Abstract
Introduction
Survival after a resected pancreatic ductal adenocarcinoma (PDAC) appears to be improving. Yet, in spite of advancements, prognosis remains disappointing. This study analyses a contemporary experience and identifies features associated with survival.
Methods
Kaplan–Meier analysis was conducted for 424 PDAC resections performed at two institutions (2001–2011). Multivariate analysis was performed to elicit characteristics independently associated with survival.
Results
The median, 1-, and 5-year survivals were 21.3 m, 76%, and 23%, with 30/90-day mortalities of 0.7%/1.7%. 76% of patients received adjuvant therapy. Patients with major complications (Clavien Grade IIIb-IV) survived equivalently to patients with no complications (P = 0.33). The median and 5-year survival for a total pancreatectomy was 32.2 m/49%; for 90 ‘favourable biology’ patients (R0/N0/M0) was 37.3 m/40%; and for IPMN (9% of series) was 21.2 m/46%. Elderly (>75 yo) and nonelderly patients had similar survival. Favorable prognostic features by multivariate analysis include lower POSSUM physiology score, R0 resection, absence of operative transfusion, G1/G2 grade, absence of lymphovascular invasion, T1/T2 stage, smaller tumor size, LN ratio <0.3, and receipt of adjuvant therapy.
Conclusion
This experience with resected PDAC shows decreasing morbidity and mortality rates along with modestly improving long-term survival, particularly for certain subgroups of patients. Survival is related to pathological features, pre-operative physiology, operative results and adjuvant therapy.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the most aggressive malignancies and is one of the most frustrating diseases to manage. With an annual death rate approaching incidence, PDAC confers a dismal prognosis. While resection provides the possibility of a long-term cure, ambiguous presentations often mean that the disease is not diagnosed until it has reached an advanced stage no longer amenable to surgery. For the ∼15% of pancreatic cancer patients who undergo resection,1 the required procedures are among the most challenging in the realm of abdominal surgery, with substantial post-operative morbidity and mortality. In the 1960s and 70s, mortality rates as high as 25% led some authors to suggest that pancreatic resections for PDAC should be abandoned because of an unacceptable operative risk.2,3 Furthermore, initial reports of 1-year survival after resection were dismal (around 50%),2 largely indicative of the severity of the operative endeavour and perhaps less refined patient selection. As recently as 1985, a study of national cancer data in the United States reported a 9.4% operative mortality rate for pancreaticoduodenectomy, and showed an actual 5-year survival of 3% for cancer patients treated with this surgery.4
The context for surgical resection of pancreatic cancer, however, has changed dramatically in the past three decades. Studies from the 1990s demonstrated dramatically lower mortality rates in high-volume centres, and pancreatic resections are now increasingly performed by specialists with reported mortality rates well under 5.0%.5–9 As operative performance has continued to improve, surgeons have gradually expanded the indications for surgery. Advanced age and venous invasion (portal vein and superior mesenteric vein) are no longer considered surgical contraindications, and some groups have even suggested a role for arterial resections and total pancreatectomies in selected situations.10 In addition, recent data have suggested that at specialty practices with significant hospital volume, lower post-operative complication rates can be achieved in spite of rising patient acuity.11,12 Specialty training in pancreatic surgery has also expanded in scope and content significantly over the last 15 years and, although hard to measure, has undoubtedly contributed to incremental improvements in peri-operative outcomes.13,14
Post-operatively, patients are now treated with a broader array of chemotherapeutic agents, and gemcitabine therapy has replaced 5-fluorouracil as the standard of care in the United States since its introduction in 1996. Patients have new options for the delivery of radiation therapy, as a result of technological advances in radiation oncology, such as intensity modulated radiation therapy, Cyberknife and proton-beam technology. Furthermore, the use of post-operative adjuvant therapy in the United States has increased over the last two decades.15 Yet, in spite of modest progress in these ancillary oncological modalities, surgical intervention, the primary contributor to potential cure, is largely underutilized for pancreatic cancer in the United States.16 This may reflect outdated beliefs regarding post-operative morbidity and mortality rates from the physician referral base.17
Furthermore, the reporting standards for surgical outcomes have changed in recent years to address ambiguities in surgical terminology. The Toronto group has developed and subsequently refined an increasingly popular complication grading scale based on clinical impact.18–20 Pancreatic fistulae and delayed gastric emptying have been codified and graded through international study group consensus to provide further consistency in the literature.21,22 Pathological analysis is becoming increasingly sophisticated, with features such as lymphovascular invasion, perineural invasion and lymph node ratios commonly being reported and correlated with patient outcomes.23 Verbeke et al. have proposed a new approach for processing specimens and ascribing resection margins, and further reports have demonstrated its relevance in survival studies.24–26 Finally, the potential utility of peri-operative risk assessment scores and nomograms are suddenly coming into focus.27–30
In light of the advances attained by these multidisciplinary contributions, and in the context of modern reporting standards, this report aims to provide a contemporary analysis of a combined experience with resected pancreatic adenocarcinoma at two high-volume specialty centres. This study seeks to identify factors that currently drive long-term survival as well as to scrutinize certain scenarios now regularly faced in managing this disease.
Methods
This study was approved by the Institutional Review Boards at both the Hospital of the University of Pennsylvania (HUP) and the Beth Israel Deaconess Medical Center (BIDMC). A retrospective review of two prospectively maintained databases identified 424 resections for confirmed PDAC performed between 2001 and 2011 by five surgeons who specialize in pancreatic surgery. Resections included only pancreaticoduodenectomies, distal pancreatectomies and total pancreatectomies. Patients with duodenal, ampullary, bile duct and other periampullary neoplasms were excluded from the study.
