who did not. Second, the distinct neural basis underlying each of the various types of delusions needs to be examined. The limited number of patients with Alzheimer’s disease with misidentification delusions (n = 4) did not allow such an analysis in the present study. Third, our study included a relatively small number of patients. However, the rate of dropout (14%; n = 9) was relatively small. The results of this study will contribute to new findings regarding the neural basis for the expression of delusions in patients with Alzheimer’s disease. A larger cohort study is needed to allow multiple topics to be studied. Fourth, apolipoprotein E is known to influence the development of specific neuropsychiatric symptoms.33,34 Unfortunately, in this study, we did not examine the apolipoprotein E genotypes in the patients with Alzheimer’s disease. Further studies are needed to clarify the effect of apolipoprotein E on the development of delusions in patients with the disease. Fifth, although individual differences in brain volume exist among patients with Alzheimer’s disease, the voxel-based morphometry technique involves spatial normalization to remove inter-individual variations in brain size and shape.35 However, we analyzed the MRI data only at baseline to compare brain abnormalities that predated the onset of delusions. We could not longitudinally assess the structural changes in patients with Alzheimer’s disease who developed delusions with those who did not using the MRI data. Thus, in this study, we could not detect dynamic changes in the brain structures of patients with Alzheimer’s disease during the 2-year follow-up period. However, some of the neuroanatomical abnormalities might have predated the expression of delusions in these patients.
Despite these limitations, the current study implies that structural brain abnormalities in the frontal and medial temporal lobes may be linked with the subsequent onset of delusions in patients with Alzheimer’s disease. The ability to predict the onset of delusions in patients with the disease based on MRI findings is clinically relevant for early intervention in those who develop delusions.
Acknowledgment
The authors gratefully acknowledge funding from a Grant-in-Aid for Scientific Research (22530750, 22591293) from the Ministry of Education, Culture, Sports, Sciences, and Technology in Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Matsumoto N, Ikeda M, Fukuhara R, et al. Caregiver burden associated with behavioral and psychological symptoms of dementia in elderly people in the local community. Dement Geriatr Cogn Disord. 2007;23(4):219–224. doi: 10.1159/000099472. [DOI] [PubMed] [Google Scholar]
- 2.Matsui T, Nakaaki S, Murata Y, et al. Determinants of the quality of life in Alzheimer’s disease patients as assessed by the Japanese version of the Quality of Life-Alzheimer’s disease scale. Dement Geriatr Cogn Disord. 2006;21(3):182–191. doi: 10.1159/000090744. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aalten P, de Vugt ME, Lousberg R, et al. Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord. 2003;15(2):99–105. doi: 10.1159/000067972. [DOI] [PubMed] [Google Scholar]
- 5.Wilkosz PA, Miyahara S, Lopez OL, Dekosky ST, Sweet RA. Prediction of psychosis onset in Alzheimer disease: the role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. Am J Geriatr Psychiatry. 2006;14(4):352–360. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]
- 6.Tsoi T, Baillon S, Lindesay J. Early frontal executive impairment as a predictor of subsequent behavior disturbance in dementia. Am J Geriatr Psychiatry. 2008;16(2):102–108. doi: 10.1097/JGP.0b013e318151fb42. [DOI] [PubMed] [Google Scholar]
- 7.Hirono N, Mori E, Ishii K, et al. Alteration of regional cerebral glucose utilization with delusions in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1998;10(4):433–439. doi: 10.1176/jnp.10.4.433. [DOI] [PubMed] [Google Scholar]
- 8.Fukuhara R, Ikeda M, Nebu A, et al. Alteration of rCBF in Alzheimer’s disease patients with delusions of theft. Neuroreport. 2001;12(11):2473–2476. doi: 10.1097/00001756-200108080-00037. [DOI] [PubMed] [Google Scholar]
