Abstract
Background
By 2033, the number of people aged 85 years and over in the UK is projected to double, accounting for 5% of the total population. It is important to understand the surgical outcome after a pancreatic resection in the elderly to assist decision making.
Methods
Over a 9-year period (from January 2000 to August 2009), 428 consecutive patients who underwent a pancreatic resection were reviewed. Data were collected on mortality, complications, length of stay and survival. Patients were divided into two groups (younger than 70 and older than 70 years old) and outcomes were analysed.
Results
In all, 119 (27.8%) patients were ≥ 70 years and 309 (72.2%) patients were < 70 years. The median length of stay for the older and younger group was 15 days (range 3–91) and 14 days (range 3–144), respectively. The overall mortality was 3.4% in the older group and 2.6% in the younger group (P = 0.75). The older cohort had a cumulative median survival of 57.3 months (range 0–119), compared with 78.7 months (range 0–126) in the younger cohort (P < 0.0001). In patients undergoing a pancreatic resection for ductal adenocarcinoma and cholangiocarcinoma there was a significant difference in survival with P-values of 0.043 and 0.003, respectively. For ampullary adenocarcinoma, the older group had a median survival of 47.1 months compared with 68.3 months (P = 0.194).
Conclusion
Results from this study suggest that while elderly patients can safely undergo a pancreatic resection and that age alone should not preclude a pancreatic resection, there is still significant morbidity and mortality in the octogenarian subgroup with poor long-term survival with the need for quality-of-life assessment.
Introduction
The demographics of the UK population are changing with the fastest change in those aged 85 years and over. By 2033, the number of people aged 85 years and over is projected to be more than double, accounting for 5% of the total population.1 Over the last 30 years the number of centenarians (people aged 100 years or more) in the UK has increased five-fold from 2500 in 1980 to 12 640 in 2010.2 Similarly, the eldest elderly group are expected to account for 24% of the elderly population and 5% of the overall American population by 2050.3
Pancreatic cancer studies show that the age-specific incidence rates are higher in men from age 40 years onwards and that the incidence rates rise dramatically from age 65years.4 Pancreatic cancer risk is very strongly related to age with 85% of patients being over 60 years at time of diagnosis.4 Therefore, it is important to understand the surgical outcome in the elderly population to assist surgical decision making. Over the last decade, outcomes after a pancreatic resection have improved with most studies reporting mortality rates of less than 5%.5–7,10,12,23 Recently, studies have tried to show that age alone should not be a contraindication to pancreatic resection.12 However, the age groups analysed have varied among the studies, making it difficult to compare and reach viable conclusions.8–13
The majority of the studies report statistically higher morbidity rates in the designated older patients when compared with younger patients.8–11 When analysing mortality, some studies show no difference12 whereas other groups report higher mortality in the older group.11,13
Studies have shown that almost 40% of the patients referred to specialist centres fall into the 70 years or older category.8,14 In an attempt to determine whether pancreatic resection is justified in elderly patients, this study retrospectively reviewed the pancreatic resection database of a single tertiary pancreatic unit to compare outcomes with their younger counterparts with relation to mortality, long-term survival, length of hospital stay and morbidity.
Methods
Study population
Over a 9-year period (from January 2000 to August 2009), 428 pancreatic resections were carried out at a single tertiary pancreatic unit. They were retrospectively analysed using a prospectively collected database. The patients were divided into those aged 70 years and older and those younger than 70 years, and the demographics, post-operative diagnosis, tumour characteristics and outcomes of the two groups studied were compared. There was further sub group analysis for malignant cases divided in decades of < 60, 60–69, 70–79 and >80 years.
The surgical resections studied were: pancreaticoduodenectomy, distal pancreatectomy, subtotal pancreatectomy, total pancreatectomy, central pancreatectomy, duodenal sparing pancreatectomy and enucleation.
