Abstract
Nutrient allocation and usage plays an important part in regulating the onset and progression of age-related functional declines. Here, we describe a heterozygous mutation in Drosophila (dFatp) that alters nutrient distribution and multiple aspects of physiology. dFatp mutants have increased lifespan and stress resistance, altered feeding behavior and fat storage, and increased mobility. Concurrently, mutants experience impairment of cardiac function. We show that endurance exercise reverses increased lipid storage in the myocardium and the deleterious cardiac function conferred by dFatp mutation. These findings establish a novel conserved genetic target for regulating lifespan and physiology in aging animals. These findings also highlight the importance of varying exercise conditions in assessing aging functions of model organisms.
Keywords: aging, cardiac declines with age, drosophila, life span, senescence, training
Introduction
Fatty acids (FA) are a significant dietary component in diverse animal species, necessary to form precursors for cell membranes and chemical messengers (Grammatikos et al., 1994). In addition, FA act as signaling molecules to regulate multiple physiological processes, including electrical impulse transmission, signal transduction, and gene expression (Pegorier et al., 2004).
Dysregulation of fatty acid levels or activities contributes to several important pathologies that are associated with the aging process, particularly the interrelated network of obesity, cardiovascular disease, and type 2 diabetes that comprises the metabolic syndrome (Ford, 2010). Extensive epidemiological evidence exists that high-fat diets and obesity can induce pathological processes leading to increasing atherosclerosis, inflammation, hypertension, and dyslipidemia (Short et al., 2009; King et al., 2010). Additionally, lipids are observed to accumulate during aging in cardiac myocytes (Slawik & Vidal-Puig, 2006). These changes promote insulin resistance and cardiovascular disease, which, in turn, are enhanced by changes brought about through normal aging.
Aging per se causes reduction in cardiac performance, both indirectly through alterations in vascular health and directly through changes in myocardial physiology (Lakatta, 2003). These changes are mediated in part by alterations in fatty acid usage. During human aging, a decrease in fatty acid usage in the myocardium has been observed (Kates et al., 2003), suggesting a decrease in FA-derived ATP production, perhaps leading to energetically compromised hearts. These changes are mediated in part by age-related changes in myocardial gene expression of factors essential for beta-oxidation of FA (Weindruch, 2003). In turn, changes in cardiac gene expression and physiology are modulated by dietary intake, because they can be reversed by caloric restriction (Dhahbi et al., 2006).
Dysregulation of fatty acid uptake and usage is therefore a potentially important factor in the etiology of several health problems prevalent in the elderly population. One potential area of regulation that could be targeted for potential interventions is fatty acid transport. Uptake of long-chain FA (LCFAs) is thought to occur by two different mechanisms: diffusion and protein-mediated transport (Ehehalt et al., 2006). Several proteins have been identified that have a high affinity for LCFAs. These include FABPpm, FAT/CD36, and the fatty acid transport protein (FATP) family (Stahl, 2004).
Several mammalian FATPs have been identified, and recent work suggests that these proteins may not influence cellular uptake of FA by direct transport, but instead may regulate the usage by virtue of their intrinsic acyl-CoA synthetase activity (Ehehalt et al., 2006). In addition to regulating the concentration of FA available for β-oxidation, such activity also facilitates passive transport mechanisms by altering the gradient of fatty acid availability inside the cell, a process known as vectorial acylation (Black & DiRusso, 2007). The six mammalian FATP family members, despite a high degree of sequence similarity, exhibit different functional properties by virtue of their different expression patterns (Stahl, 2004). FATP6 is the predominant FATP in the mammalian heart, while FATP1 is expressed in the heart, skeletal muscle, as well as highly in adipose tissue (Hirsch et al., 1998; Stahl et al., 2001; Stahl, 2004).
In cultured fibroblasts, overexpression of mouse FATP1 leads to an increase in long-chain fatty acid uptake (Schwenk et al., 2010). Conversely, disrupting the FATP1 homologue fat1 in yeast Saccharomyces cerevisiae impairs long-chain fatty acid uptake (Zou et al., 2002). FATP1 has been shown to possess acyl-CoA synthetase activity, and the ability of FATP1 to regulate fatty acid uptake is dependent on such activity (Richards et al., 2006).
Flies have previously been shown to be effective models for the study of aging (Hughes & Reynolds, 2005), fat metabolism (Teleman, 2010), adult cardiac function (Piazza & Wessells, 2011), and endurance exercise (Piazza et al., 2009). Endurance exercise has long been known to improve cardiovascular function in vertebrates (Kemi & Wisloff, 2010), and flies also respond to endurance training by improving cardiac output and stress resistance at advanced ages (Piazza et al., 2009). This, combined with the unparalleled genetic tools available for studies in the fly, presents an important opportunity to examine the impact of FATP family genes on metabolism and function at varying ages and varying degrees of exercise.
The genome project has identified three FATPs in flies, based on sequence homology. The Drosophila dFatp gene encodes a product closely related to hsFATP1 (Hirsch et al., 1998). Here, we examine the role of this FATP ortholog in metabolism, lifespan, stress resistance, mobility, and cardiac performance. We find that flies carrying one copy of a loss-of-function mutation in the dFatp gene exhibit increases in triglyceride storage and circulating free FA, with a concurrent reduction in feeding behavior. These metabolic and behavioral alterations lead to an extended lifespan as well as improved resistance to multiple stresses. However, dFatp mutants exhibit defects in cardiac performance, which can be selectively reversed by an endurance exercise program.
Results
Gene locus
We obtained a line containing a gene trap insertion in the dFatp locus dFatpK10307 (Fig. S1A). This insertion is homozygous lethal, but heterozygotes are viable with a 72% reduction in mRNA expression (Fig. S1B). We attribute the phenotypes observed in dFatpK10307 heterozygotes to the dFatp locus for the following reasons: (i) no significant alteration in mRNA expression is seen in any of three neighboring genes (Fig. S1C–E); (ii) the insertion is lethal over deletions in the region, but not over single-gene null mutants in the region other than dFatp itself; and (iii) precise excision of the insertion restores phenotypes to wild-type. These flies will be referred to as dFatp revertants. An inducible RNAi construct targeting the dFatp gene product provides an effective knockdown with a ubiquitous da-gal4 expression driver. Ubiquitous induction of this construct throughout development generates a semi-lethal phenotype with an 83% reduction in dFatp mRNA levels (Fig. S1B).
