Abstract
Background
To potentially improve outcomes in pancreatic resection, robot-assisted pancreatic surgery has been introduced. This technique has possible advantages over laparoscopic surgery, such as its affordance of three-dimensional vision and increased freedom of movement of instruments. A systematic review was performed to assess the safety and feasibility of robot-assisted pancreatic surgery.
Methods
The literature published up to 30 September 2011 was systematically reviewed, with no restrictions on publication date. Studies reporting on over five patients were included. Animal studies, studies not reporting morbidity and mortality, review articles and conference abstracts were excluded. Data were extracted and weighted means were calculated.
Results
A total of 499 studies were screened, after which eight cohort studies reporting on a total of 251 patients undergoing robot-assisted pancreatic surgery were retained for analysis. Weighted mean operation time was 404 ± 102 min (510 ± 107 min for pancreatoduodenectomy only). The rate of conversion was 11.0% (16.4% for pancreatoduodenectomy only). Overall morbidity was 30.7% (n = 77), most frequently involving pancreatic fistulae (n = 46). Mortality was 1.6%. Negative surgical margins were obtained in 92.9% of patients. The rate of spleen preservation in distal pancreatectomy was 87.1%.
Conclusions
Robot-assisted pancreatic surgery seems to be safe and feasible in selected patients and, in left-sided resections, may increase the rate of spleen preservation. Randomized studies should compare the respective outcomes of robot-assisted, laparoscopic and open pancreatic surgery.
Introduction
Pancreatic resection is amongst the most complex and challenging of abdominal operations. Even in highly experienced centres, open pancreatic surgery is associated with morbidity rates of 30–40% and mortality rates of approximately 2%.1,2 New, minimally invasive techniques may reduce postoperative morbidity. Therefore, in recent years, laparoscopic pancreatic surgery has been introduced as an alternative to open surgery.3 Laparoscopic techniques have potential benefits; they can decrease pain and blood loss, and result in fewer complications, faster recovery and a shorter hospital length of stay (LoS).4,5 Early experiences have shown that laparoscopic pancreatic surgery is safe and feasible in selected patients, and that morbidity rates range from 16% to 40%.6–10 Although a growing number of studies on laparoscopic pancreatic surgery have been published, it has not gained wide acceptance. This is probably explained by the known limitations of conventional laparoscopic surgery, such as the decreased range of motion this technique affords and the two-dimensional vision of the operative field, which make its practice difficult.
The use of a robotic system may overcome some of these shortcomings. Robot-assisted surgery provides three-dimensional vision and a magnified view of the operative field. These advantages, combined with the increased freedom of movement of surgical instruments and the elimination of tremor, lead to improved precision in operative technique and may lead to safer anastomoses compared with laparoscopic pancreatic surgery. Moreover, robotic systems are ergonomically better for the surgeon and cause less weariness during the operation. In other gastrointestinal procedures, such as oesophageal resection, several centres have gained extensive experience in robot-assisted surgery, including the University Medical Centre Utrecht (UMCU), with the first robot-assisted oesophagectomy reported in 2003.11,12 The first patient to undergo robot-assisted pancreatic surgery was also reported in that year.13 Since then, a small number of centres have adopted this technique and several small series have reported encouraging outcomes.
In preparation for a possible expansion of the UMCU programme in robot-assisted surgery from oesophagogastric to pancreatic surgery, a systematic review of the current literature was performed. The aim was to assess the safety and feasibility of robot-assisted laparoscopic pancreatic surgery according to early experiences of the use of this technique.
Materials and methods
Literature search strategy
A systematic search, restricted to papers published in English, was performed in MEDLINE (for articles published from 1947 to 30 September 2011) and EMBASE (for articles published from 1974 to 30 September 2011). The review protocol was developed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.14
Search terms were ‘[robot OR robotic OR (da Vinci)] AND (pancreas OR pancreatic OR pancreatectomy OR pancreaticoduodenectomy OR Whipple)’. Titles and abstracts of the identified papers were screened by two authors (MS and HCvS). Any differences in opinion were resolved by discussion and, if necessary, the input of a third author (MGB). Full-text versions of papers considered for inclusion were examined. The bibliographies of selected articles were reviewed for other potentially relevant studies.
In order to diminish selection bias, studies were required to report a cohort of at least five patients undergoing robot-assisted pancreatic surgery to be considered for inclusion. Animal studies, studies not reporting morbidity and mortality, review articles and conference abstracts published in abstract form only were excluded. If multiple studies were published by one centre, the study reporting the largest number of patients was selected unless it was clear that data did not overlap.
Data extraction
Study characteristics extracted from the selected articles included country, study design, study interval, number of patients undergoing robot-assisted pancreatic surgery, total number of patients in the study, type of operation and whether comparisons among open, laparoscopic and robot-assisted surgery were made.
Documented patient characteristics included sex, age, body mass index (BMI), preoperative health status scoring and pathology.
Data on outcomes of surgery were extracted, if available, and included operating time, estimated blood loss, conversion rate, complications and mortality as defined by the individual papers, hospital LoS, pathology findings as defined by the individual papers and, in cases of distal pancreatectomy, the rate of spleen preservation.
If a study reported the outcomes of open and/or laparoscopic procedures, these data were also collected.
Authors of studies that did not report outcomes separately for distal pancreatectomies and pancreaticoduodenectomies were contacted to obtain additional data.
Statistical analysis
If outcomes were represented as medians and ranges, means and standard deviations (SDs) were estimated according to the methods described by Hozo et al.15 A weighted mean and weighted SD were calculated. In the present review, the outcomes presented in tables are given as they were originally reported in the individual articles. If studies reported outcomes separately for pancreatoduodenectomy or distal pancreatectomy, these data were also included in an analysis of this particular type of procedure.