All pathology specimens were reviewed by a single pathologist to determine the primary histopathological diagnosis and the extent of the disease: TNM status, resection margins and portal vein invasion. This was done using the Leeds standardized Pathology Protocol (LEEPP).15,16
Peri-operative mortality was defined as in-hospital death or death within 30 days of surgery. The specific complications studied included: delayed gastric emptying, pancreatic fistula formation, intra-abdominal collection, cholangitis, pneumonia, wound infection, peptic ulceration, upper gastrointestinal bleeding and cardiac events. The complications were divided into five grades (I to V) according to the Dindo–Clavien classification.17,18
Follow-up
All patients with malignant pathology were followed up on a 6-monthly basis for 3 years and annually thereafter until they had disease recurrence or died. There was a prospectively collected follow-up database. These patients had tumour markers checked at each clinic visit and a computed tomography scan only if they were elevated or was clinically indicated. Data were also collected on patients undergoing chemotherapy, whether adjuvant or palliative.
Statistical analysis
Statistical analyses were carried out using Excel (Microsoft, Redmond, WA, USA) and XLSTAT (Addinsoft, New York, NY, USA). The statistical analyses of categorical variables were performed using Fisher's exact test. Comparisons between different groups were made using the Mann–Whitney U-test. Survival curves for the two groups were generated using the Kaplan–Meier method. Differences in long-term survival between subgroups were compared using the log-rank test. P-values less than 0.05 were considered significant.
Results
Patient characteristics
Between January 2000 to August 2009, 428 patients underwent a major pancreatic resection for a variety of malignant and benign disease. The median age of the entire cohort was 64 years (range 15 to 86). The demographic data, including age, gender, initial presentation and type of resection, are shown in Table 1. There was no statistically significant difference in the age, gender, aetiology or type of resection carried out between the two age groups.
Table 1.
Demographic data including age, gender, initial presentation and type of resection of entire cohort (% in brackets)
| Characteristics | Younger than 70 years | Older than 70 years | P-value |
|---|---|---|---|
| Age distribution | 309 | 119 | |
| Gender | |||
| Male | 170 (55) | 57 (48) | 0.196 |
| Female | 139 (45) | 62 (52) | |
| Initial Presentation | |||
| Jaundice | 111 (36) | 52 (44) | 0.149 |
| Pain and Jaundice | 12 (4) | 6 (5) | |
| Weight loss | 13 (4) | 8 (7) | |
| Incidental | 26 (8) | 16 (13) | |
| Others | 50 (16) | 12 (10) | |
| Not available | 97 (31) | 25 (21) | |
| Procedure | |||
| Pancreaticoduodenectomy | 223 (74) | 95 (80) | 0.066 |
| Distal pancreatectomy | 59 (19) | 21 (18) | |
| Total pancreatectomy | 5 (2) | 0 (0) | |
| Subtotal pancreatectomy | 6 (2) | 0 (0) | |
| Completion pancreatectomy | 2 (1) | 0 (0) | |
| Central pancreatectomy | 1 (1) | 0 (0) | |
| Duodenal Sparing pancreatectomy | 6 (2) | 1 (1) | |
| Enucleation | 6 (2) | 1 (1) | |
Pathology
Of the 119 pancreatic resections performed in the older age group, 100 (84.0%) were for malignant disease compared wuth 230 (74.4%) in the younger group (P = 0.057). The data representing post-operative pathological diagnosis are shown in Table 2. The TNM and resection margins status for malignant cases are summarized in Table 3. There was no significant difference between the two groups with relation to the proportion of node positive disease or the T stage.
Table 2.
Comparison of the pathological findings for the entire cohort (% in brackets)
| Characteristics | Total | Younger than 70 years | Older than 70 years | P-value |
|---|---|---|---|---|
| (n = 428) | (n = 309) | (n = 119) | ||
| Malignant histopathology | ||||
| Ductal adenocarcinoma | 85 | 56 (18) | 29 (24) | 0.057 |
| Cholangiocarcinoma | 60 | 42 (14) | 18 (15) | |
| Ampullary carcinoma | 58 | 36 (12) | 22 (18) | |
| IPMN | 34 | 23 (7) | 11 (9) | |
| Neuroendocrine | 30 | 27 (9) | 3 (3) | |
| Mucinous cystic neoplasms | 14 | 7 (2) | 7 (6) | |
| Solid pseudopapillary | 8 | 8 (3) | 0 (0) | |
| Duodenal adenocarcinoma | 7 | 5 (2) | 2 (2) | |
| Adenosquamous | 6 | 2 (1) | 4 (3) | |
| Acinar cell carcinoma | 6 | 5 (2) | 1 (1) | |
| Other malignant | 22 | 19 (6) | 3 (3) | |
| Benign Histopathology | ||||
| Chronic pancreatitis | 25 | 24 (8) | 1 (1) | 0.060 |
| Cystadenomas | 20 | 10 (3) | 10 (8) | |
| Groove pancreatitis | 10 | 10 (3) | 0 (0) | |
| Other benign | 43 | 35 (11) | 8 (7) | |
IPMN, intraductal papillary mucinous neoplasm.
