Abstract
Intracellular pathogens, such as Mycobacterium tuberculosis, reside in the phagosomes of macrophages where antigenic processing is initiated. Mycobacterial antigen–MHC class II complexes are formed within the phagosome and are then trafficked to the cell surface. Interferon-γ (IFN-γ) and interleukin-10 (IL-10) influence the outcome of M. tuberculosis infection; however, the role of these cytokines with regard to the formation of M. tuberculosis peptide–MHC-II complexes remains unknown. We analysed the kinetics and subcellular localization of M. tuberculosis peptide–MHC-II complexes in M. tuberculosis-infected human monocyte-derived macrophages (MDMs) using autologous M. tuberculosis-specific CD4+ T cells. The MDMs were pre-treated with either IFN-γ or IL-10 and infected with M. tuberculosis. Cells were mechanically homogenized, separated on Percoll density gradients and manually fractionated. The fractions were incubated with autologous M. tuberculosis -specific CD4+ T cells. Our results demonstrated that in MDMs pre-treated with IFN-γ, M. tuberculosis peptide–MHC-II complexes were detected early mainly in the phagosomal fractions, whereas in the absence of IFN-γ, the complexes were detected in the endosomal fractions. In MDMs pre-treated with IL-10, the M. tuberculosis peptide–MHC-II complexes were retained in the endosomal fractions, and these complexes were not detected in the plasma membrane fractions. The results of immunofluorescence microscopy demonstrated the presence of Ag85B associated with HLA-DR at the cell surface only in the IFN-γ-treated MDMs, suggesting that IFN-γ may accelerate M. tuberculosis antigen processing and presentation at the cell membrane, whereas IL-10 favours the trafficking of Ag85B to vesicles that do not contain LAMP-1. Therefore, IFN-γ and IL-10 play a role in the formation and trafficking of M. tuberculosis peptide–MHC-II complexes.
Keywords: antigen processing, interferon-γ and antigen processing, phagosomes
Introduction
Mycobacterium tuberculosis is the causative agent of tuberculosis and is a leading cause of morbidity and mortality worldwide.1 The development of a protective immune response to tuberculosis involves the stimulation of CD4+ T cells, which requires antigen processing and presentation of M. tuberculosis peptides by the MHC-encoded class II molecules.2
Antigen processing involves the internalization of pathogens and their products via endocytosis, including both phagocytosis and pinocytosis, catabolism of proteins to peptide fragments, binding of peptides to intracellular MHC-II molecules and trafficking of these complexes to the plasma membrane for presentation to CD4+ T cells. Peptides generated by proteolysis of endocytosed antigens bind to MHC-II molecules in the MHC class II compartment (MIIC) to form peptide–MHC-II complexes.3–5 Although the MIIC is the predominant compartment for the formation of peptide–MHC-II complexes, peptides can also bind to MHC-II molecules in other compartments. For example, some epitopes derived from hen egg lysozyme and bovine ribonuclease bind to MHC-II in endosomes,6,7 suggesting that the nature of the antigen can determine in which vesicular compartment some of its epitopes bind to MHC-II. For phagocytic antigens, Ramachandra et al.8 demonstrated that in M. tuberculosis-infected murine bone marrow-derived macrophages, the phagosome is a site for peptide–MHC-II complex formation. Other studies have demonstrated that peptides bind to MHC class I (MHC-I) within phagosomes to form peptide–MHC-I complexes.8–11 Therefore, phagosomes are not only a source of antigenic peptides but are also a competent organelle where both peptide–MHC-I and peptide–MHC-II complexes are formed and localized.
Using an in vitro model with THP-1 cells as antigen-presenting cells and a specific T-cell hybridoma as responding cells, we previously demonstrated that M. tuberculosis Ag85B(96–111)–DR1 complexes are formed in the phagosomes. By contrast, in human M. tuberculosis-infected monocyte-derived macrophages (MDMs), M. tuberculosis peptide–MHC-II complexes were not detected in phagosomes, but in the MIIC, as assessed by the use of an autologous M. tuberculosis-specific T-cell line.12 The observed discrepancy in antigen processing in those models may be a result of intrinsic differences in specificity between the cells used or the effect of interferon-γ (IFN-γ) added to prime THP-1 cells. Nevertheless, until now, little has been known regarding the factors that determine why peptide–MHC-II complexes are formed either in phagosomes or in MIIC endosomal compartments.
Several studies have demonstrated that cytokines affect the assembly of peptide–MHC complexes in different models. For example, stimulation of dendritic cells with an inflammatory stimulus, such as tumour necrosis factor-α (TNF-α), produced changes in endocytosis and intracellular MHC–peptide loading, suggesting that antigen processing can be affected by cytokines.13 Activation of murine macrophages with IFN-γ induced the acidification and maturation of Mycobacterium avium-containing phagosomes.14 Phagosomes from IFN-γ-treated cells contained elevated levels of proteasome subunits, MHC class I molecules and assembled components of the MHC class I–peptide complex.15,16 In contrast to the stimulatory effects of IFN-γ on the immune system, interleukin-10 (IL-10) exhibits strong immunosuppressive and anti-inflammatory activities. It has been reported that IL-10 decreases phagosomal trafficking and organelle maturation.17 It also inhibits the transport of newly synthesized MHC class II molecules to the plasma membrane by a post-translational process and interferes with the exocytosis of newly synthesized MHC class II molecules and the recycling of internalized MHC class II molecules in human monocytes.18 These findings suggest that IFN-γ enhances antigen processing and presentation, whereas IL-10 interferes with phagosomal maturation and organelle trafficking. However, the roles of IFN-γ and IL-10 in the intracellular assembly of M. tuberculosis peptide–MHC-II complexes is not well understood. In this study, we determined the effects of IFN-γ and IL-10 on the formation and intracellular trafficking of M. tuberculosis peptide–MHC-II complexes in M. tuberculosis-infected human macrophages using IFN-γ production by autologous antigen-specific CD4+ T cells as a marker of M. tuberculosis peptide–MHC-II complex recognition.
Materials and methods
Bacteria
Mycobacterium tuberculosis H37Ra was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown to log phase in Middlebrook 7H9 broth (BD Difco, Sparks, MD) supplemented with 1% glycerol and 10% Middlebrook albumin dextrose catalase enrichment (BD Difco). The bacteria were harvested and frozen at − 80° in 7H9 medium. The bacterial concentration was determined by counting colony-forming units on Middlebrook 7H10 agar plates (BD Difco). Heat-killed (HK) M. tuberculosis was prepared by incubating M. tuberculosis at 80° for 30 min, and killing was confirmed by counting colony-forming units. Before infection of the cells, all M. tuberculosis preparations were pelleted, washed and dispersed by 10 passages through a 26-gauge needle, followed by sonication at 80 cycles for 30 seconds in a sonicator (Ultrasonik 28× NEY, Yucalpa, CA). For fluorescein labelling of live or HK M. tuberculosis, 109 bacteria were pelleted, resuspended in 1 ml PBS, pH 9·1 and mixed with 25 ml of 20 µg/ml FLUOS (Boehringer, Mannheim, Germany) in DMSO for 10 min at room temperature. Fluorescein-labelled M. tuberculosis was washed twice and dispersed before use. To improve phagocytosis, the M. tuberculosis bacteria were incubated for 30 min in a medium containing 10% pooled human serum with no antibiotics at 37° before incubation with cells.