- 9.Sultzer DL, Brown CV, Mandelkern MA, et al. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry. 2003;160(2):341–349. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- 10.Nakano S, Yamashita F, Matsuda H, Kodama C, Yamada T. Relationship between delusions and regional cerebral blood flow in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21(1):16–21. doi: 10.1159/000089215. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka T, Narumoto J, Shibata K, et al. Insular hypoperfusion correlates with the severity of delusions in individuals with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;29(4):287–293. doi: 10.1159/000295115. [DOI] [PubMed] [Google Scholar]
- 12.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131(9):2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services. Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 15.Hirono N, Mori E, Ikejiri Y, et al. Japanese version of the Neuropsychiatric Inventory – a scoring system for neuropsychiatric disturbance in dementia patients. No to shinkei = Brain and nerve. 1997;49(3):266–271. Japanese. [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 19.Ismail Z, Nguyen MQ, Fischer CE, Schweizer TA, Mulsant BH. Neuroimaging of delusions in Alzheimer’s disease. Psychiatry Res. 2012;202(2):89–95. doi: 10.1016/j.pscychresns.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69(2):167–171. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 22.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 23.Hwang TJ, Masterman DL, Ortiz F, Fairbanks LA, Cummings JL. Mild cognitive impairment is associated with characteristic neuropsychiatric symptoms. Alzheimer Dis Assoc Disord. 2004;18(1):17–21. doi: 10.1097/00002093-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Wadsworth LP, Lorius N, Donovan NJ, et al. Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement Geriatr Cogn Disord. 2012;34(2):96–111. doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mentis MJ, Weinstein EA, Horwitz B, et al. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995;38(7):438–449. doi: 10.1016/0006-3223(94)00326-x. [DOI] [PubMed] [Google Scholar]
- 26.Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer’s disease: spet evidence of right hemispheric dysfunction. Cortex. 1999;35(4):549–560. doi: 10.1016/s0010-9452(08)70818-9. [DOI] [PubMed] [Google Scholar]
- 27.Serra L, Perri R, Cercignani M, et al. Are the behavioral symptoms of Alzheimer’s disease directly associated with neurodegeneration? J Alzheimers Dis. 2010;21(2):627–639. doi: 10.3233/JAD-2010-100048. [DOI] [PubMed] [Google Scholar]
- 28.Kusbeci OY, Bas O, Gocmen-Mas N, et al. Evaluation of cerebellar asymmetry in Alzheimer’s disease: a stereological study. Dement Geriatr Cogn Disord. 2009;28(1):1–5. doi: 10.1159/000228544. [DOI] [PubMed] [Google Scholar]
- 29.Chetelat G, Landeau B, Eustache F, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. NeuroImage. 2005;27(4):934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Kinkingnehun S, Sarazin M, Lehericy S, Guichart-Gomez E, Hergueta T, Dubois B. VBM anticipates the rate of progression of Alzheimer disease: a 3-year longitudinal study. Neurology. 2008;70(23):2201–2211. doi: 10.1212/01.wnl.0000303960.01039.43. [DOI] [PubMed] [Google Scholar]
- 31.Hirono N, Mori E, Tanimukai S, et al. Distinctive neurobehavioral features among neurodegenerative dementias. J Neuropsychiatry Clin Neurosci. 1999;11(4):498–503. doi: 10.1176/jnp.11.4.498. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda M, Shigenobu K, Fukuhara R, et al. Delusions of Japanese patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2003;18(6):527–532. doi: 10.1002/gps.864. [DOI] [PubMed] [Google Scholar]
- 33.Van der Flier WM, Staekenborg S, Pijnenburg YA, et al. Apolipoprotein E genotype influences presence and severity of delusions and aggressive behavior in Alzheimer disease. Dement Geriatr Cogn Disord. 2007;23(1):42–46. doi: 10.1159/000096682. [DOI] [PubMed] [Google Scholar]
- 34.Chen CS, Ouyang P, Yeh YC, et al. Apolipoprotein E polymorphism and behavioral and psychological symptoms of dementia in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26(2):135–139. doi: 10.1097/WAD.0b013e31821f5787. [DOI] [PubMed] [Google Scholar]
- 35.Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer’s disease. Expert Rev Neurother. 2008;8(11):1691–1702. doi: 10.1586/14737175.8.11.1691. [DOI] [PubMed] [Google Scholar]