Table 3.
Tumour characteristics for the entire cohort (% in brackets)
| Characteristics | Younger than 70 years | Older than 70 years | P-value |
|---|---|---|---|
| Tumour classification | |||
| T1 | 1 (1) | 2 (2) | 0.275 |
| T2 | 16 (10) | 15 (18) | 0.105 |
| T3 | 122 (79) | 57 (70) | 0.152 |
| T4 | 16 (10) | 8 (10) | 1.000 |
| Node status | |||
| N0 | 31 (20) | 20 (24) | 0.509 |
| N1 | 126 (80) | 64 (76) | 0.509 |
| Resection margins | |||
| R0 | 42 (29) | 36 (44) | 0.020 |
| R1 | 102 (69) | 45 (56) | 0.043 |
| R2 | 3 (2) | 0 (0) | 0.554 |
Morbidity and mortality
Of the 428 patients that underwent a pancreatic resection, 12 died peri-operatively (2.8%). There was no significant difference between the two age groups when comparing post-operative morbidity, post-operative mortality and median hospital stay as shown in Table 4.
Table 4.
Post-operative course
| Postoperative course | Older than 70 years (119) | Younger than 70 years (309) | P-value |
|---|---|---|---|
| Mortality | |||
| Yes | 4 | 8 | 0.745 |
| No | 115 | 301 | |
| Overall complications | |||
| Yes | 15 | 65 | 0.052 |
| No | 104 | 244 | |
| Grade of complications | |||
| Grade I | 0 | 3 | |
| Grade II | 5 | 24 | |
| Grade III | 6 | 27 | |
| Grade IV | 0 | 3 | |
| Grade V | 4 | 8 | |
| Post-operative length of stay (days) | |||
| Average | 19 ± 15.1 | 21 ± 21.2 | 0.47 |
| Median | 15 (3 to 91) | 14 (3 to 144 days) | |
Chemotherapy
Table 5 shows the patients who underwent adjuvant or palliative chemotherapy. Although the difference did not meet statistical significance, a greater number of younger people underwent adjuvant chemotherapy than the elderly (19.6% vs. 15%).
Table 5.
Adjuvant and palliative chemotherapy for the entire malignant cohort (n = 330)
| Chemotherapy | Total | Younger than 70 years | Older than 70 years | P-value |
|---|---|---|---|---|
| n = 89/330 (27.0) | n = 65/230 (28.3) | n = 24/100 (24.0) | 0.509 | |
| Adjuvant only | 60 | 45 (19.6) | 15 (15.0) | 0.355 |
| 5FU and FA only | 36 | 13 | ||
| 5FU/FA and Cisplatin | 2 | 0 | ||
| 5FU/FA and Mitomycin | 1 | 0 | ||
| Gemcitabine only | 3 | 1 | ||
| Capecitabine only | 3 | 1 | ||
| Palliative | 29 | 20 (8.7) | 9 (9.0) | 1.000 |
| Adjuvant 5FU/FA and palliative gemcitabine | 4 | 1 | ||
| Adjuvant 5FU/FA and palliative Capecitabine | 1 | 1 | ||
| Adjuvant 5FU/FA and palliative gemcitabine and Cisplatine | 1 | 0 | ||
| Adjuvant Gemcitabine and palliative gemcitabine and Cisplatine | 1 | 0 | ||
| Gemcitabine only | 7 | 4 | ||
| Gemcitabine and Capecitabine | 2 | 1 | ||
| Gemcitabine and Cisplatin | 3 | 1 | ||
| Cisplatin and Eposide | 1 | 0 | ||
| Modified de Gramont | 0 | 1 | ||
Survival analysis
The older cohort had a cumulative median survival of 57.3 months (range 0–119), compared with a median survival of 78.7 months (range 0–126) in the younger cohort (P < 0.001) as seen in Fig. 1.
