Abstract
Background
According to international guidelines [European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD)], portal hypertension (PHTN) is considered a contraindication for liver resection for hepatocellular carcinoma (HCC), and patients should be referred for other treatments. However, this statement remains controversial. The aim of this study was to elucidate surgical outcomes of minor hepatectomies in patients with PHTN (defined by the presence of esophageal varices or a platelet count of <100 000 in association with splenomegaly) and well-compensated liver disease.
Methods
Between 1997 and 2012, a total of 223 cirrhotic patients [stage A according to the Barcelona Clinic Liver Cancer (BCLC) classification] were eligible for this analysis and were divided into two groups according to the presence (n = 63) or absence (n = 160) of PHTN. The demographic data were comparable in the two patient groups.
Results
Operative mortality was not different (only one patient died in the PHTN group). However, patients with PHTN had higher liver-related morbidity (29% versus 14%; P = 0.009), without differences in hospital stay (8.8 versus 9.8 days, respectively). The PHTN group showed a worse survival rate only if biochemical signs of liver decompensation existed. Multivariate analysis identified albumin levels as an independent predictive factor for survival.
Conclusions
PHTN should not be considered an absolute contraindication to a hepatectomy in cirrhotic patients. Patients with PHTN have short- and long-term results similar to patients with normal portal pressure. A limited hepatic resection for early-stage tumours is an option for Child–Pugh class A5 patients with PHTN.
Introduction
In 1996, the Barcelona group demonstrated the presence of clinically significant portal hypertension (PHTN), defined as a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg, to be the most powerful predictor of post-operative liver decompensation and, in a subsequent (1999) publication, of poor long-term outcome in Child–Pugh A cirrhotic patients submitted to hepatic resection (HR).1,2 However, measurement of HVPG is invasive and requires technical expertise, whereas other clinical parameters are more easily evaluated.3 Indeed, the presence of oesophageal varices as determined by endoscopy or significant splenomegaly (major diameter >12 cm) with a platelet count of <100 000/mm3 are considered surrogate markers of clinically significant PHTN, regardless of portal pressure measurements.4 As a consequence of these studies, the European Association for the Study of the Liver (EASL) and American Association for the Study of Liver Diseases (AASLD) guidelines consider PHTN to be a relative contraindication to HR because of the high risk of post-operative liver decompensation.4,5
The literature is conflicting on the prognostic role of PHTN in patients undergoing HR, as well as other important factors (e.g. the degree of liver decompensation or type of HR) that seem to influence the overall survival.6–11 The aims of this study were to assess the results of HR in cirrhotic patients with HCC with or without clinically significant PHTN, and the relationship in terms of survival between liver function parameters and the presence of clinical PHTN.
Patients and methods
Prospective databases (459 patients submitted to HR for HCC) from two institutions (San Paolo Hospital, Milan, Italy and Hopital Henri Mondor, Creteil, Paris) between February 1997 and May 2012 were analysed. All patients referred to the Italian Centre with the diagnosis of HCC were assessed for disease staging with a pre-established protocol until 2000;12,13 it was then updated according to the Barcelona Clinic Liver Cancer (BCLC) criteria.14 In Creteil, a similar algorithm has been used:15 selection criteria for HR in patients with transplantable HCC included a solitary tumour <5 cm, chronic hepatitis or Child's class A cirrhosis, no oesophageal varices and a platelet count ≥100 × 109/l for major HR and varices <grade 2 for minor HR, and an estimated remnant liver volume >50%. Until 1998, all HR were performed through a subcostal incision. Since 1998, the laparoscopic approach has been used in selected patients for limited resection of peripheral HCC <5 cm located in segments 2 to 6.