Cells and medium
Unless otherwise specified, cells were cultured in a 37° incubator with a 5% CO2 atmosphere in RPMI-1640 culture medium (BioWhitaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 50 mm 2-mercaptoethanol, 1 mm sodium pyruvate, 2 mm l-glutamine, 10 mm HEPES buffer, 100 U/ml penicillin and 100 mg/ml streptomycin (BioWhitaker). The human monocytic cell line (THP-1) was obtained from the American Type Culture Collection (ATCC No. TIB 202). Activated THP-1 cells, which express high levels of DR1 molecules, were generated by supplementing medium with 10 ng/ml PMA (Sigma, St Louis, MO) during 24 hr and then with 50 U/ml recombinant human IFN-γ (Endogen, Woburn, MA) for an additional 24 hr.
Monocyte-derived macrophages
Peripheral blood was obtained with informed consent from healthy donors who donated blood at the blood bank of the Instituto Nacional de Enfermedades Respiratorias. Approval to perform these studies was obtained from the Institutional Review Board of the Instituto Nacional de Enfermedades Respiratorias. Heparinized blood was diluted 1 : 2 with RPMI-1640, layered on Lymphoprep (Axis-Shield PoC AS, Oslo, Norway) and centrifuged at 300 g for 45 min at room temperature. The peripheral blood mononuclear cells were harvested and monocytes were enriched by plastic adherence for 1 hr at 37°. Monocytes were cultured in culture medium for 7 days. At this point, the cells had acquired macrophage morphology and were called MDMs.
Generation of M. tuberculosis-specific CD4+ T cells
Autologous CD4+ T cells specific for M. tuberculosis were generated from the peripheral blood of tuberculin skin-test-positive (TST+) donors and were stimulated as described by Tan et al.19 Briefly, peripheral blood mononuclear cells (2 × 106 cells/well) were stimulated in 24-well plates with live M. tuberculosis at a multiplicity of infection (MOI) of 1 : 10. The cultures were supplemented with 50 U/ml of IL-2 (Pierce Endogen, Rockford, IL) on days 3, 5 and 7. After 7–10 days, CD4+ T cells were positively selected using immunomagnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The mean purity of the positively selected CD4+ T cells was 96 ± 2%, as determined by flow cytometry. The cells were allowed to rest for 24 hr before use in antigen-processing assays. For each experiment, the specificity of the autologous CD4+ T cells was assessed using autologous monocytes infected with M. tuberculosis, tetanus toxoid or uninfected cells. Interferon-γ production was detected using only the M. tuberculosis-stimulated monocytes. For some experiments, we used T hybridoma cells specific to M. tuberculosis Ag85B(96–111)–DR1 complexes (DB1 cells) generated in HLA-DR1 transgenic mice as described before.20 The production of IL-2 was used as the read-out of T-cell hybridoma DB1 complex recognition.12
Antibodies
Rabbit anti-Ag85B was a gift from Dr C.I. Espitia-Pinzón (Instituto de Investigaciones Biomedicas, UNAM, México). Mouse anti-human HLA-DR was purchased from Caltag (Burlingame, CA), the mouse anti-rat LAMP-1 was purchased from Calbiochem (EMDBiosciences, San Diego, CA), the goat anti-rabbit Alexa-Fluor 594 F(ab′)2 and rabbit anti-mouse Alexa-Fluor 488H+L were purchased from Molecular Probes (Eugene, OR).
Detection of M. tuberculosis peptide–MHC-II complexes and determination of M. tuberculosis processing kinetics
Human MDMs were cultured in 96-well flat-bottom plates (1·5 × 105 cells/well) in culture medium. The MDMs were pre-treated with either 50 U/ml IFN-γ or 5 ng/ml IL-10 or left untreated for 24 hr and then washed with RPMI-1640. The MDMs were infected with M. tuberculosis at an MOI of 30. To ensure that maximal phagocytosis could be achieved despite cytokine treatment, the cells were incubated for 1 hr and washed with ice-cold RPMI-1640 to remove any extracellular bacteria. Pre-warmed medium was added and the cells were incubated at 37° for different periods of time (chase time), ranging from 0 min to 2 hr. Antigen processing was stopped by fixing the MDMs with 1% paraformaldehyde, as previously described.12 The expression of peptide–MHC-II complexes on the plasma membrane of fixed MDMs was detected by incubation with M. tuberculosis-specific CD4+ T cells (1 × 105 cells/well), and IFN-γ release was measured after 24 hr. ELISA was used to detect the IFN-γ in the culture supernatants using anti-human IFN-γ antibodies according to the manufacturer's instructions (Pierce Endogen). The absorbance at 405 nm was measured using a Multiskan MCC/340 microplate reader (Labsystems, Helsinki, Finland).
Subcellular fractionation of macrophages for biochemical analysis and T-cell assays
The MDMs were cultured in a 75 cm2 culture flask in culture medium at a density of 30 × 106/50 ml and pre-treated with either 50 U/ml of IFN-γ or 5 ng/ml IL-10 or left untreated for 24 hr. Cells were then pulsed with heat-killed M. tuberculosis at an MOI of 30 for 60 min with chase incubations of 0, 30 and 60 min. The cells were washed, detached by scraping and suspended in 1 ml homogenization buffer (0·25 m sucrose and 10 mm HEPES, pH 7·2). Samples were homogenized in a Dounce homogenizer to obtain 60–70% lysis of cells. Intact cells and nuclei were removed by three consecutive rounds of centrifugation at 200 g for 10 min at 4°. The nuclei-free supernatants were collected, layered on 9 ml of 27% Percoll (Pharmacia, Uppsala Sweden) in homogenization buffer and centrifuged in a Sorvall RC-100 ultracentrifuge at 28 000 g for 60 min at 4°. Gradients were manually fractionated from top to bottom into 30 fractions of 333 µl each in a density range of 1·037–1·107. Gradient density was determined by parallel centrifugation of a tube with Percoll and Density Marker Beads (Amersham Biosciences, Uppsala, Sweden). The fractions were biochemically characterized, and the presence of peptide–MHC complexes was detected by autologous CD4+ T cells using T-cell-based analysis.
