Abstract
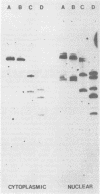
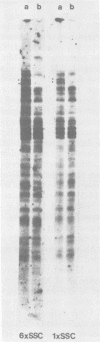
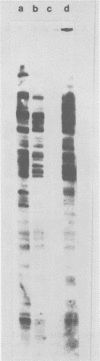
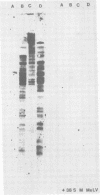
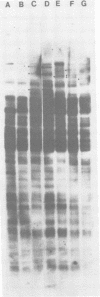
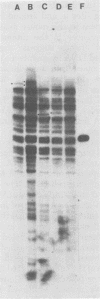
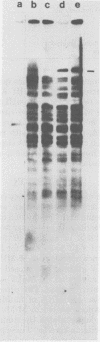
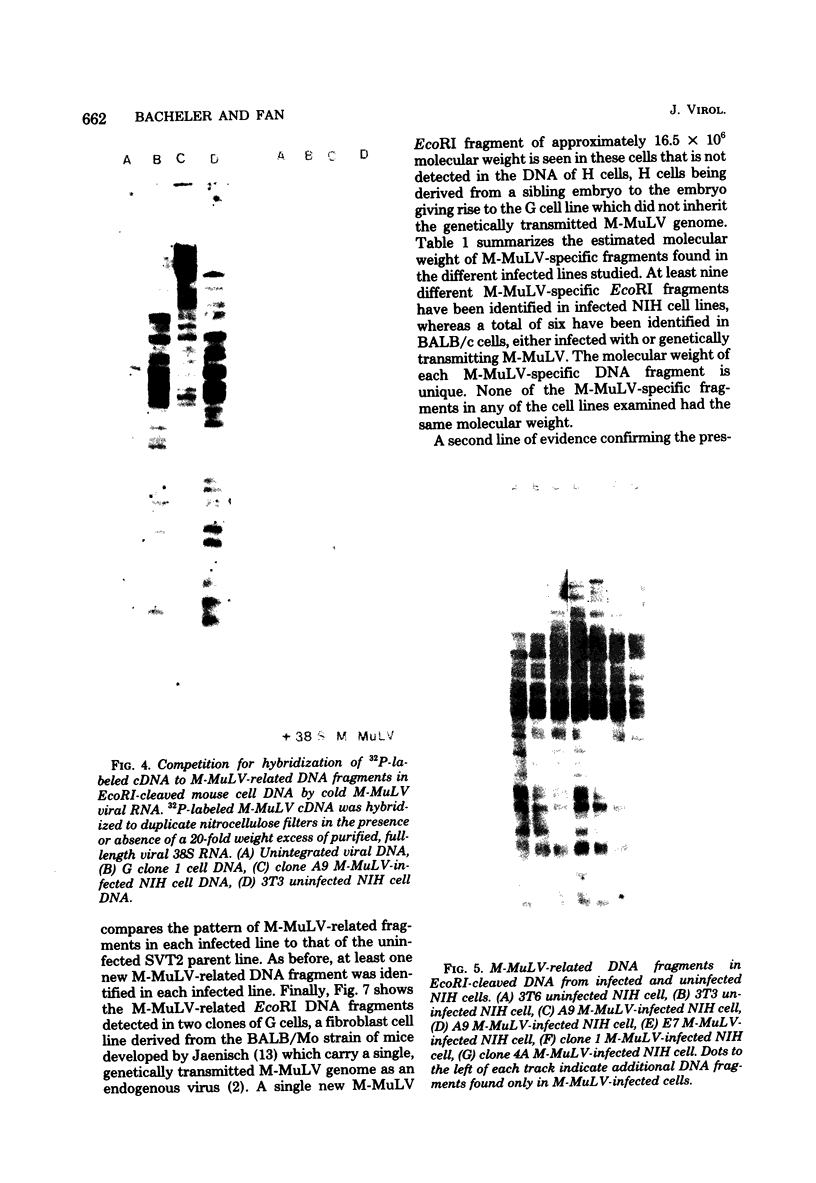
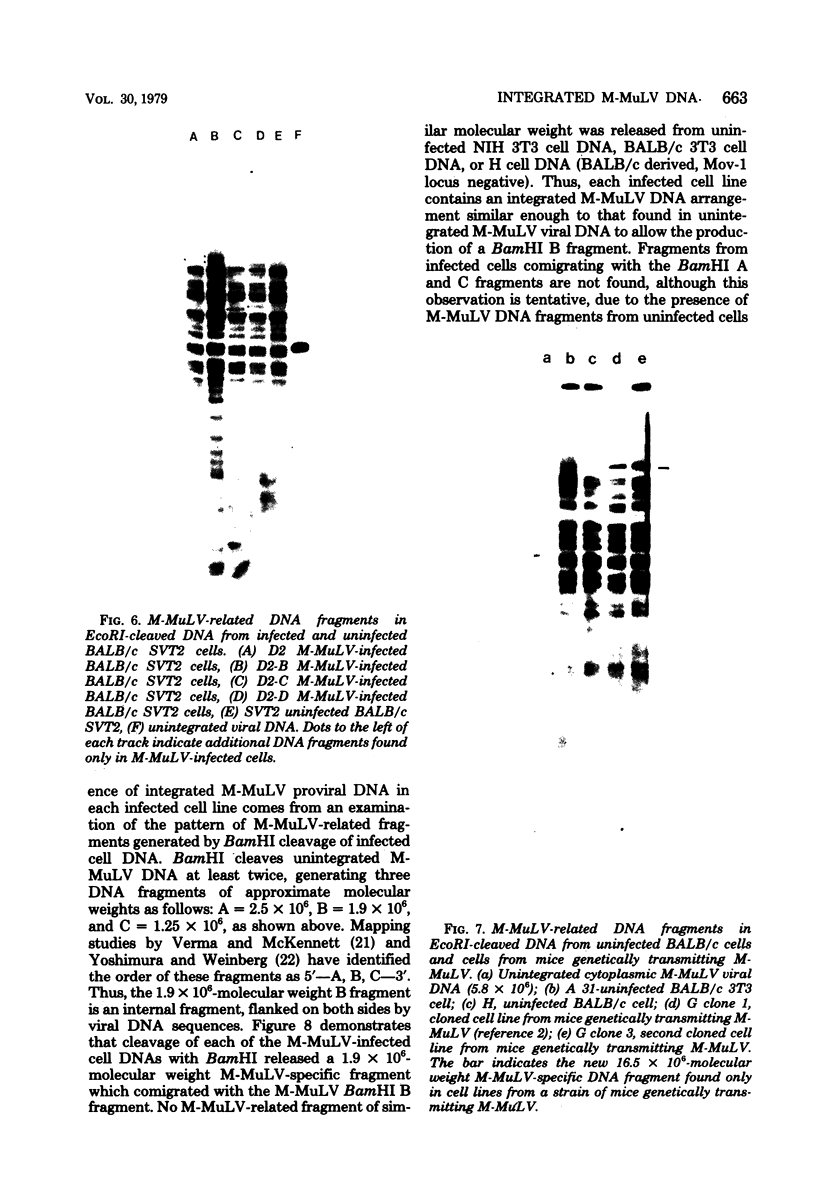
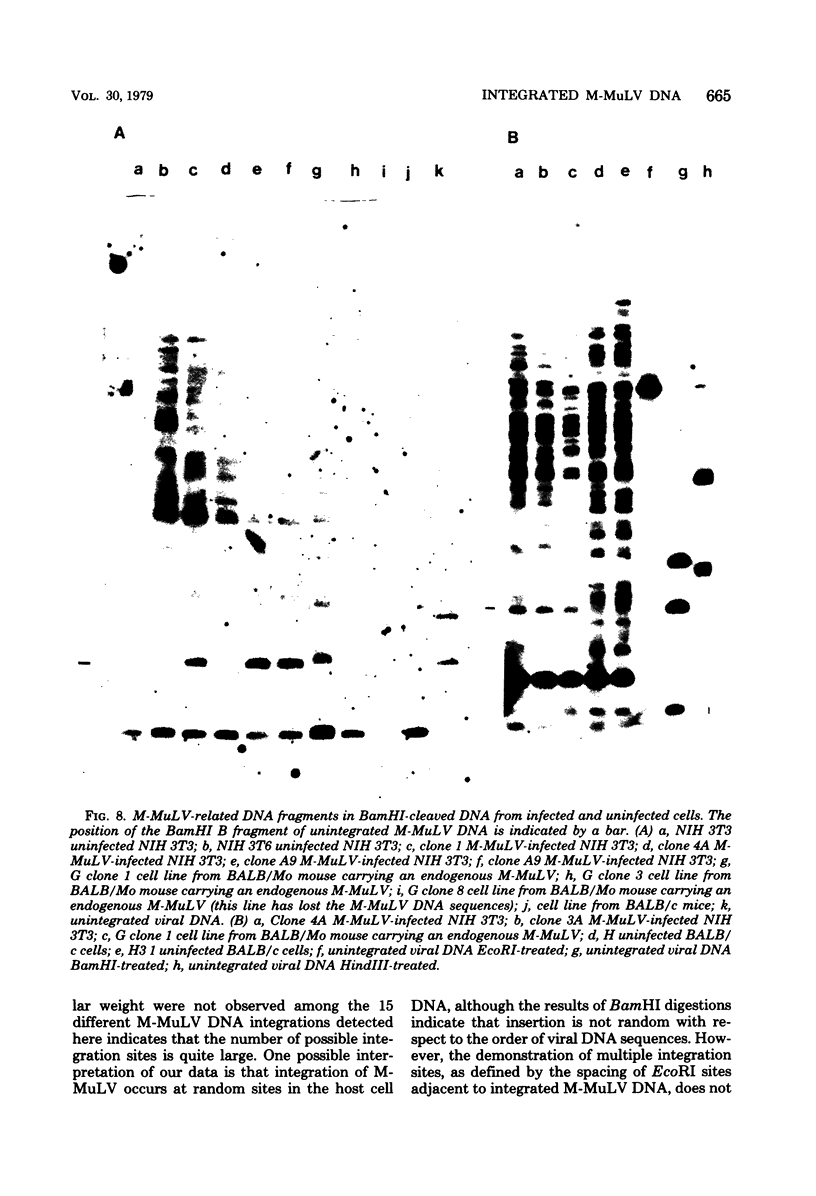
The integration sites for viral DNA in cells infected with Moloney murine leukemia virus (M-MuLV) were studied by restriction endonuclease cleavage of cellular DNA followed by electrophoresis in agarose gels, blot transfer to nitrocellulose, and detection by M-MuLV-related sequences by hybridization with high-specific-activity 32P-labeled M-MuLV complementary DNA. When EcoRI was used to cleave cellular DNA, numerous DNA fragments with sequence homology to M-MuLV were detected in uninfected mouse cell DNA. These endogenous sequences are mouse specific since they are not detectable in rat cell DNA, and are related to the 38S genomic RNA of M-MuLV. Infected cells contain additional M-MuLV-specific DNA fragments which are not detected in uninfected cells. Different patterns of M-MuLV-specific DNA fragments were detected in each cloned infected line examined. These data suggest the existence of multiple sites for integration of M-MuLV DNA in infected mouse fibroblasts. Cleavage of infected cell DNA with BamHI, which cleaves M-MuLV viral DNA at least twice, released