To avoid complicating effects from mutations contained on balancer chromosomes, we have performed all experiments described below on flies in which the dFatpK10307 insertion has been backcrossed to the starter line in which the original insertion was isolated. The insertion was backcrossed 10 times, using the w+ associated with the insertion as a selectable marker. y1w67c23;dFatpK10307/+ flies will henceforth be referred to as yw;dFatphet and compared to background and age-matched y1w67c23 flies. y1w67c23 flies will be referred to as yw. To account for the possibility that the effects of the dFatpk10307 insertion are specific to the y1w67c23 background, we also outcrossed the dFatpk10307 insertion into the y1w1 background using similar methods, except only one round of additional backcrossing was employed in this case. We separately performed a single outcross of the dFatpk10307 insertion into the white Canton S (w[cs]) background. w[cs] is the common Canton S background with a white mutation backcrossed in (Cook-Wiens & Grotewiel, 2002). This stock allows for transgenes marked with a w+ to be backcrossed into a healthy background for physiological experiments. The aforementioned outcrosses will henceforth be referred to as y1w1;dFatphet and w[cs];dFatphet. y1w1;dFatphet is compared to y1w1, while w[cs];dFatphet is compared to F1 progeny of a cross between dFatp revertant and w[cs]. Progeny of this cross are controls for the hybrid background created by the single outcross and are referred to as w[cs];dFatp revertant.
dFatp expression has been reported to be enriched in the fat body, heart, and in multiple regions in the gut (http://www.flyatlas.org/). Here, we take advantage of the reduction in dFatp expression conferred by the dFatpK10307 insertion to examine the resulting metabolic and physiological effects.
Triglyceride storage and feeding rate
As fatty acid transporters are thought to participate in fatty acid metabolism, we measured the effect of reduction in dFatp expression on triglyceride storage. Whole yw;dFatphet males contain nearly fourfold higher triglyceride levels than age-matched yw males. Female yw;dFatphet flies have nearly twofold higher triglyceride levels than controls. Precise excision of the dFatp insertion returns triglyceride levels to wild-type (Fig. 1a). w[cs];dFatphet male flies display increased triglyceride levels (Fig. S2A,B), similar in magnitude to yw;dFatphet flies. TAG was normalized to total protein to control for differences in cell number or volume. Both yw;dFatphet and w[cs];dFatphet males have elevated levels of protein (Fig. S2C,D). Triglycerides normalized to total protein levels confirm increased TAG in dFatpk10307 heterozygotes regardless of genetic background (Fig. S2E,F). Similarly, triglyceride levels were increased when an inducible RNAi construct targeting the dFatp locus was expressed in adipose tissue, albeit to a lesser extent (Fig. 1b).
Fig. 1.
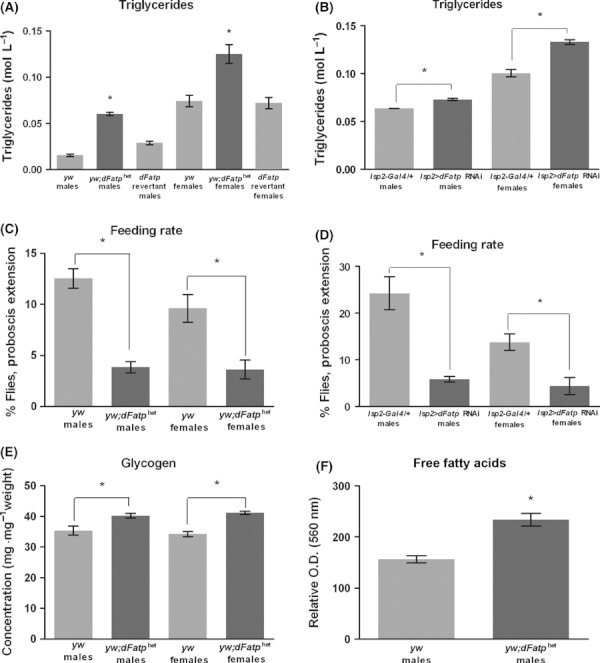
Reduction in dFatp expression increases energy storage while lowering feeding rate. (A) Triglyceride levels of yw;dFatphet flies are compared to yw background controls. Mutants have significantly higher levels of TAG (t-test: P < 0.001, males, P = 0.013, females). dFatp revertant flies return to wild-type triglyceride levels (t-test P = 0.646). (B) Knockdown of dFatp in fat (lsp2 > dFatp RNAi) causes an increase in TAG compared to control flies (t-test: P = 0.013 males and 0.002 females). (C) yw;dFatphet mutants have a significantly reduced feeding rate compared to yw background controls (t-test: P = 0.022 males and P = 0.016 females). (D) Knockdown of dFatp in fat (lsp2 > dFatp RNAi) causes a significant decrease in feeding rate (t-test: P = 0.006 males and P = 0.02 females). (E) Glycogen levels in total fly homogenate are significantly higher in both male and female mutants (t-test: P = 0.027 males and P < 0.001 females). (F) Free fatty acid levels in circulating hemolymph are significantly higher in male yw;dFatphet mutants than in yw controls (t-test: P = 0.0085). All experiments performed on 10- to 14-day-old flies.
The increased triglyceride storage could conceivably be explained by an increase in feeding behavior. To test this, we observed the feeding rate of yw;dFatphet and yw flies using the proboscis extension method (Wong et al., 2009). dFatphet flies instead display an approximately threefold reduction in feeding rate (Fig. 1c). Inducible RNAi against dFatp also produced a similar phenotype when expressed in adipose tissue (Fig. 1d).
Circulating glucose levels are marginally increased in both starved (Fig. S2G: t-test: P = 0.0476) and fed (Fig. S2H; t-test: P = 0.0073) female mutants. Glycogen levels in total fly homogenate are also mildly increased in both sexes (Fig. 1e). Additionally, dFatphet males have significantly increased free fatty acid levels in circulating hemolymph (Fig. 1f).
Lifespan extension
Changes in lipid metabolism are likely to have pleiotropic affects on animal physiology. We assessed the net effect of these changes on health and lifespan by measuring survival under standard laboratory conditions as well as several stress conditions.
During several survival experiments, we noted that background controls displayed substantial early-life mortality in several cases. The reason for this mortality is unclear. Recognizing that early control deaths might cause an overestimation of lifespan extension in mutants, we plotted and analyzed all survival curves with the first 20 days censored. In this way, we focus the statistical analysis and graphical display on the deaths that are related to aging rather than developmental or early-life events.