Intracellular organelles in the subcellular fractions were detected by biochemical tests and specific labelling as follows: the plasma membrane was labelled before homogenization with the membrane impermeable reagent, EZ-Link sulfo-NHS-biotin, followed by streptavidin–FITC, and the fluorescein-labelled membrane fractions were detected using a fluorometer. To detect the phagosome/phagolysosome fractions, β-hexosaminidase activity was measured by a colorimetric assay, as previously described.21 Briefly, 50-μl fractions were incubated with 150 μl β-hexosaminidase buffer containing 0·1 m morpholine-ethanesulphonic acid, 0·2% Triton X-100, pH 6·5, and 50 μl of 1·13 mg/ml p-nitrophenyl-acetyl-β-d-glucosaminidase (Sigma-Aldrich, St Louis, MO). After 90 min at 37°, the reaction was stopped by the addition of 50μl of 0·5 m glycine, pH 10, and the optical density was measured at 405 nm.
To identify phagosomes containing fluorescent bacteria, cells were infected with M. tuberculosis-fluorescein-labelled (FLUOS 56-carboxyfluorescein-N-hydroxysuccinimide ester; Boehringer Mannheim, Germany) before fractionation. Briefly, 50 µl of each fraction obtained from the subcellular fractionation experiments was transferred into a 96-well clear-bottom black plate (Costar, Cambridge, MA) and analysed on a fluorometer (Labsystems Fluoroskan Ascent FL, Waltham, MA). The results are expressed as arbitrary units of fluorescence.22 To identify the MIIC, uninfected MDMs were incubated with soluble 85B M. tuberculosis antigen (Ag85B) for 1 hr before fractionation. Fractions were then assessed for the presence of M. tuberculosis antigen–class II complexes using M. tuberculosis-specific autologous CD4+ T cells, as detailed in the next section. For other assays, the subcellular fractions were stored at −70° until use.
T-cell assay for the detection of M. tuberculosis antigen-class II complexes in subcellular fractions
To detect M. tuberculosis antigen–MHC-II complexes, a T-cell assay was developed in which IFN-γ production was assessed as a measure of M. tuberculosis antigen-specific stimulation. A total of 50 µl of each subcellular fraction was disrupted by freezing and thawing to expose the luminal antigen-presenting domain of the MHC-II molecules and then probed with M. tuberculosis-specific autologous CD4+ T cells (105 cells/well) in a final volume of 200 µl. After 24 hr, the culture supernatants were harvested, and IFN-γ production was measured by ELISA. For some experiments, in the same supernatants, we also assessed the production of IL-2, IL-4, IL-6, IL-10, IFN-γ and TNF-α using a T helper type 1/type 2 Cytokine kit-II, cytometric bead array (CBA) (BD Biosciences, San Jose, CA) according to the manufacturer's instructions. The FACS Canto II (BD Biosciences) was used for sample acquisition, and the data were analysed using the FCAP Array v.1.0.1 (Soft Flow software, Hungary Ltd for CBA BD Biosciences). Fluorescence values for each cytokine were extrapolated from their respective standard concentration curves and are expressed as pg/ml.
Flow organellometry of phagosomes
The MDMs were pulsed with fluorescein-labelled HK M. tuberculosis at an MOI of 30 for 60 min and the subcellular fractions were obtained as described above. The phagosomal fractions were collected, combined with equal volumes of 2% paraformaldehyde and fixed for 10 min. This suspension was mixed with an equal volume of 0·4 m lysine in PBS and the phagosomes were washed twice by pelleting and resuspended in 0·5 ml PBS, followed by permeabilization of phagosomes using a 0·1% saponin-containing buffer to allow access to luminal epitopes.22 Phagosomes were then labelled with phycoerythrin-conjugated human anti-HLA-DR monoclonal antibodies or with phycoerythrin-conjugated mouse IgG1 isotype control antibodies (BD Biosciences) in 96-well plates. The organelles were fixed with 2% paraformaldehyde in PBS, and 10 000 events were acquired with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) for each sample. The organelles were analysed with a size/granularity gate using the CellQuest program (Becton Dickinson).
Indirect immunofluorescence microscopy
Fresh human monocytes were cultured on sterile glass coverslips placed in Petri dishes. The monocytes were seeded at 1 × 106 cells/plate for 7 days until differentiation into MDMs. Subsequently, MDMs were stimulated with either 50 U/ml IFN-γ or 5 ng/ml IL-10. After 24 hr of incubation, the cells were washed and infected with M. tuberculosis at an MOI of 30 and incubated for 1 hr. Extracellular bacteria were then removed by washing with ice-cold RPMI-1640. Pre-warmed medium was added and the cells were incubated for different periods of time (chase time), ranging from 0 to 30 min. Antigen processing was stopped by fixing the MDMs with 2% paraformaldehyde. Incubation with antibodies was carried out in the presence or absence of 0·1% saponin for cell membrane or intracellular staining. In the first step, cells were incubated with anti-Ag85B and anti-HLA-DR or anti-Ag85B and anti-LAMP1 antibodies for 1 hr at room temperature, followed by three washings with PBS and 20 min with normal (non-immunized) rabbit serum. In the second step, cells were incubated with goat anti-rabbit Alexa Fluor 594 F(ab′)2 and the rabbit anti-mouse Alexa Fluor 488H+L in the presence of rabbit serum. After 1 hr of incubation, the coverslips were washed and mounted on microscopy slides in Mowiol-DAPI (Calbiochem, San Diego, CA) and examined with an Olympus BX51 microscope (Tokyo, Japan) equipped for epifluorescence with an UPlanAPO 100 × objective.23
Results
Effects of IFN-γ and IL-10 on antigen-processing kinetics of live and HK M. tuberculosis
To gain insight into the effects of IFN-γ and IL-10 on M. tuberculosis antigen processing in MDMs, we first determined the kinetics of M. tuberculosis antigen presentation in whole cells in the presence or absence of cytokines. As shown in Fig. 1, the IFN-γ production by autologous M. tuberculosis-specific T cells was higher in MDMs infected with HK M. tuberculosis compared with live M. tuberculosis. Interferon-γ treatment accelerated the processing of both live and HK M. tuberculosis (Fig. 1a,b) because IFN-γ production by the autologous M. tuberculosis-specific CD4+ T cells responding to the IFN-γ pre-treated MDMs was higher and occurred more quickly compared with the untreated control MDMs (30 min). Notably, M. tuberculosis peptide–MHC-II complex expression decreased more quickly in IFN-γ-treated MDMs than in the untreated controls (Fig. 1a,b).
Figure 1.