Variables accrued and analysed include patient characteristics, comorbidities, pre-operative labs, tumour features, operative details, post-operative management and complications, and post-operative therapy. Pre-operative weight loss was defined as a decrease in body weight of 10% or more. Median operating time was defined as the time from incision to skin closure. Operative transfusions were defined as transfusions received intra-operatively or within the first 24 h thereafter. Duration of stay for the index admission was computed from the operation date to the discharge date. Total duration of stay reflects the additive time of the index admission and any readmission time within 30 days of discharge. The post-operative course of each patient at both institutions was reviewed by a single pancreatic surgeon (C.M.V.) and scored according to the Clavien complication scale, the International Study Group on Pancreatic Fistula (ISGPF) Pancreatic Fistula Classification Scheme and the ISGPS Delayed Gastric Emptying Classification Scheme.19,21,22 Charlson, The Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity (POSSUM) and the Surgical Outcomes Analysis and Research (SOAR) risk prediction scores were calculated for each patient.27,28,30,31 Tumour specimens were analysed by an attending pathologist and staged according to the American Joint Committee on Cancer (AJCC) 7th edn but did not regularly follow the technique recently proposed by Verbeke et al.24 Resection margins were defined as follows: R0, final margins devoid of a tumour; R1, microscopic tumour present at the margin(s); and R2, gross residual tumour present at the margin(s). The lymph node ratio (LNR) was defined as the ratio of positive nodes to nodes examined. Pre-operative CA 19-9 values were inconsistently accrued (20% of patients); therefore, they were not included in the analysis.
Operatively placed drains were routinely removed on post-operative day (POD) 5–7 (upon tolerance of a soft mechanical diet) if fluid output remained low and amylase remained below 1000 IU/l. The standard oncological approach at both institutions was to provide post-operative chemoradiation therapy, predominantly gemcitabine based. Standard radiation treatment consisted of 50 Gy with chemosensitization by continuous infusion with 5-fluorouracil (5-FU). Neoadjuvant therapy was infrequently employed (<3%), in each case in the setting of initially locally-unresectable tumours. Survival was identified through hospital charts, contact with primary physicians and oncologists, and confirmed by the Social Security Death Index. Disease-specific survival was not accrued.
Statistical analyses were conducted using IBM SPSS, version 20 (SPSS Inc., Chicago, IL, USA). Summary statistics have been provided. The primary outcome measure was overall survival, defined as the time from surgery to death or last known follow-up. Univariate survival differences between dichotomized variables were tested using the log-rank test, and the survival impact of continuous variables was analysed using Cox's regression. Survival curves were generated according to the Kaplan–Meier method.32 To assess factors independently associated with survival, a Cox proportional hazard model was constructed from all univariate factors with significance P < 0.1, utilizing forward conditional modelling. In all, 107 variables (53 pre-operative, 18 peri-operative, 24 post-operative and 12 pathological) were individually tested, and 23 were used to construct the multivariate model. Comparisons between groups were assessed by Fisher's exact test, Pearson's chi-squared test, or the Mann–Whitney U-test as indicated. Independently associated differences were assessed by logistic regression. All tests were two-sided and were considered significant at P < 0.05.
Results
Patient characteristics
Four hundred and twenty-four patients were equally distributed between two institutions (HUP, n = 202; BIDMC, n = 222). In total, 341 (80.4%) pancreaticoduodenectomies, 61 (14.4%) distal pancreatectomies and 22 (5.2%) total pancreatectomies were performed. Pre-operative characteristics and comorbidities for these patients are presented in Table 1.
Table 1.
Patient characteristics and comorbidities
| n | (%) | |
|---|---|---|
| Characteristics | ||
| Gender | ||
| Male | 214 | (50.5%) |
| Female | 210 | (49.5%) |
| Age (median/IQR) | 67 | (60–74) |
| Elderly (≥75) | 101 | (23.8%) |
| BMI (median/IQR) | 25.7 | (23.0–29.5) |
| Obese (BMI > 30) | 86 | (20.3%) |
| ASA Score | ||
| ASA I | 8 | (1.9%) |
| ASA II | 147 | (34.7%) |
| ASA III | 262 | (61.8%) |
| ASA IV | 7 | (1.7%) |
| Charlson score with age (median/IQR) | 5 | (4–6) |
| POSSUM physiological score (median/IQR) | 18 | (15–23) |
| SOAR score (median/IQR) | 11 | (9–12) |
| Pre-operative weight loss (≥ 10%) | 133 | (31.4%) |
| Pre-operative jaundice | 281 | (66.3%) |
| Pre-operative biliary stenting | 145 | (34.2%) |
| Comorbidities | ||
| HTN | 233 | (55.0%) |
| Diabetes | 127 | (30.0%) |
| GERD | 118 | (27.8%) |
| CAD | 72 | (17.0%) |
| Connective tissue disease | 46 | (10.8%) |
| Myocardial infarction | 34 | (8.0%) |
| COPD | 33 | (7.8%) |
| Asthma | 26 | (6.1%) |
| Peripheral vascular sisease | 17 | (4.0%) |
| Renal disease | 17 | (4.0%) |
| Cerebrovascular disease | 16 | (3.8%) |
| Congestive heart aailure | 11 | (2.6%) |
| Ulcer disease | 9 | (2.1%) |
| Liver disease | 8 | (1.9%) |
| Dementia | 2 | (0.5%) |
IQR, interquartile range; BMI, body mass index, ASA, American Society of Anesthesiologists; HTN, hypertension; GERD, gastroesophageal reflux disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease.
Tumour features
Pathological features are presented in Table 2. Metastatic tumours were all discovered intra-operatively – usually representing a limited burden of metastatic disease with favourable resection scenarios. Among just the node-positive patients the median LNR was 0.19 [interquartile range (IQR): 0.10–0.32].
Table 2.
Tumor features
| Parameter | n | (%) |
|---|---|---|
| T-Stage | ||
| Tx | 2 | (0.5%) |
| T1 | 29 | (6.8%) |
| T2 | 54 | (12.7%) |
| T3 | 329 | (77.6%) |
| T4 | 10 | (2.4%) |
| Nodal invasion (N1) | 290 | (68.4%) |
| Metastatic disease (M1) | 11 | (2.6%) |
| LNs examined (median/IQR) | 14 | (10–20) |
| Positive LNs (median/IQR) | 1.5 | (0–3) |
| LNR ≥ 0.3 | 83 | (19.6%) |
| Arising in IPMN | 40 | (9.4%) |
| Differentiatioa | ||
| Well | 45 | (10.8%) |
| Moderate | 207 | (49.5%) |
| Poor | 166 | (39.7%) |
| Lymphovascular invasionb | 162 | (43.8%) |
| Perineural invasionc | 341 | (83.0%) |
| AJCC Stage, 7th edm | ||
| I A | 16 | (3.8%) |
| I B | 29 | (6.8%) |
| II A | 82 | (19.3%) |
| II B | 275 | (64.9%) |
| III | 10 | (2.4%) |
| IV | 11 | (2.6%) |
6 missing values.