Survival was measured in three different backgrounds on 10% yeast, 10% sucrose diet. Male survival was increased in yw;dFatphet and y1w1;dFatphet backgrounds (Fig. 2a,b), but male lifespan was not significantly increased in a second yw;dFatphet repetition (Fig. S3A) or in the w[cs];dFatphet hybrid background (Fig. S3D). Survival of females was increased compared to background in yw;dFatphet (two repetitions) and in y1w1;dFatphet (Figs 2a,b and S3B,C log-rank for all female lifespans P < 0.0001). Fertility was not affected (Fig. S3E; t-test: P = 0.309). Comparison of female yw;dFatphet survival to revertant lines was inconclusive, however, as female revertants lived longer than yw controls (Fig. S3B), indicating that the survival phenotype of the insertion is not fully rescued by precise excision.
Fig. 2.
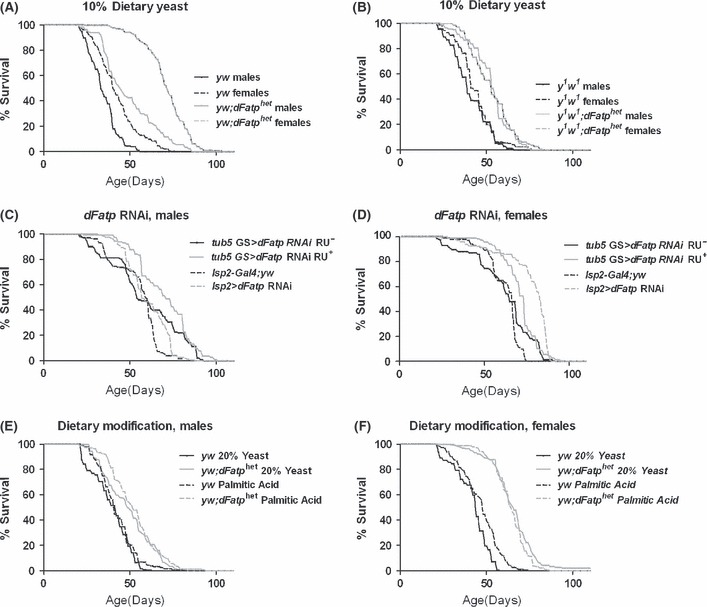
Reduction in dFatp expression extends lifespan and protects against high-nutrient diets. (A) Both male and female yw;dFatphet flies live longer than yw flies on a 10% sucrose, 10% yeast laboratory diet (log-rank: males: P = 0.0183, females: P < 0.0001). (B) Both male and female y1w1;dFatphet flies live longer than y1w1 flies on a 10% sucrose, 10% yeast laboratory diet (log-rank: P < 0.0001 for both). (C, D) dFatp RNAi flies of both sexes live longer than background controls on a 10% sucrose 10% yeast diet. dFatp expression was knocked down in adult flies ubiquitously (tub5 GS-gal4) and in adipose tissue (lsp2-gal4) (log-rank: tub5 GS > dFatp RNAi males: P = 0.0017, lsp2Gal4 males: P = 0.0002, P < 0.0001 for all females). (E, F) Both male and female yw;dFatphet flies aged on high-yeast or high-fatty-acid diets live longer than yw controls (log-rank: P < 0.0001 for all). Both yeast and palmitic acid were supplemented to 20% weight/volume. n ≥ 140 for all survival analyses. All statistical analysis here and in subsequent figures was performed with the first 20 days of life censored to remove deaths related to early mortality or developmental effects. Complete data can be visualized in the mortality curves shown in supplemental data and in accompanying life tables.
w[cs];dFatphet females lived dramatically longer than w[cs] females (Fig. S3C Iog rank: P < 0.0001). However, w[cs];dFatphet females did not have a significantly extended lifespan when compared to the hybrid w[cs];dFatp revertant controls, indicating that lifespan extension in this hybrid background can be accounted for by heterosis.
Age-specific mortality plots are in agreement with left-censored survival results, with low aging-related mortality in all backgrounds where lifespan extension is significant (Fig. S4A and S6A–C).
We examined the effects of adult-specific reduction in dFatp by inducing ubiquitous expression of RNAi against dFatp after adult eclosion. Both male and female flies expressing RNAi against dFatp had an extended lifespan as compared to background controls (Fig. 2c,d log-rank for males P = 0.0017, females: P < 0.0001), indicating that adult-specific interference with dFatp expression is sufficient to generate lifespan extension. Because dFatp expression has been reported to be enriched in the fat body, we induced RNAi against dFatp in adipose tissue. Male and female flies expressing RNAi against dFatp in the fat body displayed a significant lifespan extension compared to background controls (Fig. 2c,d, log-rank for males P = 0.002, females P < 0.0001). A plot of age-specific mortality reveals that females expressing dFatp RNAi have low mortality across ages (Fig. S4D). Males, by contrast, are extended primarily due to high mortality in controls at early ages (Fig. S4C). Taken together, this indicates that dFatp RNAi treatment is effective at slowing aging in females.
Because Fatp proteins are thought to be responsive to circulating fatty acid content, we wondered whether increasing dietary fatty acid content would reverse the lifespan advantage conferred by the mutation. We tested this by supplementing our standard laboratory diet of 10% dietary yeast, 10% dietary sucrose with an additional 10% weight/volume of yeast. On this high-nutrient diet, both mutants and control flies lived less long than on our standard diet. However, yw;dFatphet flies retained a substantial lifespan extension compared to the yw background (Fig. 2e,f; log-rank for males and females: P < 0.0001). Because yeast is a source of many nutrients, and not only lipids, we specifically supplemented the standard laboratory diet with 5 g L−1 per liter of palmitic or linoleic acid and measured survival of flies aged on these diets. Palmitic and linoleic acid were chosen because they represent the vast majority of fatty acid content in the brewer’s yeast used in this study (Mee et al., 1979). The increase in fatty acid percentage did not significantly alter media pH. yw;dFatphet flies retain a statistically significant survival extension on a diet supplemented with excess palmitic acid (Fig. 2e,f; log-rank for males and females: P < 0.0001), or linoleic acid (data not shown; log-rank for males and females: P < 0.0001). Mortality plots reveal a trend toward reduced mortality across ages in both male and female mutants subjected to either dietary treatment (Fig. S4E,F).