All patients were discussed at a weekly multidisciplinary meeting at which surgeons, hepatologists and radiologists exchanged opinions. The diagnosis and staging of HCC was based on the appropriate imaging studies including triple-phase computed tomography (CT) and/or magnetic resonance (MR) according to the Barcelona-2000 EASL Conference, and histological assessment when required.16 Eligibility for liver transplantation [according to age, aetiology, Child–Pugh and model for end-stage liver disease (MELD) score] or HR was evaluated. Unlike the BCLC treatment protocol,12,13 in the time interval of this study, we did not consider nodule size and number as absolute exclusion criteria from surgical treatment. If no resection options were feasible, patients were considered for laparoscopic or percutaneous interstitial therapy, the latter for patients at higher surgical risk. Transarterial chemoembolization was considered when patients could not otherwise be treated by surgery or ablation.
In this cohort analysis, the residual liver function was evaluated using the Child–Pugh classification17 and MELD.18 Upon referral, laboratory tests including complete blood cell count, coagulation profile, liver functions, plasma levels of alpha-fetoprotein (AFP) and a chest X-ray were performed.
Patients were included in the present cohort analysis if they fulfilled all of the following criteria on presentation: no previous HR for HCC, a single lesion and tumour size less than 5 cm, Child–Pugh class A and BCLC stage classes A1 to A3. Patient characteristics and follow-up were recorded in a dedicated database.
The presence of pre-operative portal hypertension1,4,5 was evaluated retrospectively until 2000; from 2001, a clinical evaluation has been used and portal hypertension was arbitrarily defined as oesophageal varices detected by endoscopy or a splenomegaly (major diameter >12 cm) with a platelet count <100 000/mm3, according to the BCLC group criteria.4,5 Direct measurement of venous pressure was not performed routinely in the current series,
All surgical procedures included intra-operative ultrasonography (IOUS) examinations with intra-operative or laparoscopic probes equipped with a multi-frequency linear-array transducer. A laparotomy was performed using a standardized technique.13,15 Laparoscopic HR was the first choice in patients with HCC lesions limited to the left lateral section of the liver or segments IVB, V and VI.19–21 The specimens were evaluated histologically according to Ishak et al.22 for fibrosis stage (0–6). Tumour grade was assessed using the system outlined by Edmondson and Steiner,23 and was based on the area showing the highest grade.24
Liver US and CT (and/or MRI) were performed within 3 months of treatment to assess the response to HR. Patients were further followed locally by an expert hepatobiliary team every 6 months. Physical examination, liver function tests, serum AFP level, liver US (twice a year) and CT (twice a year) were included.
Statistical analysis
The primary outcome was overall survival. Liver decompensation was defined by the presence of ascites, acute encephalopathy and/or jaundice (bilirubin level more than 3 mg/dl on postoperative biochemical examinations). The follow-up time was defined as the number of months from surgical treatment of HCC to death, or last contact with the patient.
Comparison of continuous variables between and within groups was performed using the Mann-Whitney U-test and Wilcoxon's matched pairs test. Continuous variables were also compared by one-way analysis of variance (ANOVA) for normally distributed variables between staging groups. Comparison of proportions was performed with Fisher's exact probability test. Bonferroni's correction for multiple comparisons was applied. Data following a normal distribution were expressed as mean ± standard deviation; if non-parametric, median and range were reported.
The univariate association of each parameter with survival rates was estimated by comparing actuarial curves according to the Kaplan–Meier product-limit method and log-rank test, which more accurately characterizes the final outcome. The test trend of the survivor function across the ordered groups was also calculated: a relative hazard using the Cox regression-based test was used to evaluate the weight of each subgroup in determining significance.
Initial evaluation and subsequent follow-up data were collected in a dedicated database (FileMaker Pro for Macintosh; FileMaker Inc., Santa Clara, CA, USA) and subsequently analysed (Intercooled Stata 10.0 for Macintosh, Stata Corp., College Station, TX, USA).
The study was approved by the both hospitals' Ethics Committees, and written informed consent for recording and analysis of data were obtained from all patients.