54 missing values.
13 missing values.
IQR, interquartile range; LN, lymph node; LNR, lymph node ratio; IPMN, intraductal papillary mucinous neoplasm.
Perioperative features
Negative resection margins (R0) were achieved in 276 (65.1%) of all patients. There were no R2 resections performed. The median operating time was 5.9 h (IQR: 4.4–7.3). While the median estimated blood loss (EBL) was 400 ml (IQR: 300–700), excessive blood loss (≥ 1000 ml) occurred in 60 (14.2%) operations. This was balanced by 73 operations (17.2%) where the EBL was < 250 ml. The overall operative transfusion rate was 27.4%. The overall in-hospital transfusion rate was 39.4%. Resection of the portal vein or SMV to achieve negative margins occurred in 21 patients (5.0%), of which 14 actually demonstrated venous tumour invasion. Although almost all patients (n = 400) were drained intra-operatively, percutaneous drains were required postoperatively for 13 patients (3.1%), whereas reoperations, usually for bleeding complications, were required for 17 patients (4.0%). Parenteral nutrition was used to manage 68 patients (16%) post-operatively. ICU transfers in the recovery period were required 5.9% of the time. The median duration of stay after surgery was 8 days (IQR: 7–11), and 72 (17%) patients were readmitted within 1 month of the index operation. Readmitted patients had a median total duration of stay (index and readmission) of 15 days (IQR: 11–19). Only 22 patients (5.2%) were hospitalized for longer than 3 weeks. Post-operative complications are presented in Table 3.
Table 3.
Post-operative complications
| Parameter | n | (%) |
|---|---|---|
| Clavien-Dindo complication score | ||
| None | 178 | (42.0%) |
| Grade I | 52 | (12.3%) |
| Grade II | 131 | (30.9%) |
| Grade III a | 22 | (5.2%) |
| Grade III b | 5 | (1.2%) |
| Grade IV a | 26 | (6.1%) |
| Grade IV b | 3 | (0.7%) |
| Grade V (death) | 7 | (1.7%) |
| ISGPF delayed gastric emptying gradea | ||
| None | 149 | (73.8%) |
| Grade A | 35 | (17.3%) |
| Grade B | 7 | (3.5%) |
| Grade C | 11 | (5.4%) |
| ISGPS pancreatic fistula grade | ||
| None | 358 | (84.4%) |
| Grade A | 39 | (9.2%) |
| Grade B | 24 | (5.7%) |
| Grade C | 3 | (0.7%) |
| Wound infection | 45 | (10.6%) |
| Respiratory distress | 37 | (8.7%) |
| Pneumonia | 27 | (6.4%) |
| UTI | 27 | (6.4%) |
| Ileus | 23 | (5.4%) |
| Bleeding | 20 | (4.7%) |
| Sepsis | 12 | (2.8%) |
| Wound dehiscence | 9 | (2.1%) |
| Biloma | 8 | (1.9%) |
| Neurological distress | 7 | (1.7%) |
| MI | 5 | (1.2%) |
| Acute renal failure | 3 | (0.7%) |
From the University of Pennsylvania Experience Only (n = 202).
ISGPS, The International Study Group on Pancreatic Fistula; UTI, urinary tract infection; MI, myocardial infarction.
Application of adjuvant therapy
Information on oncological treatment was accrued on all but 24 patients (5.7%). Eleven patients (2.6%) received pre-operative downstaging chemoradiation for initially unresectable tumors. Otherwise, 324 patients (76.4%) received adjuvant chemotherapy or chemoradiation therapy (CRT) with curative intent after the operation. A small portion of patients (2.8%) received palliative chemotherapy for metastatic disease discovered operatively or before the intended initiation of formal adjuvant therapy. Another 53 patients (12.5%) did not receive adjuvant therapy by choice, the discretion of their oncologist or owing to an unfavourable post-operative course (complications/death) which precluded further treatment. Among the 324 patients who received post-operative adjuvant therapy, 248 (76.5%) received CRT, 23 (7.1%) received chemotherapy only and for 53 (16.4%) it was uncertain if they received radiation in addition to documented chemotherapy. No patient received radiation therapy alone.
Overall mortality and survival
Of 157 censored patients, the median follow-up was 27.7 months (IQR: 13.9–45.4). Thirty- and 90-day mortality rates were 0.7% (n = 3) and 1.7% (n = 7). Two additional deaths occurred in the hospital beyond 90-days. In all, seven (1.7%) of these nine deaths were attributable to surgical complications (Clavien Grade V). Surgical deaths were caused by bleeding (n = 1), hepatic failure (n = 1), infection with resulting organ failure (sepsis/bacteraemia, n = 3) and unknown causes in the immediate post-operative period (n = 2). The median survival for all patients was 21.3 months, and 1-, 2-, 3-, 4- and 5-year actuarial survival rates were 76%, 45%, 34%, 28% and 23%, respectively. Of 156 patients with actual 5-year follow-up, 32 (21%) survived that long. There were no differences in survival based on surgeon (P = 0.632), institution (P = 0.288) or operation (P = 0.572, comparison of pancreaticoduodenectomies vs. distal pancreatectomies).
Surgical audit
The median POSSUM physiological and operative scores were 18 (IQR: 15–23) and 17 (IQR: 17–19), respectively. POSSUM scores predicted a 61.4% morbidity rate and Portsmouth POSSUM predicted a 6.9% operative mortality rate. This corresponds to a 0.94 O/E ratio for morbidity and a 0.24 O/E ratio for mortality (using all surgical-related deaths).
Survival analysis
In studying 107 discreet variables across the full spectrum of surgical care, factors associated with poor survival were identified (Table 4). Patients with node negative disease and negative margins (n = 97) achieved a 37.3-month median survival and had a 40% 5-year survival.
Table 4.