In summary, we find that reduction in dFatp expression by genomic mutation confers female lifespan extension in two of three genetic backgrounds tested. In addition, two different RNAi treatments against dFatp expression confer lifespan extension. Males, by contrast, display a more inconsistent and generally smaller lifespan extension when dFatp expression is reduced. Lifespan extension in neither sex is sensitive to increased dietary nutrient content, including increased fatty acid content.
Stress resistance
Lifespan extension under standard laboratory conditions may or may not involve increased resistance to more extreme conditions. Therefore, we tested the survival of yw;dFatphet flies under a variety of stressful conditions. Mutant males experience a statistically significant lifespan extension when aged at 28 °C, a condition of chronic heat stress (Fig. 3a; P < 0.0001, Fig. S4A). In the opposite case, when animals were aged at 4 °C, female, although not male, mutants exhibited a significant extension of lifespan (Fig. 3b; P < 0.0001 for females, Fig. S4A).
Fig. 3.
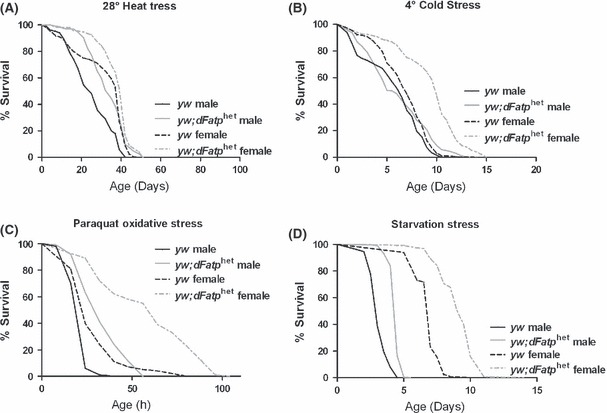
dFatphet flies display an increased resistance to multiple stresses. (A) Male yw;dFatphet flies live longer than yw flies under heat stress (28 °C) (log-rank: P < 0.0001). (B) Female dFatphet flies live longer than yw flies under cold stress (4 °C) (log-rank: P < 0.001). (C) Both male and female dFatphet flies were more resistant to paraquat than yw flies (log-rank: P < 0.0001). (D) Male and female dFatphet flies live longer than yw under agar-only starvation conditions (log-rank: P < 0.0001).
In order to test resistance to oxidative stress, we treated adults with paraquat and measured survival. Both male and female yw;dFatphet flies were long-lived compared to controls under paraquat treatment (Fig. 3c; P < 0.0001, Fig. S4A). In order to test resistance to starvation conditions, we aged yw;dFatphet flies and controls on agar-only food conditions. Both male and female yw;dFatphet flies were long-lived compared to controls (Fig. 3d; P < 0.0001 S4D). A potential caveat of the paraquat study is that reduced feeding rate in mutants may contribute to resistance by reducing ingestion of paraquat.
We conclude that reduction in dFatp expression is sufficient to provide protection simultaneously against multiple stresses.
Cardiac function
Mutations or interventions that extend lifespan and stress resistance may have either positive or negative effects on general vigor or function of major organ systems. Flies aged on a diet high in fat accumulate lipid in the heart and show symptoms of impaired cardiac function similar to human lipotoxic cardiomyopathy (Birse et al., 2010). As dFatphet flies have abnormally high triglyceride storage (Figs 1a and S2A) and high free FA in their circulation (Fig. 1f), we tested various indices of cardiac performance to assess potential impairment.
Using an external heart pacing method (Wessells & Bodmer, 2004), we tested cardiac stress resistance. The rate of cardiac failure in response to pacing stress is significantly higher in yw;dFatphet flies than in yw flies across ages (Fig. 4a; two-way anova: P < 0.0001). The slope of age-related decline, however, is identical between mutant and wild-type flies (multivariate regression; genotype-by-age effect: P = 0.7714), consistent with an acute defect rather than a progressive age-related impairment. To assess whether cardiac phenotypes of dFatp mutants are tissue-autonomous, we used a cardiac-specific driver (GMH5-Gal4) to perform heart-specific dFatp knockdown. Such flies also display an increased failure rate when subjected to external electrical pacing (Fig. 4b; two-way anova: P < 0.0001).
Fig. 4.
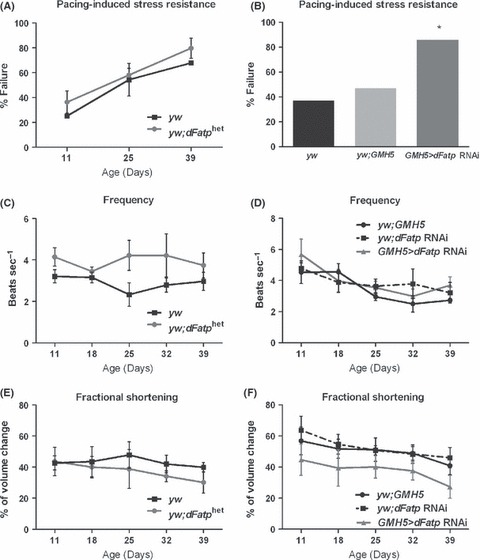
Reduction in dFatp in the heart impairs cardiac performance. (A) yw;dFatphet flies have an increased sensitivity to cardiac pacing across 5 weeks of age compared to yw (two-way anova: P < 0.0001). (B) Knockdown of dFatp specifically in the heart (GMH5 > dFatp RNAi) causes an increase in pacing-induced failure rate of 12-day-old flies (two-way anova: P < 0.0001). (C) Cardiac frequency is increased in yw;dFatphet flies across ages when compared to yw controls (two-way anova: P < 0.0001). (D) Knockdown of dFatp specifically in the heart does not significantly alter cardiac frequency (two-way anova: P = 0.5649). (E) yw;dFatphet hearts display a reduced fractional shortening across ages (two-way anova: P = 0.0343). (F) Knockdown of dFatp in the heart causes a reduction in fractional shortening across ages compared to background controls (two-way anova: P < 0.0001). n ≥ 10 flies for all cardiac analyses. Both male and female flies were analyzed and were not found to be significantly different. Therefore, male and female data were combined for all graphs in Figure 4.