Results
On the basis of inclusion criteria, 223 (48.6%) out of 459 patients were included in this analysis: 160 (71.7%) patients were included in a group without portal hypertension (no PHTN) whereas the other 63 (28.3%) patients comprised the portal hypertension (PHTN) group. In this group, 48 (76%) patients had oesophageal varices detected by endoscopy (only 5 patients with F2 esophageal varices) whereas the remaining 15 (24%) patients had indirect signs of portal hypertension as previously defined. Direct measurement of HVPG was performed in only 21 out of 223 patients.
Differences in characteristics of patients with PHTN and those without PHTN are shown in Table 1. In particular, patients with PHTN had more evident signs of liver dysfunction even if all patients were classified as liver function in Child–Pugh A class: they had lower serum albumin levels, higher total bilirubin levels, higher INR (International Normalized Ratio) values resulting in higher MELD scores and degree of fibrosis; conversely, they had similar tumour diameter and AFP levels. Regarding fibrosis, Fig. 1 shows that 90% of PHTN patients had severe fibrosis (grade 6). Table 2 shows intra-operative findings: patients with PHTN underwent a lower rate of HR of ≥2 segments (17% versus 31%; P = 0.038), whereas no differences were found regarding the other parameters including the number of patients transfused (11% versus 5.6%; P = NS).
Table 1.
Baseline characteristics of patients submitted to hepatic resection for hepatocellular carcinoma (HCC) with portal hypertension (PHTN) or without portal hypertension (no PHTN)
| Preoperative data | 160 no-PHTN | P | 63 PHTN |
|---|---|---|---|
| Gender M/F | 112/48 70%/30% | NS | 45/18 71%/29% |
| Age (years) | 66.8 ± 9.6 | NS | 65.3 ± 9.5 |
| Aetiology (HCV, HBV or other) | 91; 29; 40 57%; 18%; 25% | NS | 44; 9; 10 70%; 14%; 16% |
| Liver cirrhosis | 153 (96%) | NS | 63 (100%) |
| Child–Pugh class A | 100% | NS | 100% |
| MELD | 7.9 ± 1.4 | 0.001 | 9.5 + 2.8 |
| BCLC (A1; A2; A3) | 160/0/0 | 0.001 | 0/37/26 |
| Varices (F0) | 0% | 0.001 | 48 (76%) |
| Fibrosis (Ishak) | 5.1 ± 1.2 | 0.001 | 5.9 ± 0.4 |
| Bilirubin level (mg/dl) | 0.80 ± 0.36 | 0.001 | 1.39 ± 1.55 |
| Abnormal bilirubin (>1 mg/dl) | 28 (19%) | 0.001 | 36 (57%) |
| Albumin level (g/dl) | 4.13 ± 0.50 | 0.001 | 3.71 ± 0.49 |
| Abnormal albumin (<3.5 g/dl) | 12 (9%) | 0.001 | 21 (36%) |
| Prothrombin time (INR) | 1.09 ± 0.10 | 0.001 | 1.18 ± 0.12 |
| Abnormal INR (> 1.2) | 7 (7%) | 0.001 | 19 (36%) |
| HCC Diameter (mm) | 28.3 ± 10.9 | NS | 28.5 ± 11.1 |
| Edmondson–Steiner's classification (I-II; III-IV) | 143; 9 (94%; 6%) | NS | 53; 6 (90%; 10%) |
| Vascular invasion (present) | 49 (32%) | NS | 14 (23%) |
| AFP > 20ng/dl | 50 (31%) | NS | 19 (30%) |
MELD, model for end-stage liver disease; BCLC, Barcelona Clinic Liver Cancer; INR, international normalized ratio; HCC, hepatocellular carcinoma.
Figure 1.