Figure 1.
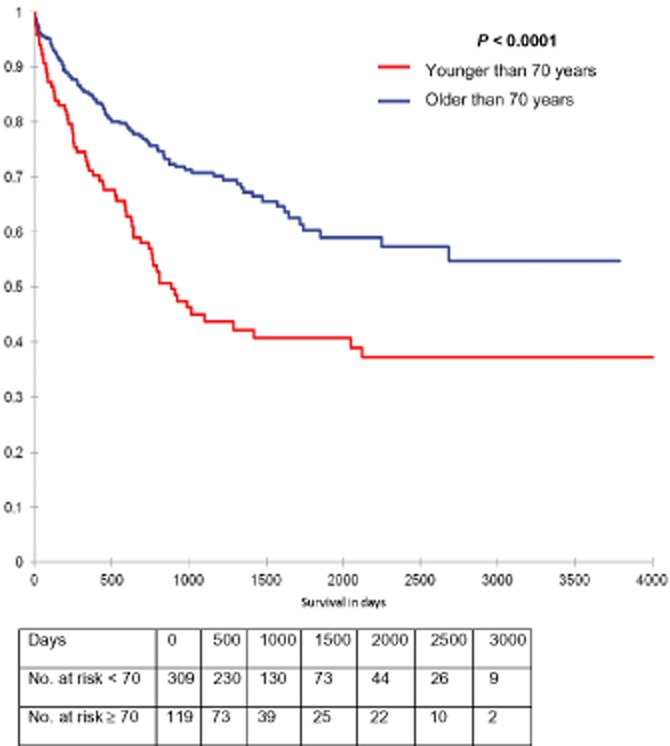
Survival curve comparing all patients 70 years of age and older undergoing a pancreatic resection compared with patients younger than 70 years old
Long-term survival was compared between different age groups. Patients younger than 60 years old (n = 167) had a median survival time of 79.6 months, between 60 and 69 years old (n = 143) had a median survival of 73.5 months, and between 70 and 79 years old (n = 102) had a median survival of 48.4 months and older than 80 years old (n = 16) had a median survival time of 23.8 months.
Survival curves were generated for different age groups (younger than 60 years old, 60 to 69 years, 70 to 79 years and older than 80 years old) in patients that underwent a pancreatic resection for ductal adenocarcinoma, cholangiocarcinoma and ampullary adenocarcinoma (Fig. 2) (P = 0.014). In the 80 years and older age group, nearly 44% of the patients died within the first year, beyond which the survival curve was similar to those aged 70 to 79 years.
Figure 2.
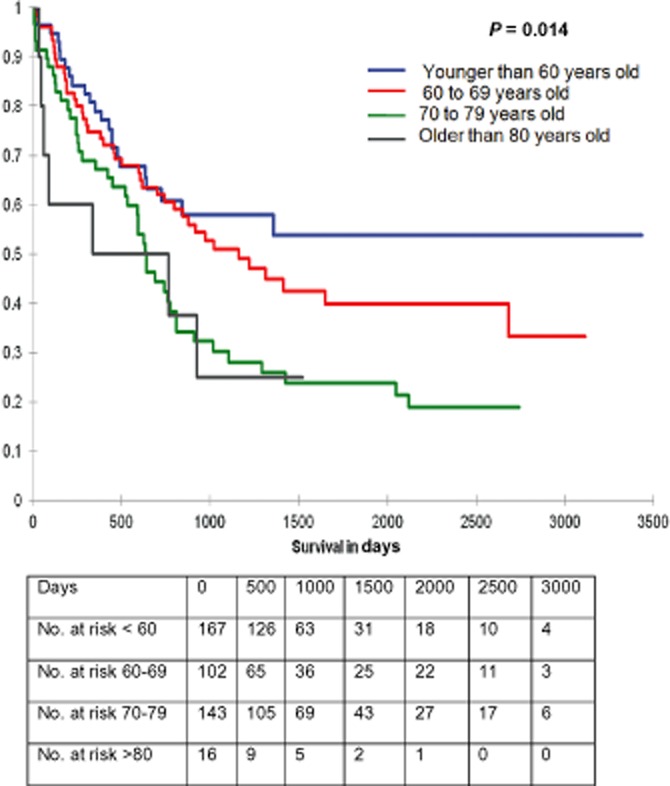
Survival curve comparing all patients divided into four groups, younger than 60 years old, 60 to 69 years old, 70 to 79 years old and older than 80 years old that underwent a pancreatic resection for ductal adenocarcinoma, Cholangiocarcinoma and ampullary adenocarcinoma
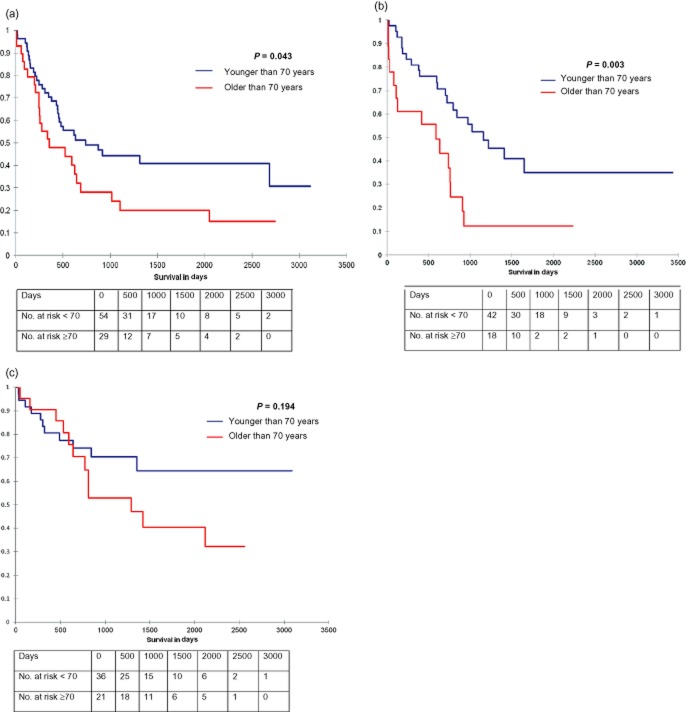
In patients undergoing pancreatic resection for ductal adenocarcinoma, cholangiocarcinoma and ampullary adenocarcinoma, long-term survival was compared (Fig. 3) between patients younger than 70 years and those 70 years or older. There was a significant difference in survival with relation to age in the ductal adenocarcinoma (P = 0.043) and cholangiocarcinoma (P = 0.003) groups but not for the ampullary adenocarcinoma category (P = 0.194) as shown in Fig. 3.
Figure 3.
Survival curves comparing all patients 70 years of age and older undergoing a pancreatic resection for ductal adenocarcinoma compared wuth patients younger than 70 years old
Discussion