Results
The literature search identified a total of 499 potentially relevant articles, of which eight studies were ultimately included in this systematic review (Fig. 1).
Figure 1.
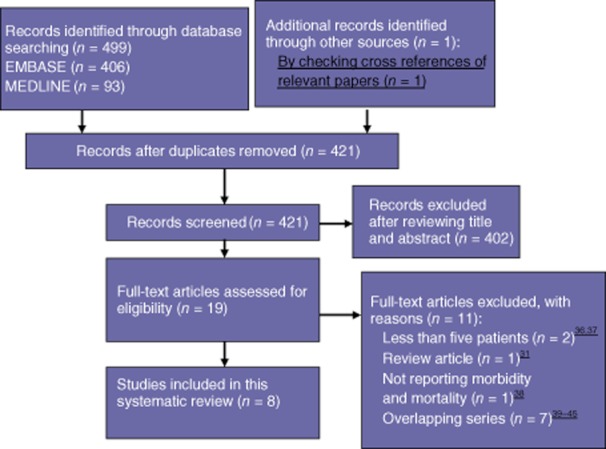
Study selection
Characteristics of the included studies are presented in Table 1. All studies were retrospective, non-controlled case series (Oxford level 4 evidence)16 and hence methodological quality was not determined. Some studies reported that data had been collected prospectively.17–19 Four studies compared outcomes in patients who underwent robot-assisted surgery with outcomes in patients who underwent laparoscopic and/or open pancreatic resections.17,20–22 Two studies reported outcomes in a single cohort undergoing different types of procedure.18,23 The authors of these studies were contacted to provide the specific outcomes of surgery for each operation type. Together, the eight studies referred to a total of 251 patients undergoing robot-assisted pancreatic surgery. The median number of relevant patients included in each study was 15 (range: five to 134 patients). Resections included 131 pancreatoduodenectomies (52.2%) and 85 distal pancreatectomies (33.9%).
Table 1.
Characteristics of included studies
| Authors | Year | Country | Study design | Study interval | Relevant patientsa, n | Patients in studyb, n | Procedure | Comparison |
|---|---|---|---|---|---|---|---|---|
| Chan et al.18 | 2011 | China | Retrospective non-controlled case series | May 2009 to December 2010 | 12 | 55 | Pancreatoduodenectomy (n = 8), distal pancreatectomy (n = 2), double bypass (n = 1), cystojejunostomy (n = 1) | None |
| Giulianotti et al.23 | 2010 | USA | Retrospective non-controlled case series | October 2000 to January 2009 | 134 | 134 | Pancreatoduodenectomy (n = 60), distal splenopancreatectomy (n = 23), distal pancreatectomy (spleen-preserving) (n = 23), central pancreatectomy (n = 3), total pancreatectomy (n = 1), enucleation (n = 3), other (n = 21)c | None |
| Kang et al.20 (DP) | 2011 | South Korea | Retrospective non-controlled case series | March 2006 to July 2010 | 20 | 45 | Distal pancreatectomy | Robot-assisted (n = 20) versus laparoscopic (n = 25) |
| Kang et al.21 (CP) | 2011 | South Korea | Retrospective non-controlled case series | Robot-assisted: December 2007 to December 2009 Open: January 1990 to November 2007 | 5 | 15 | Central pancreatectomy | Robot-assisted (n = 5) versus open (n = 10) |
| Narula et al.24 | 2010 | USA | Retrospective non-controlled case series | May 2006 to June 2007 | 5 | 5 | Pancreatoduodenectomy | None |
| Waters et al.17 | 2010 | USA | Retrospective non-controlled case series | August 2008 to August 2009 | 17 | 57 | Distal pancreatectomy | Robot-assisted (n = 17) versus laparoscopic (n = 18) versus open (n = 22) |
| Zeh et al.19 | 2011 | USA | Retrospective non-controlled case series | October 2008 to December 2010 | 50 | 50 | Pancreatoduodenectomy | None |
| Zhou et al.22 | 2011 | China | Retrospective non-controlled case series | January–December 2009 | 8 | 16 | Pancreatoduodenectomy | Robot-assisted (n = 8) versus open (n = 8) |
Patients undergoing robot-assisted pancreatic resection.
All patients in study, including patients undergoing open pancreatic surgery or robot-assisted resections of other organs.
Other: cystoduodenostomy (n = 1), cystogastrostomy (n = 14), cystojejunostomy (n = 3), pancreaticogastrostomy (n = 2), pancreaticojejunostomy (n = 1).
DP, distal pancreatectomy; CP, central pancreatectomy.
With regard to definitions of complications and mortality, four studies provided no definitions at all.17,18,20,24 In the remaining studies definitions varied widely. For example, the four papers reporting pancreatic fistula all used different definitions.19,21–23 Two of the studies used the internationally accepted International Study Group on Pancreatic Fistula (ISGPF) definition,19,21 one of these reported grade B and C fistulae only.21 Another study used the John Hopkins definition to identify fistulae, but graded some of the fistulae according to the ISGPF classification.23 The fourth study used a definition based on that of Bertrand.22 Delayed gastric emptying (DGE) was defined in only one study,19 which used the International Study Group on Pancreatic Surgery (ISGPS) classification.25 Bleeding was defined in none of the studies. Complication rates were also reported differently. One study presented 30-day morbidity,19 but others did not report the time period in which complications occurred. Only one study used a validated classification system (i.e. the Clavien–Dindo system26) to grade complications.19 None of the studies provided a definition of pathological margins.