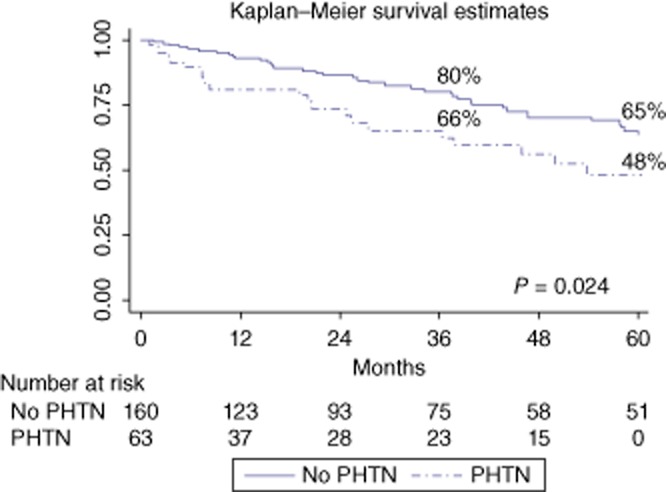
Overall survival curves of the whole study population of 223 cirrhotic patients undergoing a hepatic resection (HR) for hepatocellular carcinoma (HCC) with the portal hypertension (PHTN) or without PHTN (P = 0.024)
Table 2.
Intra-operative findings
| 160 no-PHTN | P | 63 PHTN | |
|---|---|---|---|
| HR > 2 segments | 50 (31%) | 0.038 | 11 (17%) |
| Laparoscopic approach | 56 (35%) | NS | 26 (41%) |
| Operation time (min) | 196 ± 64 | NS | 189 ± 69 |
| Pringle manoeuver | 88 (55%) | NS | 31 (49%) |
| Pringle timing (min) | 30.5 ± 26 | NS | 30.2 ± 21 |
| Intra-operative bleeding (ml) | 292 ± 262 | NS | 340 ± 340 |
HR, hepatic resection; PHTN, portal hypertension, NS, not significant.
During the immediate post-operative period, signs of liver decompensation (the presence of ascites, encephalopathy and bilirubin levels more than 3 mg/dl) were observed more frequently in the PHTN group without influencing post-operative hospital stay (Table 3): in fact, 16 out of 18 complicated patients in the PHTN group (89%) and 18 out of 22 complicated patients in the no-PHTN group (82%) had class I-II complications according to Clavien.25 One patient died in the PHTN group as a result of liver failure resulting in a 30-day mortality rate of 0.5% (no statistical difference with the no-PHTN group which had no mortality). Also no statistical differences were found for 90-days mortality: 3 (2%) patients in the no-PHTN group and 4 (6%) patients in the PHTN group died (P = NS).
Table 3.
Post-operative results
| 160 no-PHTN | P | 63 PHTN | |
|---|---|---|---|
| Post-operative mortality | 0 | NS | 1 (0.5%) |
| Total number of blood transfusions | 34 | NS | 14 |
| Patients transfused | 4 (5.6%) | NS | 7 (11%) |
| Liver decompensation | 22 (14%) | 0.009 | 18 (29%) |
| Ascitesa | 18 (11%) | 0.035 | 14 (22%) |
| Encephalopathya | 4 (3%) | NS | 5 (8%) |
| Jaundicea | 2 (1%) | NS | 3 (5%) |
| Post-operative hospital stay (days) | 9.8 ± 7.5 | NS | 8.8 ± 5.5 |
PHTN, portal hypertension, NS, not significant.
More than one complication for each patient.
The median follow-up period after HR was 25 months (range: 1–170). Long-term overall survival (Fig. 1) was poorer in the PHTN group than in the no-PHTN group (3-/5-year: 66%/48% versus 80%/65%, respectively; P = 0.024). However, among patients with BCLC stage classification from A1 to A3 (introducing bilirubin into the univariate analysis) (Fig. 2a), the 3-/5-year overall survival rates were, respectively, 77%/65% for the BCLC A1 class (no-PHTN), 70%/50% for the BCLC A2 class (PHTN with normal bilirubin) and 58%/46% for the BCLC A3 class (PHTN with abnormal bilirubin) (P = 0.070). The crude differences in overall survivals were significant for comparisons between the BCLC A1 and A3 groups (P = 0.021), but not between the BCLC A1 and A2 and BCLC A2 and A3 groups (P = NS). Introducing the albumin value into the univariate analysis (Fig. 2b), the 3-/5-year overall survival rates were, respectively, 80%/65% in the no-PHTN group, 70%/70% in the PHTN group with normal albumin and 56%/22% in the PHTN group with abnormal albumin (P = 0.001). Finally, introducing the INR value in to the univariate analysis (Fig. 2c), the 3-/5-year overall survival rates were, respectively, 80%/65% in the no-PHTN group, 69%/50% in the PHTN group with normal INR and 57%/43% in the PHTN group with an abnormal INR (P = 0.010). Among patients with Child–Pugh stage A5 and A6 (Fig. 3), the 3-/5-year overall survival rates were, respectively, 80%/65% in the no-PHTN group (with Child–Pugh A5 or A6 class), 75%/75% in the PHTN group with Child–Pugh A5 and 53%/24% in the PHTN group with Child–Pugh A6 (P = 0.006).