The results of this study show that the risk of developing pancreatic and peri-ampullary malignancy dramatically increases with age.4 As a result, the epidemiology of the disease combined with the growth of the older population results in an increasing number of elderly patients eligible for a pancreatic resection. In spite of the limited survival benefit of surgery, a pancreaticoduodenectomy with or without adjuvant chemotherapy remains the standard treatment in the absence of other effective treatment modalities.19
In the last three decades the mortality rates after a pancreatic resection for benign and malignant periampullary and pancreatic diseases have dropped to less than 2% at experienced centres.6,14,20,21 However, a pancreatic resection still carries a significant risk with reported morbidity rates exceeding 30%.6 Recently, centres in America and Europe started to report their results after pancreatic surgery in the elderly.8–12,20 The age groups studied vary among the studies. The majority of the studies report statistically higher morbidity rates in the older group compared with the younger patients.9,11,14 Some reports demonstrated that the difference between younger and older groups are not statistically significant.9,10,12,14 Interestingly, Brozetti et al. have reported statistically significant higher rates of mortality among the elderly group.11
While considerable discussion exists in published literature about outcome in the elderly, the results have been very varied with widespread inconsistency as a result of the different age groups compared. This study not only compares outcome in all pancreatic resections but also breaks it down into age groups with benign and malignant subgoups. The current results showed a significantly lower median survival in the 70 years and older (57.3 months) compared with 78.7 months in the younger cohort, although this was for all benign and malignant patients pooled together. Such a comparison of overall survival is evidently influenced by the significant differences in age between the two groups (younger than 70 years old and older than 70 years old), in addition to the higher percentage of patients in the younger group undergoing resection for benign disease (25.6%), as compared with 16.0% of patients undergoing resection for benign disease in the older group.