the internal BamHI B fragment from each infected line, confirming the presence of integrated M-MuLV DNA sequences in each infected cell line which retain some features of the sequence organization of unintegrated M-MuLV DNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacheler L. T. Virus-secific transcription in 3T3 cells transformed by the ts-a mutant of polyoma virus. J Virol. 1977 Apr;22(1):54–64. doi: 10.1128/jvi.22.1.54-64.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacheler L., Jaenisch R., Fan H. Highly inducible cell lines derived from mice genetically transmitting the Moloney murine leukemia virus genome. J Virol. 1979 Mar;29(3):899–906. doi: 10.1128/jvi.29.3.899-906.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A., Jaenisch R. Increase of AKR-specific sequences in tumor tissues of leukemic AKR mice. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2448–2452. doi: 10.1073/pnas.73.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. k., Rowe W. P., Levine A. S. Quantitative studies of integration of murine leukemia virus after exogenous infection. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4095–4099. doi: 10.1073/pnas.73.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fan H., Jaenisch R., MacIsaac P. Low-multiplicity infection of Moloney murine leukemia virus in mouse cells: effect on number of viral DNA copies and virus production in producer cells. J Virol. 1978 Dec;28(3):802–809. doi: 10.1128/jvi.28.3.802-809.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Hutton J. R., Smotkin D., Weinberg R. A. Proviral DNA of Moloney leukemia virus: purification and visualization. Science. 1976 Feb 13;191(4227):569–571. doi: 10.1126/science.1251192. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Fan H., Croker B. Infection of preimplantation mouse embryos and of newborn mice with leukemia virus: tissue distribution of viral DNA and RNA and leukemogenesis in the adult animal. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4008–4012. doi: 10.1073/pnas.72.10.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Sites of integration of reticuloendotheliosis virus DNA in chicken DNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3372–3376. doi: 10.1073/pnas.75.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M., Campo M. S., Gummerson K. S. Conservation of cytoplasmic poly (A)-containing RNA in mouse and rat. Nature. 1975 Dec 25;258(5537):682–686. doi: 10.1038/258682a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Verma I. M., McKennett M. A. Genome organization of RNA tumor viruses II. Physical maps of in vitro-synthesized Moloney murine leukemia virus double-stranded DNA by restriction endonucleases. J Virol. 1978 Jun;26(3):630–645. doi: 10.1128/jvi.26.3.630-645.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F. K., Weinberg R. A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979 Feb;16(2):323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]