Processing and presentation of Mycobacterium tuberculosis (Mtb) antigens by monocyte-derived macrophages (MDMs) pre-treated with interferon-γ (IFN-γ) or interleukin-10 (IL-10). The MDMs were pre-treated with or without 50 U/ml IFN-γ or with 5 ng/ml IL-10 for 24 hr, washed and pulsed with heat-killed (a and c) or live (b and d) Mtb for 60 min at a multiplicity of infection of 30 and fixed after a 30, 60 or 120min incubation at 37°. Polyclonal autologous Mtb-specific CD4+ T cells were used to detect Mtb peptide–MHC-II complexes, and the supernatants were assessed for IFN-γ by ELISA. (a and b) Processing by IFN-γ-treated MDMs; (c and d) processing by IL-10-treated MDMs. Data are representative of four independent experiments.
In contrast to the accelerating effect of IFN-γ on antigen processing, IL-10 pre-treatment completely inhibited M. tuberculosis antigen processing and presentation in both live and HK M. tuberculosis-infected MDMs, as indicated by the lack of peptide–MHC-II complex detection up to 120 min after infection (Fig. 1c,d). The levels of phagocytosis of HK and live M. tuberculosis were similar inside MDMs, as assessed by the identification of acid-fast bacilli with Ziehl–Neelsen staining (data not shown).
In addition, we explored the effect of IL-4 and TNF-α on the kinetic of mycobacterial processing and found that IL-4, in a similar way to IL-10, completely inhibited HK and live M. tuberculosis processing; in contrast, TNF-α inhibited the processing of HK M. tuberculosis but transiently increased the processing of live M. tuberculosis in the first 30 min as in the case of IFN-γ and then it was decreased (data not shown). Because the effects of IFN-γ and IL-10 on M. tuberculosis antigen processing and presentation to autologous T cells was similar for both HK and live M. tuberculosis but the magnitude of the response was greater in the HK M. tuberculosis-infected MDMs, we further analysed only the M. tuberculosis peptide–MHC-II complexes in the subcellular fractions containing HK M. tuberculosis.
Identification of plasma membrane, phagosomal and MHC-II compartments in M. tuberculosis-infected-MDMs fractionated on a 27% Percoll gradient
To determine the mechanism by which IFN-γ and IL-10 impact the intracellular assembly and trafficking of M. tuberculosis peptide–MHC-II complexes, we first determined the distribution of M. tuberculosis peptide–MHC-II complexes in the intracellular compartments of untreated cells using subcellular fractionation over Percoll gradients. Plasma membranes, phagosomes and MHC-II compartments were subsequently identified in isolated fractions by a combination of fluorescence-based and biochemical techniques. Fractions containing fluorescein-labelled M. tuberculosis were consistently detected in the high-density region of the gradient and corresponded to fractions 24 through 30. These high-density fractions also displayed the highest β-hexosaminidase activity, indicating that these fractions contain phagosomes and lysosomes (phagolysosomes) (Fig. 2a). Plasma membrane-EZ-Link sulpho-NHS-biotin-associated fluorescence was detected by fluorometric assay in the low-density fractions 6 through 13 (Fig. 2a) and corresponded to the plasma membranes. MIIC endocytic vesicles were detected in fractions 18 through 25 because they induced an increment on IFN-γ production in the experiments using soluble Ag85B and autologous CD4+ T cells (Fig. 2a).
Figure 2.

Characterization of subcellular fractions of macrophages isolated on 27% Percoll density gradients. (a) Plasma membrane fractions were detected by cell surface labelling of monocyte-derived macrophages (MDMs) with sulpho-NHS-LC-biotin/streptavidin-fluorescein. Fractions were analysed using a fluorometer (filled circle). The results are expressed as arbitrary units of fluorescence. The phagosome/phagolysosome fractions were identified by incubating MDMs with fluorescein-labelled Mycobacterium tuberculosis and analysing fractions for fluorescence (open triangle) and for hexosaminidase activity using a colorimetric assay (filled triangle). Fractions containing MHC class II compartments were identified by incubating MDMs with soluble Ag85B and measuring the presence of M. tuberculosis–Ag85B complexes using M. tuberculosis-specific autologous CD4+ T cells (open circle). Data are representative of three independent experiments. (b) The cytokine production by autologous M. tuberculosis-specific CD4+ T cells in response to a subcellular fraction of MDM treated (left) or not treated with interferon-γ (right) was assessed by flow cytometry using a CBA kit. Results are expressed as pg/ml, and only detectable cytokines are shown.
To rule out the interference of IFN-γ used for the MDM pre-treatment on the detected levels of IFN-γ produced as the read-out of the T-cell assay, we also measured the production of the cytokines IL-2, IL-4, IL-6, IL-10 and IFN-γ by using the CBA assay. These experiments also helped to ensure that the specific stimulation with the subcellular fractions activated T cells. We tested autologous M. tuberculosis-specific CD4+ T responding to subcellular fractions from IFN-γ-treated or untreated MDMs and measured the aforementioned cytokines. Our results indicated that autologous polyclonal CD4+ T cells specific to M. tuberculosis produce high levels of IFN-γ in response to fractions 22 through 29 (phagosomes) and among the cytokines tested, only low levels of IL-2 and TNF-α were detected responding to the same fractions. Hence, the detected IFN-γ was produced by specific stimulation and was not a result of contamination of the IFN-γ used for the MDM treatment (Fig. 2b). Taken together, these experiments showed the feasibility of using our system to study the intracellular processing of M. tuberculosis antigens.
Mycobacterium tuberculosis peptide–MHC-II complexes appear in the phagosomes of MDMs pre-treated with IFN-γ
We had previously observed that Ag85B(96–111)–DR1 complexes were detected in THP-1 phagosomes whereas the equivalent complexes were found in MIIC of human MDM,12 so in the present study we performed experiments with whole bacteria to localize Ag85B(96–111)–DR1 or peptide–MHC-II complexes in subcellular organelle. Interferon-γ-activated THP-1 cells and MDMs, primed or not with IFN-γ, were infected with HK M. tuberculosis and subcellular fractions were obtained by Percoll gradient centrifugation. Fractions were frozen and thawed to disrupt isolated organelle membranes and to expose the luminal content with antigen-presenting domain of the MHC-II molecules; then these subcellular fractions were tested for the presence of M. tuberculosis peptide–MHC-II complexes with a T-cell assay using autologous CD4+ T cells or the T-cell hybridoma (DB1) specific for Ag85B(96–111)–DR1 complexes.