Survival analysis and cox regression model
| Parameter | Category | Survival | Cox model | |||
|---|---|---|---|---|---|---|
| Mediana | 5-years | P | Hazard | P | ||
| Gender | Male | 21.6 | 23% | 0.566 | ||
| Female | 21.2 | 24% | ||||
| Age | <75 years | 22.1 | 26% | 0.138 | ||
| ≥ 75 years | 19.4 | 15% | ||||
| Pre-operative jaundice | No | 26.5 | 20% | 0.204 | ||
| Yes | 19.7 | 24% | ||||
| Pre-operative weight loss | None or <10% | 22.3 | 25% | 0.088 | ||
| ≥10% | 18.9 | 20% | ||||
| HTN | No | 22.7 | 27% | 0.105 | ||
| Yes | 20.4 | 21% | ||||
| Diabetes | No | 22.7 | 25% | 0.101 | ||
| Yes | 19.5 | 21% | ||||
| ASA | 1 or 2 | 26.4 | 32% | 0.020 | ||
| 3 or 4 | 19.7 | 19% | ||||
| Charlson comorbidity score | Continuous | <0.001 | ||||
| POSSUM physiological score | Continuous | 0.001 | 1.03 | 0.036 | ||
| POSSUM operative score | Continuous | <0.001 | ||||
| SOAR score | Continuous | 0.054 | ||||
| Resection | PPPD | 21.3 | 24% | 0.475 | ||
| Classic PD | 18.5 | 24% | ||||
| Distal | 20.7 | 13% | ||||
| Total | 32.2 | 49% | ||||
| OR time | Continuous | 0.641 | ||||
| Estimated blood loss | 0–999 ml | 21.6 | 25% | 0.023 | ||
| ≥1000 ml | 16.6 | 15% | ||||
| Vascular resection | No | 21.2 | 24% | 0.901 | ||
| Yes | 21.3 | 17% | ||||
| Operative transfusion | No | 24.5 | 27% | 0.002 | 1 | 0.001 |
| Yes | 18.8 | 14% | 1.66 | |||
| Any hospital transfusion | No | 24.8 | 27% | 0.007 | ||
| Yes | 19.2 | 18% | ||||
| Reoperation | No | 21.3 | 24% | 0.464 | ||
| Yes | 21.1 | 20% | ||||
| Duration of stay | <21 days | 21.6 | 25% | 0.012 | ||
| ≥21 days | 13.3 | 0% | ||||
| Clavien complication scoreb | None | 21.3 | 22% | 0.43 | ||
| Grade I | 19.7 | 24% | ||||
| Grade II | 23.8 | 27% | ||||
| Grade III a | 21.2 | 31% | ||||
| Grade III b | 27.4 | 36% | ||||
| Grade IV a | 19.1 | 12% | ||||
| Grade IV b | 16.4 | 0% | ||||
| ISGPS pancreatic fistula | None | 20.3 | 22% | 0.133 | ||
| Grade A | 27.8 | 20% | ||||
| Grade B | 32.2 | 45% | ||||
| Grade C | 6.5 | 0% | ||||
| ISGPS delayed gastric | None | 19.2 | 21% | 0.328 | ||
| Emptyingc | Grade A | 19.4 | 16% | |||
| Grade B | NR | 54% | ||||
| Grade C | 27.9 | 19% | ||||
| Post-operative sepsis | No | 21.5 | 24% | 0.012 | ||
| Yes | 5.8 | NA | ||||
| Post-operative bleeding | No | 21.5 | 24% | 0.202 | ||
| Yes | 17.7 | 15% | ||||
| Margins | R0 | 27.1 | 29% | <0.001 | 1 | <0.001 |
| R1 | 16.8 | 14% | 1.67 | |||
| T-Stage | T1/T2 | 45.0 | 46% | <0.001 | 1 | 0.003 |
| T3/T4 | 19.7 | 18% | 1.90 | |||
| N-Stage | N0 | 31.4 | 31% | 0.001 | ||
| N1 | 19.2 | 20% | ||||
| LNR | <.3 | 24.8 | 27% | <0.001 | 1 | <0.001 |
| ≥.3 | 15.5 | 9% | 1.97 | |||
| Tumour size (cm) | Continuous | <0.001 | 1.10 | 0.034 | ||
| Distant metastases | No | 21.3 | 25% | 0.173 | ||
| Yes | 16.6 | 0% | ||||
| Differentiation | Well/Moderate | 27.8 | 31% | <0.001 | 1 | <0.001 |
| Poor | 16.8 | 12% | 1.94 | |||
| Lymphovascular invasion | No | 27.8 | 28% | 0.001 | 1 | 0.006 |
| Yes | 18.8 | 16% | 1.5 | |||
| Perineural invasion | No | 31.0 | 34% | 0.014 | ||
| Yes | 20.1 | 21% | ||||
| Arising in IPMN | No | 21.3 | 22% | 0.354 | ||
| Yes | 21.2 | 46% | ||||
| Oncological therapy | None | 9.4 | 23% | 0.002 | ||
| Adjuvant | 22.6 | 26% | ||||
| Palliative | 16.6 | 0% | ||||
| Neoadjuvant | 17.8 | NA | ||||
| Unknown | 16.3 | 17% | ||||
| Oncological therapy | Any (adj, pal, neo) | 22.2 | 24% | <0.001 | 1 | <0.001 |
| None | 9.4 | 23% | 3.31 | |||
| Adjuvant therapy | Chemotherapy Only | 15.4 | 13% | <0.001 | ||
| Chemoradiation | 26.4 | 30% | ||||
| Unknown | 17.3 | 6% | ||||
Statistically significant values shown in bold.
In months.
Excludes grade V complications(deaths).
From The University of Pennsylvania experience only.
ASA, American Society of Anesthesiologists; HTN, hypertension; ISGPS, The International Study Group on Pancreatic Fistula;.IPMN, intraductal papillary mucinous neoplasm.; LNR, lymph node ratio.
A Cox's Proportional Hazard model was constructed to identify factors independently associated with survival (Table 4). Only the 343 patients (81%) with complete datasets were used in the model. The most frequently missing data value was lymphovascular invasion (LVI) (n = 54). Kaplan–Meier curves for select variables are presented in Fig. 1.
Figure 1.