Figure 2.
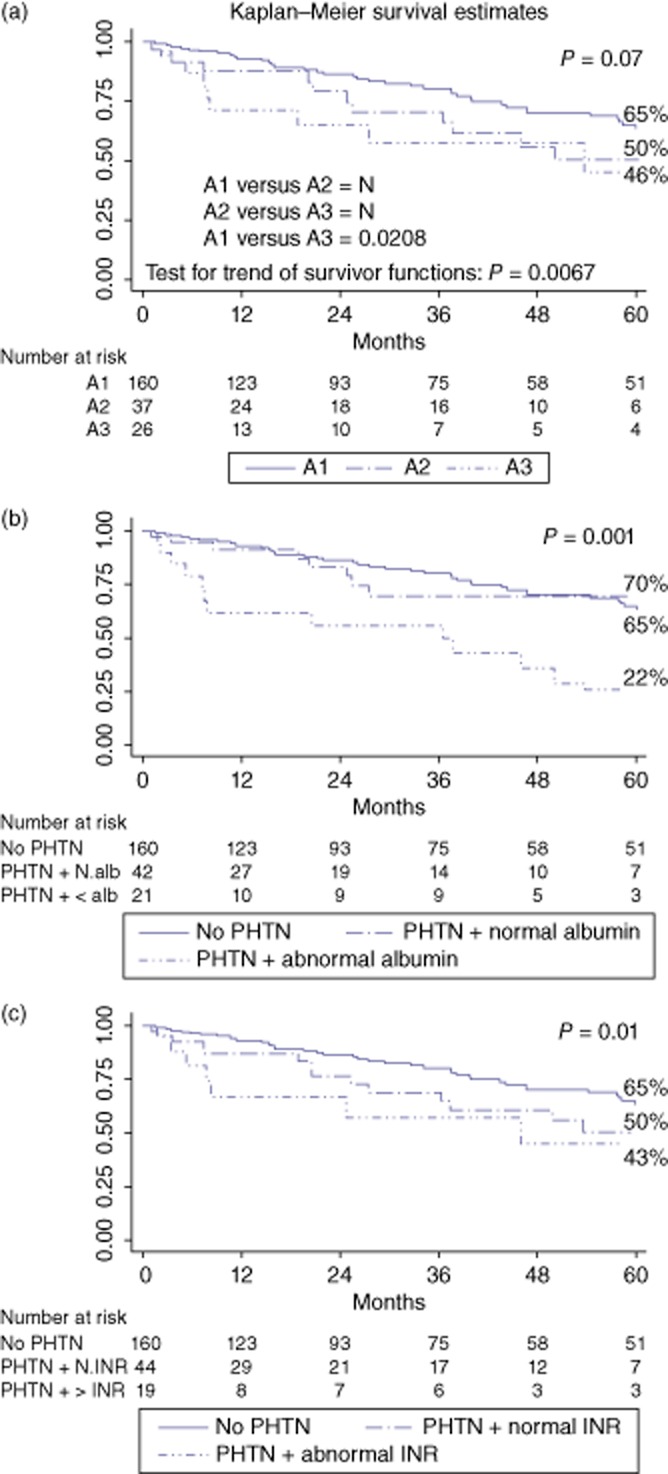
Survival curves according to the portal hypertension (PHTN) and biochemical parameters: (a) Barcelona Clinic Liver Cancer (BCLC) A1 class (no-PHTN) versus BCLC A2 class (PHTN and normal bilirubin) versus BCLC A3 class (PHTN and abnormal bilirubin); (b) no-PHTN vesus PHTN and normal albumin versus PHTN and abnormal albumin; (c) no-PHTN versus PHTN and normal international normalized ratio (INR) versus PHTN and an abnormal INR
Figure 3.