We observed that immediately after infection (chase time 0), M. tuberculosis peptide–MHC-II complexes were detected in phagosomes (fractions 25 through 29, Fig. 3a) when MDMs were pre-treated with IFN-γ, while complexes appeared in the MIIC in untreated MDMs (fractions 16 through 24, Fig. 3a). In THP-1 cells, Ag85B(96–111)–DR1 complexes were detected also in phagosomes (fractions 25 through 29, Fig. 3d). After 30 min chase, M. tuberculosis peptide–MHC-II complexes were detected in the phagosomes of IFN-γ-treated MDMs (fractions 25 through 29) and were also trafficked to the plasma membrane (fractions 6 through 12, Fig. 3b); however, in untreated MDMs, these complexes remained in the MIIC (fractions 17 through 24, Fig. 3b). In THP-1 cells, following a 30 min chase, Ag85B(96–111)–DR1 complexes were detected in phagosomes, MIIC and plasma membrane (fractions 25 through 29, 20 through 24 and 9 through 12, respectively). At 60 min chase, the M. tuberculosis peptide–MHC-II complexes were undetectable in phagosomes and were instead localized to the MIIC and plasma membrane fractions of MDMs pre-treated with IFN-γ (Fig. 3c), but complexes were detected in phagosomes and cell membrane fractions from cells not treated with IFN-γ (Fig. 3c). Antigen processing at time 60 min in THP-1 showed Ag85B(96–111)–DR1 complexes mainly in plasma membrane (fractions 8 through 12, Fig. 3f). Therefore, our results showed first that our experimental system for antigen processing and presentation with human primary MDMs detects M. tuberculosis peptide–MHC-II complexes in a similar way to that in which the THP-1 model detects a specific peptide–MHC complex. In IFN-γ pre-treated MDMs, the M. tuberculosis peptide–MHC-II complexes are primarily detected in the phagosomes, in a similar fashion to how Ag85B(96–111)–DR1 complexes were detected in THP-1-phagosomes, whereas in untreated MDMs, these complexes are primarily detected in the MIIC that receives the phagosome-derived antigen fragments. These results suggest that IFN-γ may accelerate M. tuberculosis antigen processing and presentation by primary MDMs, by altering the dynamics of peptide–MHC-II assembly and trafficking into intracellular compartments.
Figure 3.
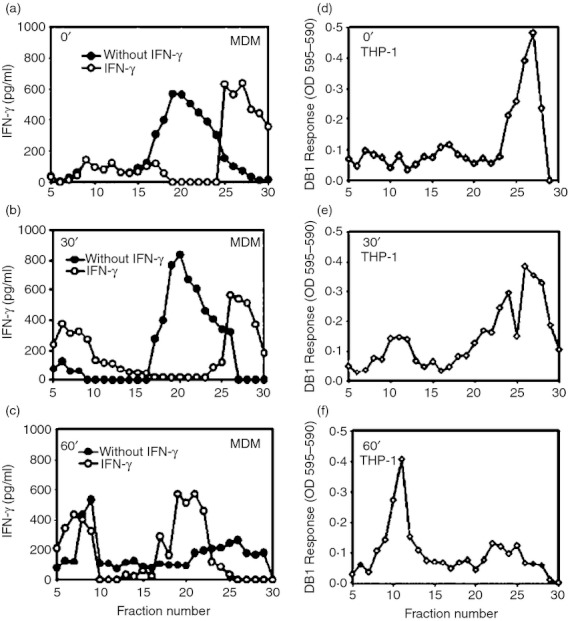
Mycobacterium tuberculosis peptide–MHC-II complexes are detected by autologous M. tuberculosis-specific T cell lines in phagosomes of interferon-γ (IFN-γ) -treated monocyte-derived macrophages (MDMs) and Ag85B(96–111)–DR1 complexes are evidenced in THP-1 phagosomes by the specific hybridoma DB1. The MDMs were pre-treated or not with 50 U IFN-γ, then pulsed with M. tuberculosis for 60 min, washed and chased for 0 (a), 30 (b) or 60 min (c), before homogenization. Subcellular organelles were separated on 27% Percoll density gradients and M. tuberculosis peptide–MHC-II complexes were detected using polyclonal autologous M. tuberculosis-specific CD4+ T cells. The results are expressed as IFN-γ production. THP-1 cells were pulsed with M. tuberculosis for 60 min, washed and chased for 0 min (d), 30 min (e), and 60 min (f). The M. tuberculosis Ag85B(96–111)–DR1 complexes were detected using DB1 T hybridoma cells. Culture supernatants were harvested after 24 hr and interleukin-2 (IL-2) production was measured using a CTLL-2 proliferation assay. Open symbols correspond to experiments performed with IFN-γ pre-treated cells and filled symbols indicate production without IFN pre-treatment. Data are representative of four independent experiments for MDM and THP-1 cells.
Interferon-γ increases the access of MHC-II molecules and decreases β-hexosaminidase activity in phagosomes
It has been reported that phagosomes from IFN-γ-treated cells contained high levels of proteasome subunits, MHC class II molecules and assembled components of the MHC class II peptide complex15,16; therefore, we evaluated the effects of IFN-γ treatment on β-hexosaminidase enzymatic activity and on HLA-DR molecule acquisition in phagosomes. As shown in Fig. 4(a), β-hexosaminidase activity was significantly decreased (P < 0·05) in the phagosomes of MDMs pre-treated with IFN-γ. β-Hexosaminidase activity was also decreased in the phagosomes of MDMs pre-treated with IL-10; however, this change was not significant. In addition, the number of phagosomes containing FLUOS-M. tuberculosis and the percentage of HLA-DR-positive staining in M. tuberculosis phagosomes isolated from MDMs pre-treated with IFN-γ was higher than in the MDMs that were not pre-treated with IFN-γ (30·65% versus 5·22% of double positives, respectively, Fig. 4b). The mean fluorescence value was also higher in the MDMs pre-treated with IFN-γ than in the untreated MDMs (6·22 versus 3·18, respectively, Fig. 4b). These results indicate that IFN-γ increases the access of MHC-II molecules but limits the acquisition of β-hexosaminidase in phagosomal fractions.
Figure 4.

Distribution of β-hexosaminidase activity and MHC II molecules in subcellular fractions of monocyte-derived macrophages (MDMs) pre-treated with interferon-γ (IFN-γ). (a) The MDMs were pre-treated without (filled circles) or with 50 U IFN-γ (open circles), or with 5 ng/ml interleukin-10 (IL-10) (filled triangles). β-Hexosaminidase activity was assessed by colorometric assay in subcellular fractions from 27% Percoll gradients and results are expressed as units of fluorescence (OD 405 nm). (b) MDMs were pre-treated without or with 50 U IFN-γ and incubated with fluorescein-labelled Mycobacterium tuberculosis (Mtb). Then, phagosomes were purified on 27% Percoll gradients, fixed, permeabilized and stained with phycoerythrin-anti-HLA-DR antibody and assessed by flow organellometrics. Events were gated according to fluorescein intensity and scatter parameters. The data are representative of three independent experiments.