Patient characteristics
Characteristics of the included patients are summarized in Table 2. The weighted mean age of all patients included was 60 ± 12 years. Two studies reported patient BMI,19,20 providing a weighted mean of 26.2 ± 4.5 kg/m2. Indications for surgery varied from pseudocyst to carcinoma. Of all resections, 115 procedures were performed because of malignancy (45.8%). In 123 pancreatoduodenectomies (93.9% of all pancreatoduodenectomies) and 83 distal pancreatectomies (97.6% of all distal pancreatectomies), indications were reported separately for this type of procedure. Malignant disease was cited as the indication for surgery in 72.4% (n = 89) of pancreatoduodenectomies and 20.5% (n = 17) of distal pancreatectomies. One of the studies also reported a palliative procedure.18
Table 2.
Characteristics of patients reported in the included studies
| Authors | Male, % | Age, years | BMI | Preoperative status | Pathology |
|---|---|---|---|---|---|
| Chan et al.18 | 58% | 71.5 (45–83)d | NR | NR | ACA of pancreas (n = 4), ACA of biliary tract (n = 2), CIS of biliary tract (n = 1), IPMN (n = 1), SCN (n = 1), NET (n = 1), SPN (n = 1), pseudocyst (n = 1) |
| Giulianotti et al.23 | 38% | 58 (25–86)c | NR | NR | PD: ACA of pancreas (n = 27), ACA of biliary tract (n = 17), MCN (n = 5), chronic pancreatitis (n = 5), duodenal pathology (n = 3), IPMN (n = 1), SPN (n = 1), choledochal cyst (n = 1) DP: non-malignant (n = 29), ACA of pancreas (n = 6), NET (n = 5), cystic NET (n = 2), MCN (n = 2), metastases (n = 2) |
| Kang et al.20 (DP) | R: 40% L: 44% | R: 44.5 ± 15.9 L: 56.5 ± 13.9b | R: 24.2 ± 2.9 L: 23.4 ± 2.6b | NR | R: MCN (n = 5), SCN (n = 4), SPN (n = 4), NET (n = 3), IPMN (n = 2), pancreatitis (n = 1), IPAS (n = 1) L: IPMN (n = 10), SPN (n = 4), SCN (n = 3), NET (n = 3), MCN (n = 2), pseudocyst (n = 1), IPAS (n = 1), benign stricture (n = 1) |
| Kang et al.21 (CP) | R: 0% O: 40% | R: 50.0 ± 12.3 O: 38.7 ± 16.5b | NR | NR | R: SPN (n = 4), NET (n = 1) O: SPN (n = 3), NET (n = 3), MCN (n = 3), congenital cyst (n = 1) |
| Narula et al.24 | NR | 51.6a | NR | NR | Chronic inflammation and fibrosis (n = 4), ACA (n = 1) |
| Waters et al.17 | R: 35% L: 50% O: 45% | R: 64 L: 59 O: 59a | NR | ASA: R: 2.8 L: 2.9 O: 2.9a | R: IPMN (n = 6), NET (n = 5), MCN (n = 3), SCN (n = 1), other (n = 2) L: NET (n = 5), MCN (n = 3), SCN (n = 2), ACA (n = 2), IPMN (n = 2), other (n = 3) O: ACA (n = 11), NET (n = 4), IPMN (n = 4), MCN (n = 2), other (n = 1) |
| Zeh et al.19 | 48% | 68 ± 16b | 27 ± 5b | ASA: II: 42.0% III: 56.0% IV: 2.0% | ACA of pancreas (n = 14), ACA of biliary tract (n = 11), NET (n = 10), IPMN (n = 10), SPN (n = 2), MCN (n = 1), duodenal adenoma (n = 1), oligocystic SCN (n = 1) |
| Zhou et al.22 | R: 62.5% O: 50.0% | R: 64.4 ± 9.1 O: 59.4 ± 9.4b | NR | NR | R: ACA of biliary tract (n = 6), ACA of pancreas (n = 1), periampullar ACA (n = 1) O: ACA of biliary tract (n = 4), pancreatic ACA (n = 2), papilloma of biliary tract (n = 1), duodenal papilloma (n = 1) |
Mean.
Mean ± standard deviation.
Mean (range).
Median (range).
BMI, body mass index; R, robot-assisted; L, laparoscopic; O, open; NR, not reported; ASA, American Society of Anesthesiologists; CP, central pancreatectomy; DP, distal pancreatectomy; PD, pancreatoduodenectomy; ACA, adenocarcinoma; CIS, carcinoma in situ; IPAS, intrapancreatic accessory spleen; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; NET, neuroendocrine tumour; SCN, serous cystic neoplasm; SPN, solid pseudopapillary neoplasm.
With respect to scoring of the patients' preoperative health status, two studies provided data on preoperative American Society of Anesthesiologists (ASA) class.17,19 Zeh et al. reported ASA classes of 2 in 21 patients (42.0%), 3 in 28 patients (56.0%) and 4 in one patient (2.0%).19 Waters et al. reported only a mean ASA score of 2.8.17
Outcomes
Outcomes in the eight studies are represented in Table 3. Weighted mean operation time was 404 ± 102 min. Estimated blood loss was described in all but one series.24 Weighted mean blood loss amounted to 328 ± 334 ml.
Table 3.