This study demonstrates that major curative pancreatic resection in selected elderly healthy patients with pancreatic cancer can be carried out with satisfactory short-term outcomes. The overall operative morbidity (10%) and mortality (3.4%) rates are somewhat lower than previously reported results with resection in patients of all ages but these are for all pancreatic resections, benign or malignant (although 70% of resections were for malignancy in the elderly cohort). These factors must be considered when contemplating resection in the elderly; nevertheless, surgical resection is the only potential procedure offering long-term survival in the elderly with malignant pancreatic lesions.22,23 The reluctance to undertake radical resections in the elderly has diminished with time but the feeling always has been that geriatric patients tolerate resection less well than younger patients. Furthermore, while the overall survival after pancreatic resections for malignant lesions has not increased significantly, in the elderly patients, this study shows that to be just over 20% at 5 years although the long-term survival is significantly better in the younger age group as shown in Fig. 2.
While the post-operative mortality as demonstrated in this study is not significantly higher in the elderly patients there is still significant morbidity from such major surgery. There may not be much of a difference in the disease-free survival but the overall survival is significantly lower in the elderly patients especially the octogenarians. Another factor to consider is that the elderly patients are less likely to tolerate chemotherapy and the dropout rates will be higher with the significant age-related co-morbidities. Not surprisingly this study showed that a greater number of younger patients underwent chemotherapy (adjuvant or palliative).
First-line treatment of metastastic pancreatic adenocarcinoma discussed at the ASCO 2011 showed that the progress in treatment of pancreatic cancer has been disappointing for decades. Even with the use of gemcitabine with other anti-metabolites still shows a median survival of around 12 months at best.24 The median survival in those ≥70 years after any resection in the present study was 57.3 months, and 26.5, 16.3 and 47.1 months for patients ≥70 years with ductal adenocarcinoma, cholangiocarcinoma and ampullary adenocarcinoma, respectively. Non-resective surgical palliation in these patients would have prevented this possibility for prolonged survival.
A limitation of this study is the absence of health-related quality of life assessments which the unit is currently recording prospectively but there was no available data for this retrospective analysis. The improvement in management of pancreatic cancer in specialist high volume centres together with improved intensive care has led to an overall reduction in post-operative mortality. Unfortunately there are limited data available in the literature with relation to where the patients go after hospital discharge, whether it is to their homes, intermediate care facilities such as rest homes or to residential or nursing home with a lot of assistance required. These data are significant in the elderly population as quality of life should be a significant determining factor when making the decision to resect in these patients as although the mortality has improved the morbidity remains high. Shat et al., in a recent population study in North America, showed in a cohort of nearly 50 000 patients, that while mortality after pancreatic surgery for malignancy decreased from 7.1% to 5.2%, the number of patients needing assistance at home after discharge increased from 20% to 33% and the number of patients discharged to another facility trebled from 5.2% to 13.3%.25 These numbers were for the entire age range, but without doubt the elderly population will need the most assistance.
Currently age alone is not a contra-indication for pancreatic surgery. However, the low survival rates make decisions regarding surgery in the elderly population very difficult, when the expected average lifespan has been reached. However, anecdotally the authors are more aggressive with younger patients who have significant comorbidities. Elderly patients tend to have rigorous selection criteria applied. Every elderly patient should be selected for surgery on their own merit; unless the patient's performance status is so poor, all elderly patients should have the opportunity to discuss the risks and benefits of major pancreatic resection with the operating centre.
This study picked a cut-off age value of 70 years as our numbers of resections in octogenarians were too few (n = 16) to draw any significant conclusions. While early recurrence remains a problem occurring in approximately 20% of all resections, the present results showed that 44% of patients aged 80 years and over undergoing pancreatic resections for malignancy died in the first year. If this cohort of early recurrence could be pre-operatively defined, then the decision to take an elderly patient to surgery may be easier.
With the continued increase in the geriatric population and increased incidence of pancreatic cancer in the elderly, and with the increasing overall incidence of pancreatic cancer over the last several decades, appropriate treatment of elderly patients with a pancreatic neoplasm becomes an important consideration. Results from this study support the contention that age alone should not necessarily be a limiting factor for a potentially curative pancreatic resection but rigorous selection criteria has to be applied in the octogenarian subgroup where results from the present study have shown significantly poor results with relation to survival.