MDMs pre-treated with IL-10 retain M. tuberculosis peptide–MHC-II complexes in the MIIC
We then investigated the role of IL-10 in the subcellular distribution of M. tuberculosis peptide–MHC-II complexes in MDMs infected with HK M. tuberculosis. These experiments were evaluated with the autologous M. tuberculosis-specific CD4+ T-cell assay. Our results demonstrated that immediately after infection at time 0, M. tuberculosis peptide–MHC-II complexes were detected in the MIIC from both untreated (fractions 17 through 24) and IL-10-treated MDMs (fractions 16 through 23) (Fig. 5a). In contrast to the untreated MDMs, M. tuberculosis peptide–MHC-II complexes remained in the MIIC in IL-10-pre-treated MDMs and did not appear in the plasma membrane fractions, even after long chase periods (Fig. 5b,c). Therefore, our results show that IL-10 retains the M. tuberculosis peptide–MHC-II complexes in the MIIC.
Figure 5.

Interleukin-10 (IL-10) retains Mycobacterium tuberculosis peptide–MHC-II complexes in MHC class II compartments (MIIC). Monocyte-derived macrophages (MDMs) were pre-treated with 5 ng/ml IL-10 and were pulsed with M. tuberculosis for 60 min, washed and chased for 0 min (a), 30 min (b), or 60 min (c) before homogenization. The subcellular organelles were separated on 27% Percoll density gradients and M. tuberculosis peptide–MHC-II complexes were detected using polyclonal autologous M. tuberculosis-specific CD4+ T cells and interferon-γ (IFN-γ) production was measured by ELISA. The data are representative of three independent experiments.
Interferon-γ and IL-10 treatment have differential effects on the access of Ag85B/HLA-DR complexes in cell membranes and intracellular vesicles
To follow the intracellular trafficking of a specific mycobacterial antigen in IFN-γ or IL-10-treated or untreated MDMs infected with HK M. tuberculosis, we used immunofluorescence microscopy. This allowed us to explore the fate of Ag85B and HLA-DR molecules during antigen processing.
We did not observe the presence of Ag85B associated with HLA-DR molecules at the cell surface following infection (chase time 0) in IFN-γ or IL-10-treated or untreated MDMs. In contrast, the presence of Ag85B associated with HLA-DR at the cell surface was observed only in the IFN-γ-treated MDMs following a 30-min chase. This association was observed as a yellow colour, indicating a co-localization of antigen and the HLA-DR molecules (Fig. 6a, top). The Ag85B was always associated with HLA-DR molecules in the membrane of intracellular vesicles of MDMs, regardless of IFN-γ or IL-10 treatment. The intensity of the yellow colour increased from 0 to 30 minutes. (Fig. 6a, bottom). The expression of abundant Ag85B–HLA-DR complexes led us to investigate further the nature of the intracellular compartments where the complexes appeared. As shown in Fig. 6(b), the Ag85B was detected mostly in vesicles with diameters of 1–3 μm that contain the lysosome/endosomal LAMP1 marker in IFN-γ-treated MDMs. Therefore, these compartments are in all likelihood involved in antigen processing and peptide transport to the cell surface. Contrasting with IFN-γ pre-treated cells, in IL-10 pre-treated cells independent labels for Ag85 and LAMP-1 were seen in vesicles, indicating a weak or null association between Ag85 and LAMP-1 (Fig. 6b, bottom). Therefore, these results of the fluorescence microcopy experiments comprise a second set of evidence, which matches our results pertaining to the subcellular fractions presented here, suggesting that IFN-γ may accelerate M. tuberculosis antigen processing and presentation at the cell membrane, whereas IL-10 favours the trafficking of Ag85B to vesicles that do not contain LAMP-1.
Figure 6.

Interferon-γ (IFN-γ) and interleukin-10 (IL-10) treatment have a differential effect on the access of Ag85B/HLA-DR complexes in cell membrane and in intracellular vesicles. Monocyte-derived macrophages (MDMs) were pre-treated with IFN-γ or IL-10 for 24 hr, infected with Mycobacterium tuberculosis and chased for 0 and 30 min and subsequently double-stained. (a) With anti-HLA-DR antibodies (green) and anti-Ag85B (red) in the absence (up) or presence (bottom) of saponin. (b) With anti-Ag85B (red) and anti-LAMP1 antibodies (green) in the presence of saponin. The images represent the merged images where yellow indicates colocalization. Ag85B and HLA-DR are associated in plasma membranes of MDMs pre-treated with IFN-γ after 30-min chase. Ag85B was not present in the plasma membrane of IL-10-treated-MDM (a). Nuclei were stained with DAPI. The framework from each picture was digitally magnified using Adobe Photoshop. Bar size equals 20 μm.
Discussion
In this report, we demonstrate that pre-treatment of MDMs with IFN-γ accelerates M. tuberculosis antigen processing and presentation by increasing the early detection of peptide–MHC-II complexes by autologous M. tuberculosis-specific CD4+ T cells in phagosomes and plasma membrane. By contrast, IL-10 decreases M. tuberculosis peptide presentation by inducing the retention of peptide–MHC-II complexes in the MIIC. Our results were obtained in experiments performed with human blood cell primary cultures and using whole bacteria, which enhances the relevance of our findings.
The crucial role of IFN-γ in tuberculosis has been extensively studied and described in mouse models. The fact that IFN-γ increases mycobactericidal activity by augmenting the expression of the inducible nitric oxide synthase24 and increasing nitric oxide production is well established.25 In humans, it has been demonstrated that mutations in the genes controlling the interleukin axis of IL-12/IL-23-dependent IFN-γ production increases the susceptibility to mycobacterial infections.26,27 However, the role of IFN-γ in mycobacterial antigen processing and presentation by macrophages has been poorly studied at the subcellular level. In particular, little is known about how the prototypical T helper type 1/type 2 cytokines, IFN-γ and IL-10, affect the site of peptide–MHC class II complex assembly and trafficking in human macrophages. We experimentally addressed this question here.
In this study, we observed that IFN-γ increased the processing of both HK and live M. tuberculosis, indicating that this effect does not depend on bacterial viability. The increased processing of both HK and live M. tuberculosis in MDMs in the presence of IFN-γ could be related to the activation induced by IFN-γ and its effects on enhancing antigenic processing and promotion of antigen loading onto MHC class II molecules.15,17,28 However, the cell surface decrease in the number of peptide–MHC-II complexes after 60 min and 120 min chases in IFN-γ-treated MDMs suggests that pre-treatment with IFN-γ may induce mechanisms that promote the degradation or recycling of the M. tuberculosis peptide–MHC class II complexes, resulting in a shorter presentation time on the plasma membrane.
Then we demonstrate in this work that our model using MDMs as antigen-presenting cells and autologous M. tuberculosis cell lines as responding cells serves to detect peptide–MHC-II complexes in a similar fashion to our previously tested model with THP-1 cells as antigen-presenting cells and DB1 hybridoma as responding cells. This experimental model, coupled to subcellular fractionation, can be used to track the fate of peptide–MHC-II complexes as was shown in this work. Moreover, the use of whole bacteria in the experiments presented here represents a more physiological model of antigen processing and presentation and expands the extent of experiments performed only with soluble antigens.