Outcomes of surgery (all types of resection)
| Authors | Operating time, min | Blood loss, ml | Conversion rate, n (%) | Patients with ≥ 1 complication, n (%) | Postoperative mortality, n (%) | Hospital stay, days | Positive surgical margins | Lymph nodes harvested | Spleen-preservation in DP |
|---|---|---|---|---|---|---|---|---|---|
| Chan et al.18 | 478 (270–692)f | 200 (30–300)f | 1 (8.3%) | 5 (41.7%) | 0 | 12 (6–21)f | NR | NR | 2/2 (100%) |
| Giulianotti et al.23 | Italy: 312 (55–660) USA: 351 (73–630)e | Italy: 261 (100–600) USA: 342 (5–2000)e | Italy: 10 (13.0%) USA: 4 (7.0%) | 35 (26.1%) | Italy: 2 (2.6%) USA: 1 (1.8%) | Italy: 21.8 (6–85) USA: 9.3 (3–30)e | PD: Italy: 0 USA: 5 (20.8%) | PD: Italy: 21 (5–37) USA: 14 (12–45)e | 42/46 (91.3%) |
| Kang et al.20 (DP) | R: 348.7 ± 121.8 L: 258 ± 118.6d | R: 372.0 ± 341.5 L: 420.2 ± 445.5d | NR | R: 2 (10.0%) L: 4 (16.0%) | R: 0 L: 0 | R: 7.1 ± 2.2 L: 7.3 ± 3.0d | NR | NR | R: 19/20 (95.0%) L: 16/25 (64.0%) |
| Kang et al.21 (CP) | R: 432.0 ± 65.7 O: 286.5 ± 90d | R: 275.0 ± 221.7 O: 858.3 ± 490d | 0 | R: 1 (20.0%) O: 5 (50.0%) | R: 0 O: 0 | R: 14.6 ± 7.7 O: 22.1 ± 13.3d | NR | NR | N/A |
| Narula et al.24 | 420 (360–510)e | NR | 3/8 (37.5%) | 0 | 0 | 9.6c | 0 | 16c | N/A |
| Waters et al.17 | R: 298 (191–418) L: 224 (100–346) O: 234 (136–437)e | R: 279 (20–1200) L: 667 (50–7000) O: 681 (50–3300)e | R: 2 (11.8%) L: 2 (11.1%) | R: 3 (17.6%) L: 6 (33.3%) O: 4 (18.2%) | R: 0 L: 0 O: 0 | R: 3.8 (2–6) L: 6.4 (3–34) O: 7.7 (3–25)e | R: 0 L: 0 O: 2 (9.1%) | R: 5 L: 11 O: 14c | R: 11/17 (64.7%) L: 5/18 (27.7%) O: 3/22 (13.6%) |
| Zeh et al.19 | 568 (536–629)g | 350 (150–625)g | 8 (16.0%) | 28 (56.0%) | 1 (2.0%) | 10 (8–13)g | 4 (10.8%)a | 17 ± 7da | N/A |
| Zhou et al.22 | R: 718.8 ± 186.7 O: 420.0 ± 127.2d | R: 153.8 ± 43.4 O: 210 ± 53.2d | 0 | R: 2 (25.0%) O: 6 (75.0%) | R: 0 O: 1 (12.5%) | R: 16.4 ± 4.1 O: 24.3 ± 7.1d | R: 0 O: 1 (16.7%)b | NR | N/A |
In 37 malignant tumours.
In six malignant tumours.
Mean.
Mean ± standard deviation.
Mean (range).
Median (range).
Median (interquartile range).
R, robot-assisted; L, laparoscopic; O, open; DP, distal pancreatectomy; PD, pancreatoduodenectomy; NR, not reported; N/A, not applicable.
A total of 27 of the 254 (10.6%) robot-assisted procedures had to be converted to open surgery and one (0.4%) was converted to conventional laparoscopic surgery.17 Reasons for conversion were: bleeding (n = 1),24 arterial/venous abutment/infiltrations (n = 9),19,23 local advancement in non-vascular structures (n = 2),18,23 difficult dissection (n = 14),17,19,23,24 hypercapnia (n = 1)23 and malfunction of the robot (n = 1).23
Complications occurred in 77 of 251 patients (30.7%). One study reported the total complication rate, but did not report types of complication.20 In the 231 patients included in the remaining seven studies, 46 incidences of pancreatic fistula were identified (19.9%). Three studies used the ISGPF classification scheme in at least a proportion of patients (n = 112). When grade B and C fistulae only were considered, the fistula rate amounted to 11.6% (13 of 112 patients). Delayed gastric emptying occurred in 13 of 231 patients (5.6%). The study using the ISGPS classification (which included 10 patients with DGE) reported that six patients experienced grade B DGE and four experienced grade C events.19
Other complications included pulmonary complications (n = 13, 5.6%), bleeding (n = 10, 4.3%), intra-abdominal fluid collection (n = 9, 3.9%), cardiac complications (n = 7, 3.0%), thromboembolic events (n = 5, 2.2%), wound infection (n = 5, 2.2%), urinary tract infection (n = 4, 1.7%), gastrointestinal complications (n = 4, 1.7%), biliary leak (n = 1, 0.4%) and requirements for reoperation (n = 8, 3.5%). Four of 251 patients (1.6%) died. The cause of death was reported in three patients and included sepsis following Boerhaave syndrome (n = 1),23 colonic ischaemia (n = 1)23 and multi-system organ failure (n = 1).19 The latter occurred in a patient with an ISGPF grade C fistula, who experienced a complicated postoperative course not directly related to the pancreatic fistula.19
Weighted mean hospital LoS was 14.2 ± 10.7 days.