The frequency of the heart rate is increased across ages in yw;dFatphet flies (Fig. 4c; two-way anova: P < 0.0001), and hearts with reduced levels of dFatp display a reduction in fractional shortening (Fig. 4e; two-way anova: P = 0.0343), an indirect measure of cardiac output. Flies expressing dFatp RNAi specifically in the heart do not exhibit defects in frequency resembling those of the mutants (Fig. 4d), but do show similar defects in fractional shortening (Fig. 4f; two-way anova: P < 0.0001). In summary, dFatphet hearts beat at a higher rate than wild-type flies, but exhibit weak contractions that are unlikely to pump as much hemolymph as those of wild-type flies. Cardiac-specific knockdown is sufficient to recapitulate fractional shortening and electrical pacing phenotypes, while the frequency defect may be regulated tissue non-autonomously.
Rescue of cardiac phenotype by exercise
yw;dFatphet flies are not defective in negative geotaxis ability. Rather, they have a delayed age-related decline in this measure of mobility and vigor in comparison with controls (Fig. 5a; genotype-by-age: P = 0.0019). We have recently developed a Drosophila exercise training protocol that increases negative geotaxis and cardiac function in males (Piazza et al., 2009). We used this protocol to determine whether yw;dFatphet males are capable of responding to endurance exercise. Indeed, mutant males demonstrate a significant degree of improvement in negative geotaxis following a 3-week training period of endurance exercise (Fig. 5b; treatment by age: P < 0.0001). This improvement is similar in magnitude to previous observations of multiple wild-type strains (Piazza et al., 2009).
Fig. 5.
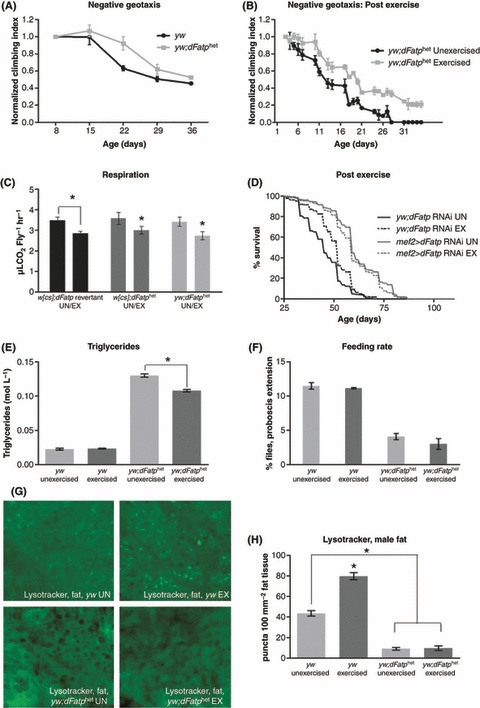
Endurance exercise rescues a subset of mutant phenotypes. (A) yw;dFatphet flies display a significantly slower age-related decline in negative geotaxis ability compared to yw (Multivariate regression: genotype-by-age: P = 0.0019). Generally, male flies of all genotypes exhibit a stronger response to endurance exercise. Therefore, exercise experiments were conducted exclusively on male flies. (B) yw;dFatphet flies show improved negative geotaxis ability during and following a 3-week time course of exercise training compared to unexercised siblings (Multivariate regression: treatment by age: P < 0.0001). (C) w[cs];dFatp revertant flies have significantly decreased CO2 production following exercise training (t-test: P = 0.013). w[cs];dFatphet and yw;dFatphet mutants have lower CO2 production than control flies following exercise training (two-way anova: P = 0.016, n ≥ 20). Respiration is not significantly different between genotypes (two-way anova: P = 0.961). (D) Flies with dFatp knockdown in the muscle/heart have extended lifespan compared to background control flies, whether exercised or not (log-rank: P < 0.0001). Survival was recorded following cessation of exercise program at 25 days old. (E) Triglyceride levels in mutant flies that were exercise-trained are significantly reduced when compared to unexercised siblings (t-test: P = 0.002). Triglyceride levels of control flies are not altered by exercise. (F) yw;dFatphet exercised flies have a similar feeding rate in comparison with unexercised siblings. However, the mutants, whether exercised or not, have a reduced feeding rate when compared to either exercised or unexercised yw controls (t-test for each: P < 0.0001). (G) Representative micrographs of lysotracker staining in fat tissue of 3-week-old flies at 40 × magnification. (H) Quantification of lysotracker stain performed in male adipose tissue shows increased staining in wild-type yw flies following an endurance exercise program. Both exercised and unexercised yw;dFatphet flies exhibit low adipose lysotracker staining compared to background.
Endurance exercise is known to alter respiration and oxidative efficiency in mammalian species (Coffey & Hawley, 2007), but the effect of chronic exercise on baseline respiration of Drosophila has not previously been examined. Therefore, we examined respiration of mutant and wild-type males, with and without an endurance exercise program. We find that w[cs];dFatp revertant flies decrease CO2 production following a 3-week exercise program (Fig. 5c; t-test: P = 0.0132). Both yw;dFatphet and w[cs];dFatphet flies have similar baseline CO2 production to control flies. Furthermore, yw;dFatphet and w[cs];dFatphet flies exhibit similarly decreased respiration following endurance exercise (Fig. 5c, two-way anova, P = 0.016), suggesting that dFatp mutation does not significantly alter either baseline respiration or the response of baseline respiration to chronic exercise in Drosophila.
Because flies with reduced dFatp expression respond readily to endurance exercise, we asked whether endurance training could rescue mutant phenotypes. First, we asked whether exercise altered the lifespan of dFatp mutants. To avoid complicating effects of injuries from exercise treatment itself, we measured lifespan beginning at 24 days, following the completion of 3 weeks of training. dFatp RNAi in muscle and heart resulted in lifespan extension, and this extension was unaffected by exercise treatment (Fig. 5d; log-rank: P < 0.0001), suggesting that exercise training in combination with dFatp reduction can improve both mobility and lifespan simultaneously. When mortality of the same flies was plotted, RNAi flies showed a distinct trend toward reduced mortality, particularly between days 20 and 80 (Fig. S6D).
The increase in triglyceride storage in dFatphet flies was somewhat reduced by exercise training (Figs 5e and S3F), although not to wild-type levels. However, the reduced feeding rate of yw;dFatphet flies persisted whether flies were exercised or not (Fig. 5f). It has been recently established that autophagy and lipid metabolism work in concert with modulate longevity pathways (Lapierre et al., 2011). In Drosophila, the effects of exercise training on autophagy and lipid metabolism have not been previously examined. Lysotracker-stained puncta in the fat of yw males increase following exercise training. However, yw;dFatphet males exhibit very little lysotracker staining whether exercised or not (Fig. 5g,h).