We demonstrated that in MDMs pre-treated with IFN-γ, the M. tuberculosis peptide–MHC class II complexes appeared early (time 0) in phagosomes, whereas in untreated MDMs, the complexes were detected in the MIIC. These results correlate with higher levels of MHC-II molecules (HLA-DR) in phagosomes derived from MDMs upon IFN-γ treatment. We hypothesized that this robust ability of MHC-II molecules to concentrate in phagosomes from MDMs pre-treated with IFN-γ indicates that the peptide–MHC-II complexes are actually formed in this compartment, instead of the peptide being trafficked from the phagosomes to the MIIC to be assembled on MHC molecules. Accordingly, it has been reported that in non-professional antigen-presenting tumour cells, IFN-γ induces the expression of HLA-DM, HLA-DR, the invariant chain (li) and the protease cathepsin S (which is necessary for li degradation), leading to the release of MHC molecules that are available to engage peptides.29 Based on these findings and our results, we propose that IFN-γ provides the phagosomes with the necessary molecules for the assembly of peptide–MHC class II molecule complexes. Therefore, in addition to the known effects of IFN-γ on the enhancement of mycobactericidal activity in macrophages,5,6,25 an additional biological role for IFN-γ is described here: IFN-γ has the capacity to render the phagosome a competent organelle for the assembly of peptide–MHC-II complexes.
We then investigated whether IFN-γ pre-treatment of MDM could account for the phagosomal acquisition of the lysosomal enzyme, β-hexosaminidase. The acquisition of lysosomal enzymes is a marker of phagosome maturation and is necessary for antigen processing.30 Previously, Yates et al.31,32 reported that activation of macrophages with IFN-γ reduces vacuolar protease activity and prevents complete proteolytic destruction of proteins and peptides but favours the generation of peptides suitable for antigen presentation. Consistent with this study, we observed that IFN-γ pre-treatment of MDMs reduces the activity of β-hexosaminidase in phagosomes. Despite the reduced activity of β-hexosaminidase, we observed that in IFN-γ-treated MDMs, the overall antigen processing and presentation was enhanced. Therefore, we suggest that fine-tuning the regulation of acquisition of lysosomal enzyme, combined with moderate proteolytic activity, is necessary for optimal peptide–MHC-II complex assembly in human phagosomes.
Another important observation from our work is that IFN-γ-pre-treatment induces peptide–MHC-II complex assembly in phagosomes and that these complexes are detected early on in the plasma membrane in comparison to untreated MDMs. This finding suggests that IFN-γ promotes the efficient trafficking of peptide–MHC-II complexes to the plasma membrane and that the complexes are transported rapidly to the plasma membrane and are able to activate specific CD4+ T cells as early as 30 min. We postulate that the early CD4+ T-cell activation could be important for the control of mycobacterial infection. However, IFN-γ production is regulated by IL-12, IL-23 and the IL12/IL-23–IFN-γ axis and is associated with defence against microorganisms of the genera Mycobacterium, Salmonella and Candida.33–35 In this regard, Happel et al.36 observed that transferring the IL-23 gene to naive mice infected with M. tuberculosis led to high local levels of IFN-γ and IL-17 expression, significantly reducing the mycobacterial burden and increasing the number of activated CD4+ T cells. These findings and our results suggest that IFN-γ can accelerate the trafficking of mycobacterial peptide–MHC-II complexes to the plasma membrane of antigen-presenting cells, which, consequently, results in increased numbers of activated CD4+ T cells. As a result of the enhancing effects of IFN-γ on antigen processing and presentation demonstrated in this work, early treatment with IFN-γ in individuals with a high risk of developing tuberculosis may help to induce a more efficient immune response for the control of infection.
In contrast to the observed enhancing effects of IFN-γ pre-treatment on antigen processing and presentation, IL-10 pre-treatment resulted in M. tuberculosis peptide–MHC class II complex assembly and retention in the MIIC. The results obtained in our experiments are consistent with a previous study published by Koppelman et al.18, demonstrating that IL-10 treatment of human monocytes led to the accumulation of MHC class II complexes in intracellular vesicles. Notably, a recent paper published by Thibodeau et al.37 showed that the immunosuppressive effect of IL-10 on antigen presentation is mediated through the induction of MARCH1, a ubiquitin ligase family member that promotes the degradation of MHC class II molecules, so establishing a molecular mechanism for the inhibitory effect of IL-10. Although we did not assess MHC class II molecule degradation in our experiments, we propose that IL-10 may promote the degradation of peptide–MHC-II complexes retained in the MIIC, and this phenomenon could be associated with the decreased detection of peptide–MHC-II complexes in both the plasma membrane and MIIC after 60 min of antigen processing.
Mycobacterium tuberculosis has developed distinct escape mechanisms, such as the induction of IL-10, to prevent the development of an efficient immune response by the host.38 In this study, we demonstrate that IL-10 pre-treatment impairs peptide–MHC-II complex trafficking to the plasma membrane. The peptide–MHC-II complexes retained in the MIIC limit the expression of functional peptide-loaded MHC molecules for antigen presentation at the plasma membrane in IL-10-pre-treated MDMs. Additionally, in vivo, this could be related to the decreased T-cell response that our group observed in peripheral mononuclear cells from patients with tuberculosis who also had high levels of IL-10 production.39,40 Moreover, in patients with a concomitant intestinal helminth infection and tuberculosis, high levels of IL-10 are associated with a decreased clinical response to tuberculosis therapy and decreased levels of CD4+ CD25+ T cells.41 It has also been observed that there is an increased susceptibility to pulmonary tuberculosis in patients who present with polymorphisms in allele 2 of SLC11A1 (Nramp1), with enhanced IL-10 production by monocytes in response to lipopolysaccharide.42
Although our results using M. tuberculosis-specific T cells suggested that peptide–MHC-II complexes from IFN-γ-treated MDMs reach the plasma membrane more quickly than in untreated MDMs or IL-10-treated MDMs, our experiments did not provide specific information regarding the antigens that are present early on the plasma membrane. To compensate for this limitation, we used immunofluorescence to investigate the trafficking of Ag85B, an immunodominant antigen involved in protective immunity. Our results indicated that Ag85B can be detected in association with HLA-DR molecules on the plasma membrane of MDMs soon after treatment with IFN-γ, and these data are consistent with the rapid T-cell response induced by the plasma membrane fraction of IFN-γ-treated MDMs. However, Ag85B was not detected in the plasma membrane of IL-10-treated MDMs. This result suggests that environmental cytokines are important in the processing and intracellular trafficking of mycobacterial antigens and Ag85B–MHC complexes.