References
- 1.Fastest increase in the ‘oldest old’. 2010. Available at: http://www.statistics.gov.uk (last accessed 19 October 2011)
- 2.Estimates of centenarians in the UK. 2011. Available at: http://www.ons.gov.uk/ons/rel/mortality-ageing/population-estimates-of-the-very-elderly/2010/sum-eve-2010.html (last accessed 19 October 2011)
- 3.Sixty-five plus in the United States. United States Census Bureau. 2001. Available at: http://www.census.gov/population (last accessed 19 October 2011)
- 4.Cancer statistics registrations: registrations of cancer diagnosed in 2001, England. 2004. Available at: http://www.statistics.gov.uk/statbase/Product.asp?vlnk=8843&More=N (last accessed 19 October 2011)
- 5.Sohn TA, Yeo C, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 210-1. [DOI] [PubMed] [Google Scholar]
- 7.Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821–831. doi: 10.1097/00000658-199806000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn TA, Yeo CJ, Cameron JL, Lillemoe KD, Talamini MA, Hruban RH, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998;2:207–216. doi: 10.1016/s1091-255x(98)80014-0. [DOI] [PubMed] [Google Scholar]
- 9.Makary MA, Winter JM, Cameron JL, Campbell KA, Chang D, Cunningham SC, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10:347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Scurtu R, Bachellier P, Oussoultzoglou E, Rosso E, Maroni R, Jaeck D. Outcome after pancreaticoduodenectomy for cancer in elderly patients. J Gastrointest Surg. 2006;10:813–822. doi: 10.1016/j.gassur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Brozzetti S, Mazzoni G, Miccini M, Puma F, De Angelis M, Cassini D, et al. Surgical treatment of pancreatic head carcinoma in elderly patients. Arch Surg. 2006;141:137–142. doi: 10.1001/archsurg.141.2.137. [DOI] [PubMed] [Google Scholar]
- 12.Hodul P, Tansey J, Golts E, Oh D, Pickleman J, Aranha GV. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001;67:270–275. doi: 10.1016/s0016-5085(00)81967-8. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 13.Riall TS, Reddy DM, Nealon WH, Goodwin JS. The effect of age on short-term outcomes after pancreatic resection: a population-based study. Ann Surg. 2008;248:459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 15.Verbeke CS, Smith AM. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg. 2010;251:776–777. doi: 10.1097/SLA.0b013e3181d57af6. [DOI] [PubMed] [Google Scholar]
- 16.Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP) HPB (Oxford) 2009;11:18–24. doi: 10.1111/j.1477-2574.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Hill JS, McPhee JT, Whalen GF, Sullivan ME, Warshaw AL, Tseng JF. In-hospital mortality after pancreatic resection for chronic pancreatitis: population-based estimates from the nationwide inpatient sample. J Am Coll Surg. 2009;209:468–476. doi: 10.1016/j.jamcollsurg.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 21.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.al-Sharaf K, Andren-Sandberg A, Ihse I. Subtotal pancreatectomy for cancer can be safe in the elderly. Eur J Surg. 1999;165:230–235. doi: 10.1080/110241599750007090. [DOI] [PubMed] [Google Scholar]
- 23.Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, Nishioka R, et al. A pancreaticoduodenectomy is acceptable for periampullary tumors in the elderly, even in patients over 80 years of age. J Hepatobiliary Pancreat Surg. 2009;16:675–680. doi: 10.1007/s00534-009-0106-6. [DOI] [PubMed] [Google Scholar]
- 24.Strimpalous AS, Syringos KN, Saif MW. Updates on first-line treatment of metastatic pancreatic adenocarcinoma. JOP. 2011;12:339–342. [PubMed] [Google Scholar]
- 25.Shah BC, Smith LM, Ullrich F, Are C. Discharge disposition after pancreatic resection for malignancy: analysis of national trends. HPB (Oxford) 2012;14:201–208. doi: 10.1111/j.1477-2574.2011.00427.x. doi: 10.1111/j.1477-2574.2011.00427.x. Epub 2012 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]