Five studies reported pathological margin status in a total of 127 patients (Table 3).17,19,22–24 The number of resected lymph nodes was described in four series.17,19,23,24 One of the series reported these pathological findings only in patients undergoing pancreatoduodenectomy.23 Two of the five studies reported findings of positive margins in the robot-assisted surgery group (nine of 127 patients, 7.1%).19,23 The weighted mean number of lymph nodes harvested was 15.3 ± 7.6. All reported positive surgical margins were found in patients undergoing pancreatoduodenectomy.
Only two studies included in the current systematic review reported on costs. Kang et al. reported mean per patient costs of US$8305 ± 870 for robot-assisted distal pancreatectomy and US$3862 ± 1724 for conventional laparoscopic surgery in South Korea.20 Waters et al., who primarily addressed the cost-effectiveness of robot-assisted distal pancreatectomy, reported the direct costs of the operation and the entire hospital stay in a US centre and cited mean per patient costs of US$11 904 for robot-assisted surgery, US$12 900 for laparoscopic surgery and US$15 521 for open surgery. This suggests that direct costs were comparable between the particular approaches.17
Median follow-up was reported in two studies,21,24 which cited a maximum duration of 19 months (range: 16–24 months).21 Giulianotti et al. reported follow-up periods in particular subgroups; the longest period mentioned was 106 months.23
Pancreatoduodenectomy
Outcomes of pancreatoduodenectomies were analysed separately. One study reported outcomes of pancreatoduodenectomies and other types of resection together18 and another study did not separate morbidity and mortality in pancreatoduodenectomy from those in other procedures.23 The authors of these studies were contacted and provided additional data.
In the 131 pancreatoduodenectomies, weighted mean operation time was 510 ± 107 min and weighted mean blood loss was 440 ± 254 ml (Table 4). Twenty-two of 134 intended robot-assisted procedures were converted to open surgery (16.4%). Complications occurred in 51 patients (38.9%) and three patients died (2.3%). Fistulae occurred in 34 patients (26.0%). Weighted mean LoS was 15.9 ± 16.7 days. Positive surgical margins were found in 10 of 115 patients in whom pathological margin status was reported (8.7%). The number of lymph nodes harvested was reported for 110 patients to give a weighted mean of 16 ± 8 lymph nodes.
Table 4.
Outcomes of robot-assisted pancreatoduodenectomy
| Authors | Relevant patientsa, n | Operation time, min | Blood loss, ml | Conversion rate | Patients with ≥1 complications, n (%) | Postoperative mortality, n (%) | Hospital stay, days | Positive surgical margins | Lymph node harvested |
|---|---|---|---|---|---|---|---|---|---|
| Chan et al.18 | 8 | 550.8 ± 119.7e | 156.3 ± 101.6e | 0 | 3 | 0 | 13.1 ± 4.6e | 4b | 12 ± 6e |
| Giulianotti et al.23 | 60 | 421 (240–660)f | 394 (80–1500)f | 11 (18.3%) | 21 (35.0%) | 2 (3.3%) | 17.7 (5–85)f | 5 (21%) | Italy: 21 (5–37) USA: 14 (12–45)f |
| Narula et al.24 | 5 | 420 (360–510)f | NR | 3/8 (37.5%) | 0 | 0 | 9.6d | 0 | 16d |
| Zeh et al.19 | 50 | 568 (536–629)g | 350 (150–625)g | 8 (16.0%) | 28 (56.0%) | 1 (2.0%) | 10 (8–13)g | 4 (10.8%)c | 17 ± 7eb |
| Zhou et al.22 | 8 | 718.8 ± 186.7e | 153.8 ± 43.4e | 0 | 2 (25.0%) | 0 | 16.4 ± 4.1e | 0 | NR |
Relevant patients: patients undergoing robot-assisted pancreatoduodenectomy.
In five malignant tumours.
In 37 malignant tumours.
Mean.
Mean ± standard deviation.
Mean (range).
Median (interquartile range).
NR, not reported.
Distal pancreatectomy
Four studies reported on distal pancreatectomy,17,18,20,23 but only two reported outcomes for this type of resection separately (including in 37 of the 85 patients undergoing robot-assisted distal pancreatectomy).17,20 Authors were contacted and provided additional data.18,23 Weighted mean operating time was 281 ± 102 min and weighted mean blood loss was 251 ± 317 ml. Three studies (including 65 patients) provided conversion rates; four resections were converted to open surgery and one to a conventional laparoscopic resection (7.7%).17,18,23 Fifteen patients suffered complications (17.6%). In the studies that provided data on pancreatic fistulae, the rate of fistulae was 16.1% (10 of 62 patients).17,18,23 There was no mortality and weighted mean hospital LoS was 6.0 ± 2.0 days. Two studies (n = 19) provided data on pathological findings; these reported no positive surgical margins. The study reporting the number of lymph nodes harvested (n = 17) cited a mean of five lymph nodes. All studies describing distal pancreatectomy reported on the percentage of spleen preservation: the spleen was preserved in 74 of 85 distal pancreatectomies (87.1%).
Discussion
The combined data provided by the eight observational cohort studies that fulfilled the criteria for inclusion in this systematic review suggest that robot-assisted pancreatic surgery is feasible and can be performed safely in selected patients.
As robot-assisted pancreatic surgery was introduced to potentially improve current outcomes of surgery, the findings of this systematic review were compared with outcomes reported for open and laparoscopic pancreatic surgery. Table 5 summarizes outcomes of recent studies of open and laparoscopic pancreatic surgery. Compared with open or laparoscopic approaches, operation times are longer in robot-assisted surgery. Hospital stay may be shorter in robot-assisted resections than in open or laparoscopic surgery. Conversion rates do not seem to be higher in robot-assisted approaches than in conventional laparoscopic pancreatic surgery.
Table 5.