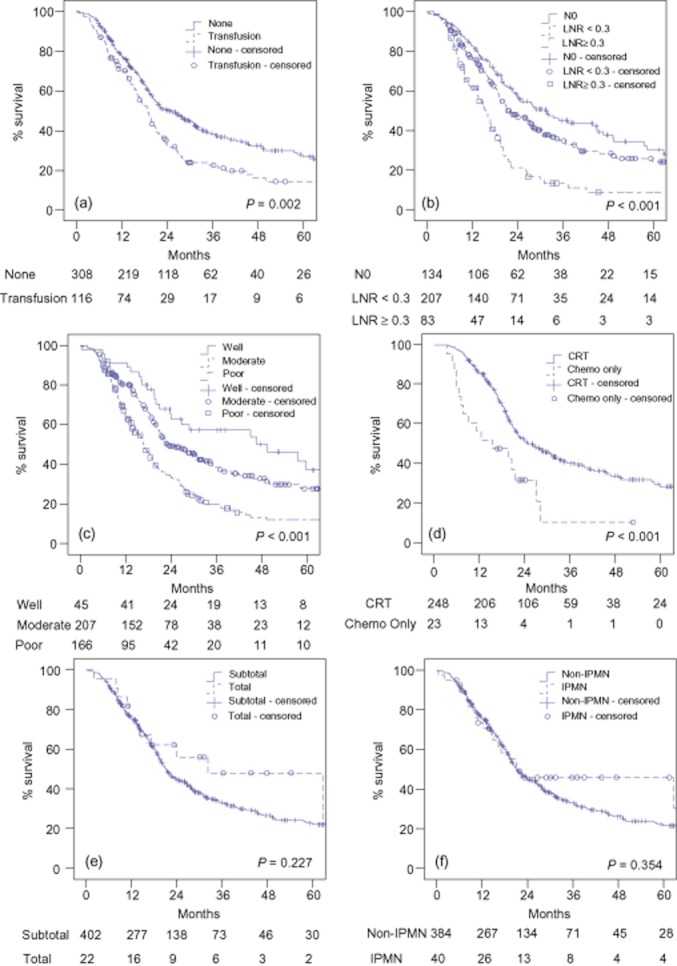
(a) Survival by use of peri-operative (operation and 24-h post-operatively) transfusion. Survival was significantly longer in patients without peri-operative transfusions (P = 0.002). (b) Survival by lymph node ratio (LNR). Survival was different between LNR groupings (P < 0.001). N1 patients with LNR < 0.3 had significantly longer survival than patients with LNR ≥ 0.3 (P < 0.001), but survival was not significantly different between N1 patients with LNR <0.3 and N0 patients (P = 0.068). (c) Survival by tumour differentiation. Survival was significantly different between grades (P < 0.001). (d) Survival by adjuvant therapy received. Among patients who received post-operative adjuvant therapy with curative intent, those who received chemoradiation therapy (CRT) had a significantly longer survival than those who received chemotherapy only (P < 0.001). (e) Survival by extent of resection. Survival was not significantly different by resection type (P = 0.227). (f) Survival by presence of intraductal papillary mucinous neoplasm (IPMN). Survival was not significantly different by presence of IPMN (P = 0.354)
The influence of operative transfusions
Patients transfused either during the operation or within the subsequent 24 h demonstrated significantly diminished 5-year survival compared with those who were not (14% vs. 27%). Further scrutiny shows that operatively transfused patients had worse pre-operative physiology and comorbidities, occurred more frequently after downstaging CRT, occurred more often in classical pancreaticoduodenectomies and had increased operative blood loss, increased rates of post-operative bleeding, respiratory distress, and sepsis, worse complication profiles, longer durations of stay, and significantly increased rates of LVI (not shown). On multivariate logistic regression, pre-operative physiology and operative blood loss were independently associated with operative transfusion (POSSUM Physiology Score, HR: 1.14, P < 0.001: EBL, HR: 36.8, P < 0.001), whereas tumour-related factors were not. Transfusions administered after the first day did not appear to have any detrimental effect on ultimate survival.
Lymph node analysis
Survival for N1 patients with a single positive node (N1-a; n = 77) was compared with those with more than one positive node (N1-b; n = 213). The two populations had similar median and 5-year survivals (N1-a 21.2 m/21%; N1-b 18.6 m/20%, P = 0.335). While the survival difference between patients with a single isolated node and N0 patients was not statistically significant, the disparity was more striking (N0 31.4 m/31%, P = 0.065).
Intraductal Papillary Mucinous Neoplasm
While intraductal papillary mucinous neoplasm (IPMN) patients had improved long-term survival over other patients, the difference was not statistically significant (5-year survival: 46% vs. 22%, P = 0.354). IPMN patients had more optimistic AJCC staging, with Stage I tumours occurring in 32% of patients compared with 8% in non-cystic PDACs (P < 0.001). Stage I tumors accounted for 10 out of 13 IPMN patients with actual 5-year survival. Lower T-stage (T1/T2) was present in 16 (40.0%) IPMN patients vs. 67 (17.5%) non-cystic patients (P = 0.002). Node negative disease was present in 19 (47.5%) IPMN patients vs. 115 (29.9%) non-cystic patients (P = 0.031). Perineural invasion was present in 21/36 (58.3%) IPMN patients with available pathological data vs. 320/375 (85.3%) non-cystic patients (P < 0.001 & P = 0.065). R0 resections were achieved in 34 (85.0%) IPMN patients vs. 242 (63.0%) non-cystic patients (P = 0.005). Differences in tumour grade and LVI rates for IPMN patients were not statistically significant.
Discussion
Results from this contemporary (2000s) combined-institution study demonstrate continued progress in patient outcomes after resection for pancreatic adenocarcinoma. The 30-day mortality rate (0.7%) is particularly encouraging, indicating that surgical safety continues to improve in high-volume centres33 (Table 5). Two key metrics of post-operative surgical management, pancreatic fistulae and major complications, were found to be limited in both scope and impact on patient survival. Furthermore 76% of patients survived the first year post-operatively, which compares favourably with historical standards and suggests significant improvements in peri- and post-operative management.
Table 5.