Increased lipid content in the myocardium has been observed to impair fly cardiac performance (Birse et al., 2010). Endurance exercise has been shown to improve Drosophila heart performance in male flies (Piazza et al., 2009). Because exercise both reduces triglycerides and improves cardiac performance, we hypothesized that exercise could rescue cardiac impairment of dFatphet flies. Further, we hypothesized that the mechanism of this rescue might be the reduction in lipid deposits in the heart muscle. Therefore, we placed yw;dFatphet flies on an exercise training program and assessed them later for lipid levels in the myocardium and for rescue of cardiac performance. Cardiac pacing assays performed on mutant and wild-type populations prior to treatment produced results indistinguishable from those in Figure 4 (data not shown). However, exercised dFatphet flies displayed a significant degree of rescue in fractional shortening (Fig. 6a; t-test: P = 0.002, Fig. S3G; t-test; P < 0.001), the critical defect displayed by the mutant flies. The increased frequency of heartbeats in dFatphet flies is also reduced by exercise (Fig. 6b; t-test: P = 0.0049, Fig. S3H; t-test P = 0.028).
Fig. 6.
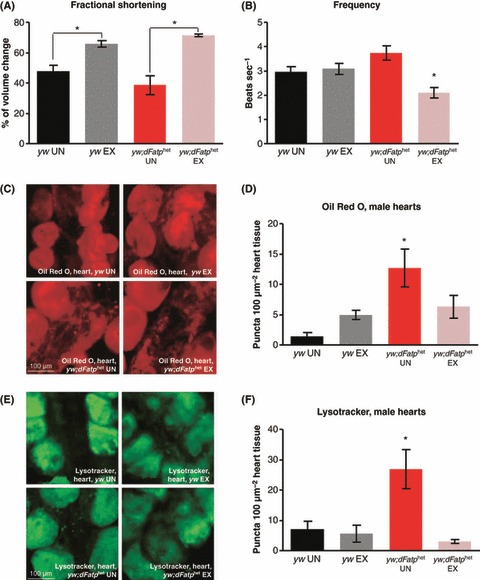
Endurance Exercise rescues cardiac phenotypes of flies with reduced dFatp. (A) Both exercise-trained control flies and exercise-trained yw;dFatphet flies display increased fractional shortening compared to unexercised siblings at 3 weeks of age (t-test: P = 0.009, control flies, P = 0.002, yw;dFatphet). (B) Control yw flies exhibit no significant change in resting heart rate following exercise training (P = 0.714). Cardiac frequency is reduced in yw;dFatphet flies following exercise training. (P = 0.049). (C) Representative 40 × micrograph of Oil Red O staining in yw and yw;dFatphet hearts with and without exercise at 3 weeks of age. (D) Quantification of Oil Red O staining shows increased lipid in 3-week-old, unexercised yw;dFatphet hearts (one-way anova: P = 0.004). Both male and female yw;dFatphet flies were found to have increased baseline Oil Red O staining when compared to background controls. Males are depicted here. Following exercise, Oil Red O staining in yw;dFatphet hearts returned to wild-type quantities (Dunnett pos t-test: P = 0.459 for exercised values when compared to unexercised control). (E) Representative 40 × micrograph of lysotracker staning in yw and yw;dFatphet hearts, with and without exercise, at 3 weeks of age. (F) Both male and female yw;dFatphet flies were found to have increased baseline lysotracker staining. Males are presented here. Quantification of lysotracker staining shows enhanced autophagy in 3-week-old, unexercised yw;dFatphet hearts (one-way anova: P = 0.0006). Following exercise, lysotracker punctae in yw;dFatphet hearts return to wild-type quantities (Dunnett pos t-test: P = 0.218 for exercised values when compared to unexercised control). n ≥ 10 flies for all stains.
Because exercised males exhibit improvement in a subset of cardiac phenotypes, we applied Oil Red O staining to dissected hearts from yw and yw;dFatphet males to determine whether reduction in lipids contributes to the mechanism of cardiac impairment and rescue. yw;dFatphet flies display increased cardiac lipid staining, which is reversed following endurance training (Fig. 6c,d). yw male hearts have low Oil Red O staining with or without exercise (Fig. 6c,d, P = 0.0042). yw males display low levels of lysotracker staining regardless of exercise (Fig. 6e,f). However, yw;dFatphet flies have increased autophagy in heart tissue, and this phenotype is reversed following exercise (Fig. 6e,f, P = 0.0006).
Discussion
Fatty acid metabolism and lifespan
The impact of fatty acid metabolism in any organism reflects a balance between dietary intake, fatty acid oxidation, and packaging into triglycerides for long-term storage. Here, we examine various effects of reduction in the expression of a proposed fatty acid transporter, dFatp. Critically, the reduction in expression provided by the dFatphet mutation is not total. Complete loss of dFatp is lethal or semi-lethal in both homozygous null mutants and animals that constitutively express RNAi against dFatp throughout development. A partial reduction in dFatp expression, however, provides lifespan extension, high exercise capacity, and resistance to multiple stresses.
Flies with reduced dFatp expression have high fat storage, perhaps providing a signal for reduced feeding rate. Increased fat storage may contribute to the enhanced stress resistance conferred by dFatp, particularly the enhanced resistance to starvation. While increased TAG levels have been previously correlated with lifespan extension [e.g., chico (Clancy et al., 2001)], it is not clear whether this reflects a causative effect.
dFatphet flies are not impaired in their response to endurance exercise and, indeed, display a modest improvement in untrained mobility. We propose that the increased fat storage in the mutant flies becomes beneficial under stress conditions, when it could be mobilized to provide protection against starvation and cold and to provide additional energy during endurance training.
However, other aspects of physiology do not benefit from these changes. In particular, cardiac performance is substantially impaired when dFatp expression is reduced, either globally or in the heart itself. Crucially, this impairment can be reversed by endurance exercise, without suppressing beneficial phenotypes. Various aspects of this model are discussed separately below.
Feeding behavior
dFatphet flies display a substantial reduction in feeding rate, accompanied by an increase in triglyceride storage. This observation is consistent with other recent reports in which mutations in worms or flies both reduce feeding rate and increase fat storage. For example, worms with a mutation in daf-7, which encodes a ligand of the TGF-β family, increase fat accumulation and reduce feeding rate (Greer et al., 2008). In flies, mutants for AKHR, which encodes a receptor for adipokinetic hormone, also display low feeding rate and high triglyceride storage (Bharucha et al., 2008). Taken together, these phenotypic observations suggest that fat itself may produce one or more signals to reduce feeding rate in adult invertebrates.