Outcomes of open and laparoscopic pancreatic surgery in international centres of excellence
| Authors | Study design | Patients, n | Type of procedure | Operation time, min | Blood loss, ml | Conversion rate | Hospital stay, days | Morbidity, n (%) | Mortality, n (%) | Spleen preservation in DP |
|---|---|---|---|---|---|---|---|---|---|---|
| Cameron et al. (2006)28 | Retrospective observational cohort study | 1970s: 2 1980s: 63 1990s: 587 2000s: 347 | Open PD | 1970s: 528 1980s: 378 1990s: 366 2000s: 330f | 1970s: 1090 1980s: 900 1990s: 700 2000s: 700f | N/A | 1980s: 17 1990s: 11 2000s: 9f | 41% | 10 (1%) | N/A |
| Winter et al. (2006)2 | Retrospective observational cohort study | 1175 | Open PD | 380 (200–790)e | 800 (150–15 000)e | N/A | 9 (4–375)e | 415 (38%) | 26 (2%) | N/A |
| Diener et al. (2011)27 | Randomized controlled trial | 352 | Open DP | 190.0 ± 80.5c | NR | N/A | 15.4 ± 14.7c | 157 (45%) | 3 (1%) | 59 (17%) |
| Gagner et al. (2009)8 | Review | 146 | Laparoscopic PD | 439.3 (284–660)d | 142.8 (50–770)d | 46% | 18 (7–39)d | 23 (16%) | 2 (1.3%) | N/A |
| Borja-Cacho et al. (2009)7 | Systematic review | 806 | Laparoscopic DP | 199.1 ± 83.5c | 235.7 ± 42.9c | 74 (9.2%) | 6.6 ± 4.1c | 284 (37.6%) | 2 (0.2%) | 400 (49.6%) |
| Present study | Systematic review | PD: 131 DP: 85 | PD and DP | PD: 510 ± 107c DP: 281 ± 102c | PD: 440 ± 254c DP: 251 ± 317c | PD: 22 (16.4%)a DP: 5 (7.7%)b | PD: 15.9 ± 16.7c DP: 6.0 ± 2.0c | PD: 51 (38.9%) DP: 15 (17.6%) | PD: 3 (2.3%) DP: 0 | PD: N/A DP: 74 (87.1%) |
Of 134 intended robot-assisted procedures.
Data for 65 patients.
Mean ± standard deviation.
Mean (range).
Median (range).
Median.
PD, pancreatoduodenectomy; DP, distal pancreatectomy; N/A, not applicable; NR, not reported.
Reported morbidity rates in open and laparoscopic surgery range from 16% to 45% and mortality rates range between 1% and 2%.2,7,8,27,28 In the studies included in the current review, complications occurred in 30.7% of patients and mortality in 1.6%. This suggests that in selected patients, morbidity and mortality rates in robot-assisted pancreatic surgery are comparable with those in laparoscopic and open surgery. The most common complication in the current study was pancreatic fistula, which occurred at a rate of 19.9%. When only fistulae of ISGPF grades B and C were considered, the rate of occurrence was 11.6%. Other large open and laparoscopic series showed rates of pancreatic fistulae of 12–36%.1,7,27 Only one study included in this review reported on the texture of the pancreas.19 As a soft pancreatic remnant is associated with a higher risk for fistula,29 this may have been important in evaluating the risk for pancreatic fistula.
Four of the studies in this review directly compared the outcomes of robot-assisted surgery with those of open and/or laparoscopic pancreatic surgery in a non-randomized fashion (Table 3).17,20,21,22 All studies showed longer operation times in robot-assisted than in open or laparoscopic surgery. Less blood loss21,22 and shorter hospital LoS17,22 were also observed. In distal pancreatectomies, rates of spleen preservation were higher in robot-assisted surgery than in open or laparoscopic approaches. One study cited spleen preservation rates of 95% in robot-assisted and 64% in laparoscopic surgery,20 and another cited rates of 65% in robot-assisted, 28% in laparoscopic and 14% in open surgery.17
With reference to comparisons of the outcomes of robot-assisted surgery with those of open or laparoscopic surgery, it should be noted that the studies on robot-assisted surgery report early experiences with this technique. This may imply that healthier patients with easier tumours were selected for robot-assisted surgery. Therefore, the resulting outcomes may be more favourable than they would have been in an unselected patient group. However, there is probably a considerable learning curve involved in the evolution of experience in robot-assisted pancreatic resections.24 Learning curves have also been reported in other types of robot-assisted surgery.12,30 Zeh et al. compared the outcomes of a first set of 20 pancreatoduodenectomies with those of a second set of 22 operations.31 In the latter group they observed reductions of 295 ml in perioperative blood loss and 3 days in hospital LoS compared with the first group. The rate of pancreatic fistula decreased from 25% to 9%. Operation time, however, did not decrease.31 Other data suggest that the set-up and docking time required in robot-assisted procedures can decrease as experience with the system increases. Iranmanesh et al. showed that over the course of 96 procedures, set-up time decreased from 35 min to 15 min.32 When docking was carried out by inexperienced surgeons (who had performed fewer than five dockings), median docking time was 17.5 min (range: 10–70 min). Experienced surgeons (who had performed more than 12 dockings) demonstrated a median docking time of 8 min (range: 2–50 min).32
The promising outcomes of robot-assisted surgery shown in this systematic review suggest that this approach offers the advantages associated with minimally invasive surgery, such as decreased pain and blood loss, fewer complications, faster recovery and a shorter hospital LoS.4,5 Moreover, robot-assisted procedures provide potential advantages over conventional laparoscopic surgery: there is increased freedom of movement of surgical instruments; tremor is eliminated, and 3D vision of the operative field is available. This leads to more accurate movements such as in resecting and suturing. Another important advantage refers to the ergonomic benefits afforded to the surgeon by a robot-assisted system.