Selected reports of comprehensive experiences with resected pancreatic ductal adenocarcinoma (PDAC), 1970 present
| Author | Years | n | Mortality | Survivala | Scope | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30d | 90d | Surgical | Median (months) | 1-year | 3-year | 5-year | 5-year, actual | ||||
| Yeo8 | 1970–1994 | 201 | 5.0b | NA | NA | 15.5 | 57 | 26 | 21 | 15 | SI – Hopkins |
| Mayo56 | 1970–2008 | 1822 | NA | NA | NA | 18 | 68.3 | 38.9 | 18 | NA | SI – Hopkins |
| Trede57 | 1972–1989 | 133 | 2.2b | NA | NA | NA | NA | NA | 24c | NA | SI – Mannheim |
| Schnelldorfer58 | 1981–2001 | 357 | 1.4b | NA | NA | 17 | NA | NA | 18 | 17.4 | SI – Mayo Clinic |
| Conlon7 | 1983–1989 | 118 | 3.4 | NA | NA | 14.3 | NA | NA | 10.2 | 10.2 | SI – MSKCC |
| Winter5 | 1983–1989 | 123 | 4.9 | 7.3 | NA | 23.2f | 58 | NA | 17f | NA | SI – MSKCC |
| Geer59 | 1983–1990 | 146 | 3.4 | NA | NA | NA | NA | NA | 24 | 19.2 | SI – MSKCC |
| Ferrone60 | 1983–2001 | 618 | 1.1 | NA | NA | NA | NA | NA | NA | 11.8 | SI – MSKCC |
| Sohn6 | 1984–1999 | 616 | 2.3b | NA | 2.3 | 17 | 63 | 25 | 17 | 17 | SI – Hopkins |
| Sener61 | 1985–1995 | 9044 | NA | NA | NA | NA | NA | NA | 23.4 | NA | NCDB |
| Wade62 | 1987–1991 | 252 | 8.3 | NA | NA | 15d | NA | NA | 9 | NA | Dept. Vet. Affairs |
| Cleary63 | 1988–1996 | 123 | NA | NA | 4.8 | 13.6 | NA | NA | 14.6 | 14.6 | SI – Toronto |
| Baxter64 | 1988–2002 | 2919 | NA | NA | NA | 17e | NA | NA | NA | NA | SEER |
| Winter5 | 1990–1999 | 399 | 1.5 | 3.0 | NA | 25.6f | 68 | NA | 20f | NA | SI – MSKCC |
| Bilimoria1 | 1992–1998 | 21512 | NA | NA | NA | 12.6 | 52 | 19.1 | 12.6 | 12.6 | NCDB |
| van Geenen65 | 1992–1998 | 108 | NA | NA | NA | 16 | NA | NA | NA | NA | SI – Amsterdam |
| Konstantinidis66 | 1993–2008 | 517 | 0.8 | NA | NA | 19.7 | NA | NA | 17.3 | NA | SI – Mass General |
| Evans67h | 1998–2001 | 64 | 1.6g | NA | NA | 34 | NA | NA | 36 | NA | SI – MD Anderson |
| Winter5 | 2000–2009 | 625 | 1.3 | 3.9 | NA | 24.5f | 68 | NA | 8f | NA | SI – MSKCC |
| Present Study | 2001–2011 | 424 | 0.7 | 1.7 | 1.7 | 21.3 | 76 | 34 | 23 | 21 | DI – Penn & BIDMC |
Actuarial.
In-hospital.
Excludes in-hospital mortalities.
Mean.
Calculated from diagnosis; only includes patients that survived 3 months from diagnosis.
Only includes patients that survived 1-year.
Peri-operative death.
Reflects only patients who completed neoadjuvant therapy and received surgical resection.
DI, dual institution; SI, single institution. NA, not applicable.
Optimism based on these improved immediate-term surgical outcomes must be tempered by a recognition that advances in post-operative oncological treatment and long-term survival have not yet been realized. While the actual 5-year survival rate (21%) corresponds with the upper limit of previously reported figures, it is comparable to outcomes achieved by some institutions in the 5-FU era of the 1980s and 1990s (Table 5). While operative results have improved, these numbers indicate that the underlying aggressive tumour biology has not been significantly altered by modern post-operative treatments.
The present study found that the 79.2% of patients received post-operative adjuvant or palliative therapy (with an additional 5.7% of unknown treatment status), which stands in sharp contrast to the national average of 49.0% reported by Bilimoria et al.34 This increased utilization of adjuvant therapy may partially explain the discrepancy between survival numbers from national sources and high-volume centres. Both institutions in this study have integrated multidisciplinary systems in place designed to successfully deliver patients from the surgeon to the oncologist, and this may represent one of the greatest assets of high-volume specialty programmes.
POSSUM has yet to be assessed as an independent predictor of survival after resection for PDAC. The current finding that poor pre-operative physiology, as measured by POSSUM, dampens survival highlights the versatility and utility of the POSSUM score and also emphasizes the importance of pre-operative physiology to achieving long-term survival. For patients demonstrating poor pre-operative physiology, the decision to operate should be approached with increased caution and efforts to bolster physical status should be considered pre-operatively. For its intended use in surgical audit, POSSUM has previously been shown to accurately estimate post-operative morbidity,35 but this study has now shown POSSUM to be inadequate at estimating post-operative mortality. The discrepancy in observed and expected mortality suggests that the POSSUM mortality equation needs to be recalibrated for pancreatic surgery.
The association of blood loss and intra-operative transfusions with long-term survival for resected PDAC has been observed previously.6,36,37 The immunosuppressive effect of an allogeneic blood transfusion was originally demonstrated in the 1970s when reports showed reduced rates of kidney-graft rejection among heavily transfused patients.38–40 More recent studies in potentially curative resection for colorectal cancer and breast cancer have associated transfusion with increased rates of recurrence, peri-operative mortality, length of stay and complications.41–43 The current study corroborates these findings, although increased peri-operative mortality was not observed in the transfused group. A more recent study has suggested a biochemical response by which blood transfusion and surgical stress may synergistically affect prognosis.44 In the current analysis, operative transfusions were adversely associated with survival, whereas subsequent post-operative (POD1 +) transfusions were not, supporting the idea that surgical stress and transfusions interact synergistically.