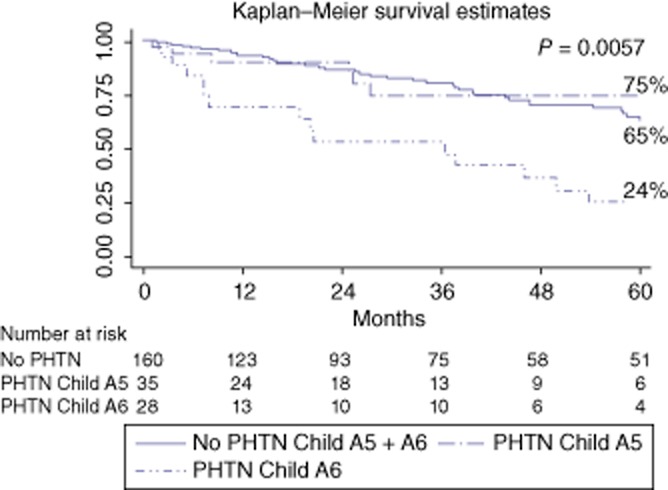
Survival curves according to portal hypertension (PHTN) and Child–Pugh A class (A5 and A6)
Multivariate analysis with Cox's regression confirmed that only the albumin was related to survival with a hazard ratio of 2.20 (P = 0.028), whereas PHTN, characteristics of tumour and type of HR were not related to survival (Table 4).
Table 4.
The results of univariate and multivariate analysis of the influence of pre- and intra-operative factors on the survival rates among the 223 patients with hepatocellular carcinoma (HCC) included in the present study
| Variables | Univariate analysis (P) | Hazard ratio | 95% CI | P |
|---|---|---|---|---|
| Aetiology (HCV versus other) | NS | |||
| BCLC (A1; A2; A3) | NS | |||
| Child-Pugh (A5 versus A6) | 0.008 | 1.540 | 0.840–2.825 | 0.162 |
| MELD (<9 versus > 9) | NS | |||
| HR (Laparoscopic versus laparotomy) | NS | |||
| HCC diameter (< versus >20 mm) | 0.009 | 1.609 | 0.902–2.869 | 0.107 |
| Edmondson classification (I-II versus III-IV) | 0.004 | 1.996 | 0.615–6.477 | 0.250 |
| Vascular invasion (absent versus present) | NS | |||
| Portal hypertension | 0.021 | 1.696 | 0.887–3.245 | 0.110 |
| INR (normal versus abnormal) | NS | |||
| Bilirubin (normal versus abnormal) | NS | |||
| Albumin (normal versus abnormal) | 0.001 | 2.204 | 1.088–4.463 | 0.028 |
| AST (normal versus abnormal) | NS | |||
| Creatinine (normal versus abnormal) | 0.039 | 1.671 | 0.615–4.539 | 0.314 |
| AFP (normal versus abnormal) | 0.030 | 1.650 | 0.929–2.929 | 0.087 |
BCLC, Barcelona Clinic Liver Cancer; MELD, model for end-stage liver disease; HR, hepatic resction; INR, international normalized ratio; AST, aspartate transaminase; AFP, alpha-fetoprotein; NS, not significant.
HCC recurrences were more frequent in the no PTHN group (54%) than in PTHN group (37%; P = 0.020).