In addition to endocrine signaling from adipose tissue, neuronal signaling is likely to be required for this regulation to occur in adult flies. Potential candidates for such signals include serotonin, whose reduction is known to reduce feeding rate without lowering negative geotaxis ability in adult flies (Neckameyer et al., 2007), a combination of phenotypes reminiscent of those seen in dFatp mutants.
It is possible that reduced feeding rate may be causally mediating lifespan extension, perhaps by a dietary restriction (DR)-dependent mechanism. The phenotypic differences between DR and dFatp reduction, especially negative geotaxis, cardiac performance, and exercise response, argue against a DR-dependent mechanism. However, we cannot rule out the possibility that feeding rate reduction contributes to lifespan extension by inducing a subset of effects induced by DR.
Cardiac functional impairment
Cardiac performance and lifespan are separable in Drosophila (Wessells & Bodmer, 2004; Piazza et al., 2009; Piazza & Wessells, 2011). Impairment of cardiac function is a well-characterized consequence of high levels of triglyceride storage in humans (Unger et al., 2010). The relationship of fat levels with cardiac performance in flies is less well understood. Flies fed a diet supplemented with coconut oil display impaired cardiac function (Birse et al., 2010), but mutations that increase triglyceride levels, such as chico (Clancy et al., 2001), can also provide substantial protection of cardiac function during aging (Wessells et al., 2004). In flies fed a high-fat diet (Birse et al., 2010) and in rodents overexpressing fatty acid transporter genes in the heart (Chabowski et al., 2007), accumulation of lipid deposits in the myocardium has been observed, leading to lipotoxic cardiac dysfunction. Here, we find that lipid levels in the heart are significantly increased in dFatphet mutants. However, exercise rescues both cardiac performance and myocardial lipid content. These results are consistent with the hypothesis that cardiac functional impairment in these mutants is a result of lipotoxicity.
Autophagy
In dFatp mutants, we observe abnormally high myocardial autophagy. In a rabbit model of myocardial infarction, compromised cardiac muscles exhibit increased autophagosome accumulation, which is ameliorated following exercise (Chen et al., 2010a). Following endurance training, yw;dFatphet males exhibit a similar response. We have yet to determine whether autophagy has a causative role or is an indirect effect of high lipid storage.
In contrast to cardiac autophagy, dFatp mutants have low autophagy in adipose tissue whether exercised or not. Several interventions have been shown to modify autophagy levels in fat. These changes have variously correlated with either lifespan extension (Partridge et al., 2011) or lifespan reduction (Scott et al., 2004). In the case of the dFatp fat body, low autophagy and high TAG correlate with increased lifespan in mutants. Autophagy responds tissue-specifically to lipid levels to protect against lipotoxicity (Singh, 2010). In wild-type flies, exercise increases autophagy in the fat body but not the myocardium, and lifespan is unaffected. These findings indicate that in flies (i) autophagy in fat can vary highly without affecting lifespan; and (ii) exercise can modify autophagy tissue- and context-specifically.
Sexual dimorphism of phenotypes
Sexual dimorphism has been frequently observed in single-gene mutations that extend lifespan (Bartke, 2011) and in environmental interventions such as DR (Chen et al., 2010b; Soh et al., 2007). Some mechanisms associated with sexual dimorphism are inherently female specific, such as the link between female infertility and enhanced lifespan. In the case of dFatp, fertility is normal, ruling this out as the mechanism of lifespan extension. In other cases, the mechanisms responsible for sexual dimorphism are not fully understood.
Here, we find that dFatphet females have a significant lifespan extension in multiple backgrounds, whereas dFatphet males have either a more modest lifespan extension or none at all, depending on background. Additionally, we observe substantially larger increases in TAG levels as well as differential increases in stress resistance in dFatphet females. Conversely, male and female dFatphet flies have similar cardiac abnormalities, reduced feeding rate, and altered autophagy levels. The correlation between sexually dimorphic phenotypes, such as TAG storage and stress resistance, and greater lifespan extension seen in females may suggest that TAG storage and stress resistance are important factors driving the lifespan extension.
Further questions remain regarding the mechanistic basis of lifespan extension associated with dFatp mutation. The relative contributions of fat storage, reduced feeding rate, and altered autophagy will require further experimentation to fully define the mechanisms of dFatp action. Another major question regards the cellular role of the dFatp protein itself. Localization of the protein under various conditions and the effect on phenotypic outputs in the fly model may have particular significance to understanding the cellular role of dFatp.
Experimental procedures
Real-time polymerase chain reaction
cDNA was prepared from 7 to 10 whole, day-old male flies. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) for each genotype. At least three independent RNA extractions were prepared for each sample. Relative message abundance was determined by amplification and staining with SYBR Green I using an ABI 7000 SDS (Applied Biosystems, Carlsbad, CA, USA). Expression of Rp49 and corresponding control y1w67c23 flies were used for normalization. Differences between genotypes were assessed by t-test or nested anova.
Primer sequences are listed below.
dFatp
Forward: 5′-AGAAACACCGAGTGCGTCTG-3′
Reverse: 5′-CCACCGTGTTGTCATGATTC-3′
CG7384
Forward: 5′-AAACCGACAGTTCTGGAAG-3′
Reverse: 5′-GCAGGAACTGACGATTGATG-3′
Lrr47
Forward: 5′-CCGCTGGTTAAATTCGAGTC-3′
Reverse 5′-ATCCGTAGATCCTCGACAGC-3′
Myo31DF
Forward: 5′-CTGGGTGGGTGATTATCTGG-3′
Reverse: 5′-AGCCTGCTTGTTGAAATGGT-3′
Rp49
Forward: 5′-ACTCAATGGATACTGCCCAAGA-3′
Reverse: 5′-CAAGGTGTCCCACTAATGCATA-3′
dFatp insertion
Forward: 5′-TGAACCTCGCACTCGGCTGAT-3′
Reverse: 5′-TTGGACAACTATGCGAACAGC-3′
Triglycerides
Following collection, treatment, and aging, five female or eight male flies were weighed and homogenized in 500 μL of 0.05% PBS/Triton X buffer. Following lipase inactivation and centrifugation, the supernatant was added to 10 volumes of preheated (37 °C) Thermo Infinity Reagent (#TR22321; Thermo Electron Corp, Waltham, MA, USA). Absorbance of 520 nm was determined following 10 min of incubation with agitation at 37 °C. Resulting triglyceride measures were normalized per fly or per μg total protein. Each data point is based on at least three replicate measures.