In conclusion, efficient antigen processing and presentation during a mycobacterial infection depends on the initial balance of IFN-γ or IL-10 levels and requires IFN-γ. If IL-10 levels predominate, M. tuberculosis peptide–MHC-II complexes are retained in MIIC and antigen presentation is blocked. By contrast, treatment of MDMs with IFN-γ results in the assembly of peptide–MHC-II complexes in phagosomes followed by the efficient trafficking of these complexes to the plasma membrane for antigen presentation and induction of the T-cell response.
Acknowledgments
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT) Grant CB 2008-101948. KB was recipient of Doctoral Fellowship CONACYT 181487 at Programa de Doctorado en Ciencias Biomédicas (PDCB), Facultad de Medicina, Universidad Nacional Autónoma de México. Funding agencies did not participate in the study design, execution, analysis, interpretation of data or writing of the manuscript.
Disclosures
The authors declare no conflict of interest.
References
- 1.Centers for Disease Control and Prevention (CDC) Mortality among patients with tuberculosis and associations with HIV status — United States, 1993–2008. MMWR Morb Mortal Wkly Rep. 2010;59:1509–13. [PubMed] [Google Scholar]
- 2.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729–41. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudensky AY, Maric M, Eastman S, Shoemaker L, DeRoos PC, Blum JS. Intracellular assembly and transport of endogenous peptide-MHC class II complexes. Immunity. 1994;1:585–94. doi: 10.1016/1074-7613(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 5.Tulp A, Verwoerd D, Dobberstein B, Ploegh HL, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–6. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- 6.Harding CV, Geuze HJ. Immunogenic peptides bind to class II MHC molecules in an early lysosomal compartment. J Immunol. 1993;151:3988–98. [PubMed] [Google Scholar]
- 7.Harding CV. Intracellular organelles involved in antigen processing and the binding of peptides to class II MHC molecules. Semin Immunol. 1995;7:355–60. doi: 10.1006/smim.1995.0040. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandra L, Noss E, Boom WH, Harding CV. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J Exp Med. 2001;194:1421–32. doi: 10.1084/jem.194.10.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramachandra L, Harding CV. Phagosomes acquire nascent and recycling class II MHC molecules but primarily use nascent molecules in phagocytic antigen processing. J Immunol. 2000;164:5103–12. doi: 10.4049/jimmunol.164.10.5103. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandra L, Simmons D, Harding CV. MHC molecules and microbial antigen processing in phagosomes. Curr Opin Immunol. 2009;21:98–104. doi: 10.1016/j.coi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grotzke JE, Harriff MJ, Siler AC, Nolt D, Delepine J, Lewinsohn DA, Lewinsohn DM. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:1–13. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres M, Ramachandra L, Rojas RE, Bobadilla K, Thomas J, Canaday DH, Harding CV, Boom WH. Role of phagosomes and major histocompatibility complex class II (MHC-II) compartment in MHC-II antigen processing of Mycobacterium tuberculosis in human macrophages. Infect Immun. 2006;74:1621–30. doi: 10.1128/IAI.74.3.1621-1630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–6. [PubMed] [Google Scholar]
- 15.Jutras I, Houde M, Currier N, et al. Modulation of the phagosome proteome by interferon-γ. Mol Cell Proteomics. 2008;7:697–715. doi: 10.1074/mcp.M700267-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. The phagosomal proteome in interferon-γ-activated macrophages. Immunity. 2009;30:143–54. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111(Pt 7):897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 18.Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II αβ peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. 1997;7:861–71. doi: 10.1016/s1074-7613(00)80404-5. [DOI] [PubMed] [Google Scholar]
- 19.Tan JS, Canaday DH, Boom WH, Balaji KN, Schwander SK, Rich EA. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol. 1997;159:290–7. [PubMed] [Google Scholar]
- 20.Canaday DH, Gehring A, Leonard EG, Eilertson B, Schreiber JR, Harding CV, Boom WH. T-cell hybridomas from HLA-transgenic mice as tools for analysis of human antigen processing. J Immunol Methods. 2003;281:129–42. doi: 10.1016/j.jim.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Levi-Schaffer F, Riesel N, Soffer D, Abramsky O, Brenner T. Mast cell activity in experimental allergic encephalomyelitis. Mol Chem Neuropathol. 1991;15:173–84. doi: 10.1007/BF03159954. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandra L, Sramkoski RM, Canaday DH, Boom WH, Harding CV. Flow analysis of MHC molecules and other membrane proteins in isolated phagosomes. J Immunol Methods. 1998;213:53–71. doi: 10.1016/s0022-1759(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez-Mata A, Michalak C, Mendoza-Hernandez G, Leon-Del-Rio A, Gonzalez-Noriega A. Annexin VI is a mannose-6-phosphate-independent endocytic receptor for bovine β-glucuronidase. Exp Cell Res. 2011;317:2364–73. doi: 10.1016/j.yexcr.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 25.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 26.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 27.Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–61. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Lah TT, Hawley M, Rock KL, Goldberg AL. γ-interferon causes a selective induction of the lysosomal proteases, cathepsins B and L, in macrophages. FEBS Lett. 1995;363:85–9. doi: 10.1016/0014-5793(95)00287-j. [DOI] [PubMed] [Google Scholar]
- 29.Meissner M, Whiteside TL, Kaufmann R, Seliger B. CIITA versus IFN-γ induced MHC class II expression in head and neck cancer cells. Arch Dermatol Res. 2009;301:189–93. doi: 10.1007/s00403-008-0922-6. [DOI] [PubMed] [Google Scholar]
- 30.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–70. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–50. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 32.Yates RM, Russell DG. Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome. Methods Mol Biol. 2008;445:311–25. doi: 10.1007/978-1-59745-157-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 35.Pedraza S, Lezana JL, Samarina A, et al. Clinical disease caused by Klebsiella in 2 unrelated patients with interleukin 12 receptor β1 deficiency. Pediatrics. 2010;126:e971–6. doi: 10.1542/peds.2009-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Happel KI, Lockhart EA, Mason CM, Porretta E, Keoshkerian E, Odden AR, Nelson S, Ramsay AJ. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun. 2005;73:5782–8. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thibodeau J, Bourgeois-Daigneault MC, Huppe G, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38:1225–30. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–70. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 39.Torres MM-SP, Jimenez-Zamudio L, Teran L, Camarena A, Quezada R, Ramos E, Sada E. Comparison of the immune response against Mycobacterium tuberculosis antigens between a group of patients with active pulmonary tuberculosis and healthy household contacts. Clin Exp Immunol. 1994;96:75–8. doi: 10.1111/j.1365-2249.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–80. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awomoyi AA, Marchant A, Howson JM, McAdam KP, Blackwell JM, Newport MJ. Interleukin-10, polymorphism in SLC11A1 (formerly NRAMP1), and susceptibility to tuberculosis. J Infect Dis. 2002;186:1808–14. doi: 10.1086/345920. [DOI] [PubMed] [Google Scholar]