Discussion
PHTN in cirrhotic patients is considered a relative contraindication for HR according to EASL/AASLD guidelines. Bruix et al.1 analysed the outcome of 29 Child–Pugh class A patients with a HVPG greater than 10 mmHg and observed a higher likelihood of post-operative hepatic decompensation in these patients compared with those without PHTN. In a subsequent publication,2 the same authors confirmed that PHTN and serum bilirubin levels were the independent prognostic factors affecting overall survival after HR.
However, these results have not been confirmed by other studies: in fact, recent Italian and Japanese studies have reported contradictory results.6–11 These studies demonstrated that cirrhotic patients with both clinically significant PHTN and well-preserved liver function (evaluated by Child–Pugh classification or ICGR 15 value or MELD score) had similar short- and long-term outcomes compared with patients without PHTN, above all if resection of two or less segments were performed.
Therefore, the prognostic relevance of clinically significant PHTN after HR in patients with HCC is still a matter for debate. Furthermore, two recent articles26,27 suggested that an increased HVPG was associated with post-operative liver failure and mortality after HR in patients with HCC and liver cirrhosis, whereas indirect criteria of clinically significant PHTN were not.
The current study confirmed that the presence of clinically significant PHTN (even if evaluated only by clinical criteria) could influence the post-operative course of cirrhotic patients submitted to HR. However, if stringent pre-operative selection criteria are met (i.e. Child–Pugh class A patients undergoing limited HR, with a laparoscopic approach if indicated) and limited HR are performed (less than 2 segments in 31% of the no PHTN group and 17% of the PHTN group), the post-operative mortality rate is very low (1.59% in the PHTN group and 0.45% in all patients). Furthermore, even if post-operative complications as a result of temporary hepatic failure were more frequent in the PHTN group, they were slight and transient as evidenced by Clavien's classified complications25 and the similar post-operative hospital stay in the two groups.
As regards the long-term outcomes, the data from the present study confirm the recent publications in the literature.6–11 If patients with and without PHTN irrespective of any sign of liver decompensation are analysed, long-term survival was significantly lower in patients with clinically significant PHTN (P = 0.024). However, including laboratory parameters in the analysis of long-term survival (i.e. albumin, bilirubin and INR), it is possible to identify a subgroup of patients in which the association of clinically significant PHTN with an abnormal biochemical parameter has prognostic relevance after HR. In other words, patients with clinically significant PHTN and Child–Pugh class A6 showed worse long-term survival after HR than patients without clinical PHTN or with PHTN and normal liver function parameters (Child–Pugh A5).
Probably, clinically significant PHTN influenced the outcome of patients submitted to HR only if some degree of hepatic compensation started (Child–Pugh A6), even if methods of liver function evaluation seemed to show good hepatic reserve (Child–Pugh A class). In fact, as suggested by other publications,26,28 PHTN is well correlated with the degree of liver fibrosis and in the present series, 90.5% of patients with PHTN had severe fibrosis (grade 6 according Ishak classification) in comparison with patients without PHTN (56.5%; P = 0.004) while no patient with PHTN had mild fibrosis (grade 0–3).
In conclusion, the current study indicates that cirrhotic patients with HCC and hepatic compensation (Child–Pugh A class) being considered for HR should be evaluated not only for the presence of clinically significant PHTN, but for biochemical parameters as well: patients with clinically relevant PHTN and abnormal biochemical parameters (e.g. bilirubin, albumin or INR) should be referred for other treatment options such as radiofrequency ablation and/or OLT. Patients without PHTN or with clinically significant PHTN and preserved liver function (Child–Pugh A5 class) can undergo HR with the best chances of long-term survival without post-operative impairment of liver function. If possible, limited HR (less than 2 segments) and a laparoscopic approach should be pursued. If a major HR is necessary, non-surgical therapies should be pursued even if it is known that patients with clinically significant PHTN have a negative prognosis irrespective of the treatment choice, or liver transplantation should be considered the only valid therapeutic strategy.
Conflicts of interest
None declared.