There are several limitations to robot-assisted surgery. Firstly, there is a lack of tactile feedback,19,33 although it has been suggested that this is compensated for by improved visual feedback.23 Secondly, it may be difficult to operate in multiple quadrants of the abdomen because of the risk for interference by the robotic arms. The configuration of the ports can minimize arm interference.19 Thirdly, robot-assisted surgery is probably associated with longer operation time. Finally, robot-assisted surgery may incur greater costs.
The shortcomings of the current systematic review include the relatively small numbers of patients reported in the individual studies included, which derive from single-institution series. Only 17 articles reporting outcomes in more than five robotically operated patients were identified. The number of centres performing robot-assisted pancreatic surgery is also limited. Of 19 potentially relevant articles, 14 were published by only three centres.
Another drawback may refer to the differences among the studies in how outcomes were reported. Most of the studies did not define outcomes; when outcomes were defined, definitions differed across the studies. This makes it difficult to compare the individual studies and to make comparisons between robot-assisted surgery and other techniques.
The studies included in this review used different techniques: not all resections were performed completely robotically. For example, in some studies a hybrid approach that incorporated both robot-assisted and conventional laparoscopic techniques was used, or hand assistance was applied. This was not clearly reported in most of the studies.
Most importantly, there is a probable selection bias in studies reporting on robot-assisted surgery. Some of the studies explicitly reported that patients were selected for robot-assisted approaches. For example, one study cited an established diagnosis of adenocarcinoma as a reason for exclusion.24 Other studies operated only in patients with benign or borderline malignant lesions.20,21
The initial concerns about the oncological outcomes of robot-assisted surgery are not supported by the literature. In a large series of patients undergoing open pancreatoduodenectomy, 9% of resections resulted in positive margins.34 Systematic reviews of conventional laparoscopic resections have reported findings of histopathologically positive margins in 0.4% (varying from 0% to 11% in the individual studies in the review) of patients undergoing pancreatoduodenectomy9 and 6% of patients undergoing distal pancreatectomy.7 In the current systematic review, positive margins were reported in 7.1% of all resections, 8.7% of pancreatoduodenectomies and 0% of distal pancreatectomies (although these data were provided for only 19 of 85 patients undergoing distal pancreatectomy).
Given the oncological outcomes, it should be noted that comparisons of pathological margins are problematic because reported outcomes are strongly dependent on the definitions used in the different studies.35 Moreover, there is a potential selection bias and studies reported conversions that occurred for oncological reasons. In addition, the short follow-up time makes it difficult to assess the oncological safety of robot-assisted pancreatic surgery.
In conclusion, robot-assisted pancreatic surgery seems to be safe and feasible in selected patients. In distal pancreatectomy, it may increase the rate of spleen preservation compared with open and laparoscopic approaches. However, studies with larger numbers of patients, longer follow-up periods and validated definitions, such as the ISGPS definitions for complications, are needed. To assess the potential benefits of robot-assisted surgery, future studies should compare the results of robot-assisted pancreatic surgery with those of laparoscopic and open resections and should include oncological outcomes among their data.
Acknowledgments
The authors would like to thank Herbert J. Zeh III, md, Department of Surgery, University of Pittsburgh, Pittsburgh, PA, USA, for his careful review of the manuscript and Dr O. C. Y. Chan, Department of Surgery, Pamela Youde Nethersole Eastern Hospital, Hong Kong and Dr P. C. Giulianotti, Department of Surgery, University of Illinois at Chicago, Chicago, IL, USA, for providing additional data from their studies.
Conflicts of interest
None declared.
References
- 1.Kleeff J, Diener MK, Z'graggen K, Hinz U, Wagner M, Bachmann J, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245:573–582. doi: 10.1097/01.sla.0000251438.43135.fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 3.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 4.Schwenk W, Haase O, Neudecker J, Müller JM. Short-term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;(3) doi: 10.1002/14651858.CD003145.pub2. CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keus F, Gooszen HG, van Laarhoven CJ. Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD008318. CD008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc. 2011;25:2084–2099. doi: 10.1007/s00464-010-1538-4. [DOI] [PubMed] [Google Scholar]
- 7.Borja-Cacho D, Al-Refaie WB, Vickers SM, Tuttle TM, Jensen EH. Laparoscopic distal pancreatectomy. J Am Coll Surg. 2009;209:758–765;. doi: 10.1016/j.jamcollsurg.2009.08.021. quiz 800. [DOI] [PubMed] [Google Scholar]
- 8.Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg. 2009;16:726–730. doi: 10.1007/s00534-009-0142-2. [DOI] [PubMed] [Google Scholar]
- 9.Gumbs AA, Rodriguez Rivera AM, Milone L, Hoffman JP. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol. 2011;18:1335–1341. doi: 10.1245/s10434-010-1503-4. [DOI] [PubMed] [Google Scholar]