While blood loss and operative transfusions are the result of many factors, including the extent of tumour invasion, vascular involvement and surgeon skill, this study shows improved outcomes when transfusions are not applied. To this end, a blood transfusion has recently been proposed as a quality metric for pancreatic resection.45 Surgeons should strive to minimize blood loss through meticulous dissection, particularly in light of the irrelevance of operating time seen in this study, and the operative safety at relatively low Hgb counts demonstrated previously.46
Recent studies have suggested that the LNR is a more refined indicator of prognosis than node status.47,48 However, variability exists within the literature as to appropriate cut-off values.47,48 In this study, the LNR cut-off value of 0.3 was chosen for modelling after assessing the prognostic ability of the LNR as a continuous variable, as well as at all decile levels. The value of 0.3 provided the most significant discrimination in overall survival in the overall population and in the cohort of N1 patients alone. Furthermore, studies have pointed to the importance of both total lymph nodes examined and total positive nodes as significant prognostic factors.49 Of the various measures of nodal disease in this series (N-stage, LN ratio and total positive nodes), the LN ratio was the most significant predictor of survival, although all other values were highly predictive (P < 0.001). Total nodes examined was not significant (P = 0.772). This study confirms prior reports that the LN ratio provides refined predictive capacity and suggests a preferred cutoff value of 0.3 for reporting of LN ratio prognostics.
Patients with invasive PDAC arising in IPMN are felt to have improved long term survival compared with non-IPMN associated PDAC, with 5-year survival reported in the range of 42–60%.50,51 However, it has been suggested that when staging differences are taken into account, the prognosis is equivalent between tumour types.50,52 While the results of this study show an actuarial 5-year survival for IPMN in line with previous reports (46%), the overall survival was not found to be significantly different from non-cystic patients – reflective of the unusual survival curve generated from the IPMN patients, and the small number of patients present for analysis after 2-years. Additionally, IPMN patients had earlier stage tumours than non-cystic PDAC patients, along with lower rates of perineural invasion and R1 resections. These results appear to agree with earlier findings that while long-term survival is enhanced for PDAC arising in IPMN, this is likely attributable to earlier stage presentations and not actually a more indolent tumour behaviour.
Historically, the value of total pancreatectomies for adenocarcinoma patients has been questioned because of concerns about operative mortality, metabolic lability, and long-term survival. A recent study on causes of mortality after a pancreatic resection showed a total pancreatectomy, particularly in the setting of PDAC, to be highly lethal.9 On the contrary, this study has demonstrated optimistic outcomes for total pancreatectomies, with no 30-day deaths among total resections, and a lower incidence of complications than other resections (45.5% vs. 58.7%, P = 0.324). Furthermore, survival was better for total pancreatectomies than any other resection type (32.2 m median, 49% 5 year). This is reflective of the increased incidence of IPMN (41%) and stage I tumours (18%) in total pancreatectomies. These results corroborate more recent studies showing the value of total pancreatectomies when required oncologically.53,54
A national study by Bilimoria et al. reported the underutilization of surgery for early stage tumours, particularly among the elderly.16 This represents a potential area for improvement of care dependent on improved processes and systems. The short-term safety and long-term benefit of resection in the elderly is questioned today,55 as it has been for decades. While this study found a higher rate of overall complications among 101 elderly (≥75 years) patients (74.3% vs. 52.9%, P < 0.001), they accounted for none of the 30-day deaths. Furthermore, the overall survival for the elderly was not statistically different from the younger cohort (P = 0.138). These results agree with earlier findings associating old age with morbidity but do not support the association with increased mortality rates, which may indicate an improving ability to manage post-operative complications in the elderly population. These results demonstrate that elderly patients are able to withstand the stress of surgery and obtain the survival benefits achieved by younger patients.
Conclusion
Peri-operative outcomes for a resected pancreatic adenocarcinoma continue to improve, highlighting the significant achievements in surgical care over the past three decades. This series demonstrates that extended survival is affected by tumour biology, pre-operative physiology and some operative factors under the surgeon's control, specifically, negative margin resection and low blood loss/transfusion-less surgery. Operative mortality, now minimized, along with improved management of complications, currently allows for more consistent delivery of patients to the oncologist for application of adjuvant therapy. As first suggested by Peter Allen in 2007, such improvements have enhanced early outcomes, putting ‘a hump’ in the pancreatic cancer survival curve. However, long-term results remain frustratingly poor. As patients and physicians wait for effective chemotherapeutics to influence ultimate prognosis, surgeons continue to improve survival through constant refinement of their approaches to this disease.
Conflicts of interest
None declared.
References
- 1.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro TM. Adenocarcinoma of the pancreas: a statistical analysis of biliary bypass vs Whipple resection in good risk patients. Ann Surg. 1975;182:715–721. doi: 10.1097/00000658-197512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crile G., Jr The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049–1053. [PubMed] [Google Scholar]
- 4.Steele GD, Jr, Osteen RT, Winchester DP, Murphy GP, Menck HR. Clinical highlights from the National Cancer Data Base: 1994. CA Cancer J Clin. 1994;44:71–80. doi: 10.3322/canjclin.44.2.71. [DOI] [PubMed] [Google Scholar]
- 5.Winter JM, Brennan MF, Tang LH, D'Angelica MI, Dematteo RP, Fong Y, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–175. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 7.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 2000;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1996;221:721–731. doi: 10.1097/00000658-199506000-00011. discussion 31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmer CM, Jr, Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, et al. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 1995;16:89–102. doi: 10.1007/s11605-011-1753-x. discussion -3. [DOI] [PubMed] [Google Scholar]
- 10.Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Buchler MW, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2012;254:882–893. doi: 10.1097/SLA.0b013e31823ac299. [DOI] [PubMed] [Google Scholar]
- 11.Vollmer CM, Jr, Pratt W, Vanounou T, Maithel SK, Callery MP. Quality assessment in high-acuity surgery: volume and mortality are not enough. Arch Surg. 2011;142:371–380. doi: 10.1001/archsurg.142.4.371. [DOI] [PubMed] [Google Scholar]