References
- 1.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 3.De Franchis R, Dell'Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis. 2008;40:312–317. doi: 10.1016/j.dld.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capussotti L, Ferrero A, Vigano L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30:992–999. doi: 10.1007/s00268-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 7.Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, et al. Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patient with cirrhosis. Ann Surg Oncol. 2008;15:1670–1676. doi: 10.1245/s10434-008-9880-7. [DOI] [PubMed] [Google Scholar]
- 8.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 9.Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- 10.Choi GH, Park JY, Hwang HK, Kim DH, Kang CM, Choi JS, et al. Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Intern. 2011;31:485–493. doi: 10.1111/j.1478-3231.2010.02436.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruzzenente A, Valdegamberi A, Campagnaro T, Conci S, Pachera S, Iacono C, et al. Hepatocellular carcinoma in cirrhotic patients with portal hypertension: is liver resection always contraindicated? World J Gastroenterol. 2011;17:5083–5088. doi: 10.3748/wjg.v17.i46.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santambrogio R, Costa M, Barabino M, Opocher E. Laparoscopic radiofrequency of hepatocellular carcinoma using ultrasound-guided selective intrahepatic vascular occlusion. Surg Endosc. 2008;22:2051–2055. doi: 10.1007/s00464-008-9751-0. [DOI] [PubMed] [Google Scholar]
- 13.Santambrogio R, Opocher E, Zuin M, Selmi C, Bertolini E, Costa M, et al. Surgical resection versus laparoscopic radiofrequency ablation in patients with hepatocellular carcinoma and Child-Pugh class A liver cirrhosis. Ann Surg Oncol. 2009;16:3289–3298. doi: 10.1245/s10434-009-0678-z. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 15.Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, et al. Liver resection for transplantable hepatocellular carcinoma. Long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona – 2000 EASL Conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 17.Pugh RNH, Murray-Lyon M, Dawson JL, Pietroni MC, Williams R. Transection of the esophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 18.Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, et al. Prognosis of hepatocellular carcinoma: comparison of seven staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 19.Santambrogio R, Opocher E, Pisani Ceretti A, Barabino M, Costa M, Leone S, et al. Impact of intraoperative ultrasonography in laparoscopic liver surgery. Surg Endosc. 2007;21:181–188. doi: 10.1007/s00464-005-0738-9. [DOI] [PubMed] [Google Scholar]
- 20.Santambrogio R, Aldrighetti L, Barabino M, Pulitanò C, Costa M, Montorsi M, et al. Laparoscopic liver resections for hepatocellular carcinoma. Is it a feasible option for patients with liver cirrhosis? Langenbecks Arch Surg. 2009;394:255–264. doi: 10.1007/s00423-008-0349-8. [DOI] [PubMed] [Google Scholar]
- 21.Cherqui D, Laurent A, Tayar A, Chang S, Van Nhieu JT, Lotiau J, et al. Laparoscopic Liver Resection for Peripheral Hepatocellular Carcinoma in Patients With Chronic Liver Disease Midterm Results and Perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 23.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka Y, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;5:722–731. doi: 10.1097/00000658-200205000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 633 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boleslawski E, Petrovai G, Truant S, Dharancy S, Duhamel A, Salleron J, et al. hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg. 2012;99:855–863. doi: 10.1002/bjs.8753. [DOI] [PubMed] [Google Scholar]
- 27.Stremitzer S, Tamandl D, Kaczirek K, Maresch J, Abbasov B, Payer BA, et al. Value of hepatic venous pressure gradient measurement before liver resection for hepatocellular carcinoma. Br J Surg. 2011;98:1752–1758. doi: 10.1002/bjs.7672. [DOI] [PubMed] [Google Scholar]
- 28.Samonakis DN, Cholongitas E, Thalheimer U, Kalambokis G, Quaglia A, Triantos CK, et al. Hepatic venous pressure gradient to assess fibrosis and its progression after liver transplantation for HCV cirrhosis. Liver Transpl. 2007;13:1305–1311. doi: 10.1002/lt.21227. [DOI] [PubMed] [Google Scholar]