- 10.Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, et al. Left-sided pancreatectomy: a multicentre comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438–446. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 11.Horgan S, Berger RA, Elli EF, Espat NJ. Robotic-assisted minimally invasive transhiatal oesophagectomy. Am Surg. 2003;69:624–626. [PubMed] [Google Scholar]
- 12.Boone J, Schipper ME, Moojen WA, Borel Rinkes IH, Cromheecke GJ, van Hillegersberg R. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg. 2009;96:878–886. doi: 10.1002/bjs.6647. [DOI] [PubMed] [Google Scholar]
- 13.Melvin WS, Needleman BJ, Krause KR, Ellison EC. Robotic resection of pancreatic neuroendocrine tumour. J Laparoendosc Adv Surg Tech A. 2003;13:33–36. doi: 10.1089/109264203321235449. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxford Centre for Evidence-Based Medicine Levels of Evidence Working Group. 2012. The Oxford 2011 Levels of Evidence. Available at: http://www.cebm.net/index.aspx?o=5653 (last accessed 12 March 2012)
- 17.Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, Aguilar-Saavedra JR, et al. Robotic distal pancreatectomy: cost-effective? Surgery. 2010;148:814–823. doi: 10.1016/j.surg.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Chan OC, Tang CN, Lai EC, Yang GP, Li MK. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci. 2011;18:471–480. doi: 10.1007/s00534-011-0389-2. [DOI] [PubMed] [Google Scholar]
- 19.Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol. 2012;19:864–870. doi: 10.1245/s10434-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 20.Kang CM, Kim DH, Lee WJ, Chi HS. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc. 2011;25:2004–2009. doi: 10.1007/s00464-010-1504-1. [DOI] [PubMed] [Google Scholar]
- 21.Kang CM, Kim DH, Lee WJ, Chi HS. Initial experiences using robot-assisted central pancreatectomy with pancreaticogastrostomy: a potential way to advanced laparoscopic pancreatectomy. Surg Endosc. 2011;25:1101–1106. doi: 10.1007/s00464-010-1324-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhou NX, Chen JZ, Liu Q, Zhang X, Wang Z, Ren S, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot. 2011;7:131–137. doi: 10.1002/rcs.380. [DOI] [PubMed] [Google Scholar]
- 23.Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–1657. doi: 10.1007/s00464-009-0825-4. [DOI] [PubMed] [Google Scholar]
- 24.Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas. 2010;39:160–164. doi: 10.1097/MPA.0b013e3181bd604e. [DOI] [PubMed] [Google Scholar]
- 25.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomized, controlled multicentre trial. Lancet. 2011;377:1514–1522. doi: 10.1016/S0140-6736(11)60237-7. [DOI] [PubMed] [Google Scholar]
- 28.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232:419–429. doi: 10.1097/00000658-200009000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayn MH, Hussain A, Mansour AM, Andrews PE, Carpentier P, Castle E, et al. The learning curve of robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol. 2010;58:197–202. doi: 10.1016/j.eururo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Zeh HJ, III, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg. 2011;45:323–340. doi: 10.1016/j.yasu.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Iranmanesh P, Morel P, Wagner OJ, Inan I, Pugin F, Hagen ME. Set-up and docking of the da Vinci surgical system: prospective analysis of initial experience. Int J Med Robot. 2010;6:57–60. doi: 10.1002/rcs.288. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Kang CM, Lee WJ, Chi HS. The first experience of robot-assisted spleen-preserving laparoscopic distal pancreatectomy in Korea. Yonsei Med J. 2011;52:539–542. doi: 10.3349/ymj.2011.52.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2011;(5) doi: 10.1002/14651858.CD006053.pub4. CD006053. [DOI] [PubMed] [Google Scholar]
- 35.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 36.Kang CM, Choi SH, Hwang HK, Lee WJ, Chi HS. Minimally invasive (laparoscopic and robot-assisted) approach for solid pseudopapillary tumour of the distal pancreas: a single-centre experience. J Hepatobiliary Pancreat Sci. 2011;18:87–93. doi: 10.1007/s00534-010-0316-y. [DOI] [PubMed] [Google Scholar]
- 37.Kang CM, Kim DH, Lee WJ. Ten years of experience with resection of left-sided pancreatic ductal adenocarcinoma: evolution and initial experience to a laparoscopic approach. Surg Endosc. 2010;24:1533–1541. doi: 10.1007/s00464-009-0806-7. [DOI] [PubMed] [Google Scholar]
- 38.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- 39.Giulianotti PC, Addeo P, Buchs NC, Ayloo SM, Bianco FM. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumours. Pancreas. 2011;40:1264–1270. doi: 10.1097/MPA.0b013e318220e3a4. [DOI] [PubMed] [Google Scholar]
- 40.Giulianotti PC, Addeo P, Buchs NC, Bianco FM, Ayloo SM. Early experience with robotic total pancreatectomy. Pancreas. 2011;40:311–313. doi: 10.1097/MPA.0b013e3181f7e303. [DOI] [PubMed] [Google Scholar]
- 41.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 42.Buchs NC, Addeo P, Bianco FM, Ayloo S, Elli EF, Giulianotti PC. Safety of robotic general surgery in elderly patients. J Robot Surg. 2010;4:91–98. doi: 10.1007/s11701-010-0191-1. [DOI] [PubMed] [Google Scholar]
- 43.Buchs NC, Addeo P, Bianco FM, Gangemi A, Ayloo SM, Giulianotti PC. Outcomes of robot-assisted pancreaticoduodenectomy in patients older than 70 years: a comparative study. World J Surg. 2010;34:2109–2114. doi: 10.1007/s00268-010-0650-x. [DOI] [PubMed] [Google Scholar]
- 44.Zureikat AH, Nguyen KT, Bartlett DL, Zeh HJ, Moser AJ. Robotic-assisted major pancreatic resection and reconstruction. Arch Surg. 2011;146:256–261. doi: 10.1001/archsurg.2010.246. [DOI] [PubMed] [Google Scholar]
- 45.Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg. 2011;35:2739–2746. doi: 10.1007/s00268-011-1276-3. [DOI] [PubMed] [Google Scholar]