- 12.Pratt W, Callery MP, Vollmer CM., Jr Optimal surgical performance attenuates physiologic risk in high-acuity operations. J Am Coll Surg. 2007;207:717–730. doi: 10.1016/j.jamcollsurg.2008.06.319. [DOI] [PubMed] [Google Scholar]
- 13.Raptis DA, Clavien PA, International Hepato-Pancreato-Biliary Association E, Training C. Evaluation of Hepato-Pancreato-Biliary (HPB) fellowships: an international survey of programme directors. HPB. 2008;13:279–285. doi: 10.1111/j.1477-2574.2010.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zyromski NJ, Torbeck L, Canal DF, Lillemoe KD, Pitt HA. Incorporating an HPB fellowship does not diminish surgical residents' HPB experience in a high-volume training centre. HPB. 2011;12:123–128. doi: 10.1111/j.1477-2574.2009.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2010;214:33–45. doi: 10.1016/j.jamcollsurg.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2012;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodmass J, Lipschitz J, McKay A. Physician attitudes and treatment patterns for pancreatic cancer. World J Surg Oncol. 2007;9:21. doi: 10.1186/1477-7819-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 2011;111:518–526. [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 1992;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2004;250:177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2009;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2005;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2007;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 25.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2006;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson NB, Foulis AK, Oien KA, Going JJ, Glen P, Dickson EJ, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2011;251:1003–1010. doi: 10.1097/SLA.0b013e3181d77369. [DOI] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 2010;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1987;78:355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 29.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 1991;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill JS, Zhou Z, Simons JP, Ng SC, McDade TP, Whalen GF, et al. A simple risk score to predict in-hospital mortality after pancreatic resection for cancer. Ann Surg Oncol. 2004;17:1802–1807. doi: 10.1245/s10434-010-0947-x. [DOI] [PubMed] [Google Scholar]
- 31.A Simple Risk Score for Pancreatectomy. Massachusetts: University of Massachusetts Medical School; 2010. Available at http://www.umassmed.edu/surgery/panc_mortality_custom.aspx (last accessed 2 Feb 2012) [Google Scholar]
- 32.Kelin JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer-Verlag; 2012. 2003. [Google Scholar]
- 33.Sosa JA, Bowman HM, Gordon TA, Bass EB, Yeo CJ, Lillemoe KD, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, et al. Multimodality therapy for pancreatic cancer in the U.S. : utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 35.Pratt W, Joseph S, Callery MP, Vollmer CM., Jr POSSUM accurately predicts morbidity for pancreatic resection. Surgery. 2008;143:8–19. doi: 10.1016/j.surg.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621–633. doi: 10.1097/00000658-199705000-00018. discussion 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazanjian KK, Hines OJ, Duffy JP, Yoon DY, Cortina G, Reber HA. Improved survival following pancreaticoduodenectomy to treat adenocarcinoma of the pancreas: the influence of operative blood loss. Arch Surg. 2008;143:1166–1171. doi: 10.1001/archsurg.143.12.1166. [DOI] [PubMed] [Google Scholar]
- 38.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 39.Salvatierra O, Jr, Vincenti F, Amend W, Potter D, Iwaki Y, Opelz G, et al. Deliberate donor-specific blood transfusions prior to living related renal transplantation. A new approach. Ann Surg. 1980;192:543–552. doi: 10.1097/00000658-198010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glass NR, Miller DT, Sollinger HW, Belzer FO. A four-year experience with donor blood transfusion protocols for living-donor renal transplantation. Transplantation. 1985;39:615–619. doi: 10.1097/00007890-198506000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Busch OR, Hop WC, Marquet RL, Jeekel J. Prognostic impact of blood transfusions on disease-free survival in colorectal carcinoma. Scand J Gastroenterol Suppl. 1993;200:21–23. doi: 10.3109/00365529309101570. [DOI] [PubMed] [Google Scholar]
- 42.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. discussion 9–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe JP, Gordon NH, Fry DE, Shuck JM, Hubay CA. Breast cancer survival and perioperative blood transfusion. Surgery. 1989;106:836–841. [PubMed] [Google Scholar]
- 44.Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol. 2006;18:60–66. doi: 10.1016/j.clon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Ball CG, Pitt HA, Kilbane ME, Dixon E, Sutherland FR, Lillemoe KD. Peri-operative blood transfusion and operative time are quality indicators for pancreatoduodenectomy. HPB. 2010;12:465–471. doi: 10.1111/j.1477-2574.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 47.House MG, Gonen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 48.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 49.Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg. 2007;142:767–774. doi: 10.1001/archsurg.142.8.767. discussion 73–74. [DOI] [PubMed] [Google Scholar]
- 50.Poultsides GA, Reddy S, Cameron JL, Hruban RH, Pawlik TM, Ahuja N, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Partelli S, Fernandez-Del Castillo C, Bassi C, Mantovani W, Thayer SP, Crippa S, et al. Invasive intraductal papillary mucinous carcinomas of the pancreas: predictors of survival and the role of lymph node ratio. Ann Surg. 2010;251:477–482. doi: 10.1097/SLA.0b013e3181cf9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. discussion 46. [DOI] [PubMed] [Google Scholar]
- 53.Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–287. doi: 10.1097/SLA.0b013e3181ae9f93. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–578. doi: 10.1016/j.surg.2007.07.016. discussion 8–80. [DOI] [PubMed] [Google Scholar]
- 55.Haigh PI, Bilimoria KY, DiFronzo LA. Early postoperative outcomes after pancreaticoduodenectomy in the elderly. Arch Surg. 2011;146:715–723. doi: 10.1001/archsurg.2011.115. [DOI] [PubMed] [Google Scholar]
- 56.Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2011;118:2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 59.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. discussion -3. [DOI] [PubMed] [Google Scholar]
- 60.Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–706. doi: 10.1007/s11605-007-0384-8. [DOI] [PubMed] [Google Scholar]
- 61.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 62.Wade TP, el-Ghazzawy AG, Virgo KS, Johnson FE. The Whipple resection for cancer in U.S. Department of Veterans Affairs Hospitals. Ann Surg. 1995;221:241–248. doi: 10.1097/00000658-199503000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14:1320–1326. doi: 10.1245/s10434-006-9249-8. [DOI] [PubMed] [Google Scholar]
- 65.van Geenen RC, van Gulik TM, Offerhaus GJ, de Wit LT, Busch OR, Obertop H, et al. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. 2001;27:549–557. doi: 10.1053/ejso.2001.1162. [DOI] [PubMed] [Google Scholar]
- 66.Konstantinidis IT, Deshpande V, Zheng H, Wargo JA, Fernandez-del Castillo C, Thayer SP, et al. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J Gastrointest Surg. 2010;14:261–267. doi: 10.1007/s11605-009-1096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]