Feeding rate
After collection and mating as described in fly stocks and maintenance, 10-day-old adult flies were housed in vials of standard food (10% sucrose/10% yeast). Flies were separated into vials of five and left to equilibrate for 1 h. The percentage of flies engaged in proboscis extension in each vial was scored as in Wong et al. (2009). Each vial was observed 10 times in a double-blind fashion. To assess the amount of food consumed, 10-day-old adult flies were fed standard food supplemented with 0.5% (w/v) FD&C Blue #1 (Spectrum Chemical Mfg. Corp., Gardena, CA, USA). Following exposure to dyed food, five female or eight male flies were homogenized in 200 μL PBS and cleared by centrifugation at 15 700 g. After centrifugation, the absorbance of the supernantant was measured at 625 nm. Having established blue-dye feeding assays as a control, data collected from proboscis extension methods were analyzed by Student’s t-test.
Glycogen
After collection and mating as described, 30 male or female flies were weighed and homogenized in 200 μL PBS/0.05 × Triton X. Homogenate was cleared by spinning for 2 min at 13 000 rpm. Twenty microlitre of fly homogenate was added to 1 μL amyloglucosidase at 0.1 U μL−1. Sample was incubated with agitation at 37 °C for 1 h. Absorbance of 340 nm was determined within 30 min following incubation. Resulting glycogen was normalized per milligram dry weight. Data were analyzed using Student’s t-test.
Free FA
Free FA were assessed from the hemolymph of 25–30 male flies per genotype. Hemolymph was obtained by puncturing cuticle with a sterile needle and then ‘pulse’ centrifuging flies in an Eppendorf with a mesh screen to separate solid material. One microliter of hemolymph sample was combined with 49 μL BioVision Free Fatty Acid reagent (BioVision, Mountain View, CA, USA). Reagents were mixed according to manufacturer’s instructions following standardization preparation and acyl-coA synthesis. Reaction was allowed to incubate, protected from light, for 30 min at 37 °C. Absorbance of 570 nm was determined following reaction completion. Data were analyzed using Student’s t-test.
Survival assays
Background genotypes utilized in this study frequently displayed unusually high early-life mortality. Because this early mortality is unrelated to aging, we have plotted all survival assays except for stress assays with deaths from the first 20 days censored. Differences in all survival curves were assessed following censoring using log-rank analysis, applied only to post-20-day data. To provide a clear illustration of age-dependent mortality differences, we also plotted age-specific mortality for all curves in their entirety (Supporting Information.
Prior to all experiments, fly cultures were maintained at a constant density for at least two generations. Fifteen virgin females and five males were mated in 300-mL bottles with 50 mL standard 10% sucrose 10% yeast unless otherwise described. Adult progeny were synchronized by collecting within 2 h of eclosion, over a 24-h time period. Groups of 20 age- and sex-matched flies were immediately transferred into narrow polypropylene vials containing 5 mL of appropriate food medium. Food vials were changed every second day at which time dead flies were removed and counted. Flies were housed in a 25 °C incubator on a 12:12 light/dark cycle at 50% relative humidity.
For starvation resistance, flies were collected and housed as described and placed on agar-only food. Survival measurements were recorded twice per day in 12-h intervals. To assess survival on high-nutrient diet, standard 10% sucrose/yeast food was supplemented with an additional 10% weight/volume brewer’s yeast, dry palmitic acid, or dry linoleic acid. Survival measurements were recorded every second day.
Oxidative stress was induced by introducing 40 mm methyl viologen dichloride hydrate (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 5% sucrose. Deaths were recorded three times per day in 8-h intervals. Temperature stress was assessed at either 4 or 28 °C. Cold survival was assessed twice per day in 12-h intervals. Before recording deaths from flies under cold stress, flies were given 1 h to recover movement at room temperature. Heat stress was recorded every second day.
Respiration
CO2 production was measured using a flow-through respirometry system. Twenty-eight-day-old exercised and unexercised flies were immobilized 24 h before measurement by CO2 gas and separated into groups of five flies per sample. Samples were assayed in random order to evenly distribute variance between measurements. For each measurement, seven samples were transferred into 2-mL glass measurement chambers in a room kept under constant light at 25 °C, and one chamber was left empty as a blank reference. Chambers were consecutively flushed for 150 s at a flow rate of 90 mL min−1 with CO2-free, water-saturated room air through an 8-channel MUX flow multiplexer (Sable Systems International, Las Vegas, NV, USA). Flushing of all samples was consecutively repeated four times per measurement, resulting in 20 min intervals during which the chambers were sealed before the second, third, and fourth flushing. Integrated CO2 concentration over time was measured for all samples during the fourth flushing using a Li-7000 CO2/H2O Analyzer (Sable Systems International) and used to calculate CO2 output over time per fly. All n values were between 19 and 46. Results were analyzed using a two-tailed t-test (Prism; GraphPad Software, San Diego, CA, USA).
Lysotracker
Adult flies separated by age, genotype, and/or treatment were dissected, ventral side up, in room temperature PBS. Having exposed the heart and fat bodies, partially dissected flies were rinsed 3 × in fresh PBS. Lysotracker green (Molecular Probes, Eugene, OR, USA) was diluted to 0.01 μm in PBS and applied to dissected preps for 1 min. Samples were washed three additional times in PBS. Stained hearts and fat bodies were subsequently removed and mounted in one drop of antifade reagent (Molecular Probes). Slides were imaged on an Olympus BX41 compound fluorescence microscope (Olympus, Center Valley, PA, USA) using a 40 × objective. Images were analyzed using Image (National Institutes of Health, Bethesda, MD, USA). A minimum of five samples were analyzed for each tissue type. Data were subjected to Student’s t-test following quantification.
Oil Red O
Adult flies were dissected similarly to lysotracker staining with the following changes. Dissections were performed in room temperature PBS/0.05% Triton X and fixed in 4% paraformaldehyde/PBS for 10 min. Following fixation, samples were washed 3 × in PBS. Oil Red O (Sigma-Aldrich) was diluted 1:100 in isopropanol and applied to partially dissected preps for 20 min at room temperature. Following staining, samples were washed 3 × in ddH2O. Hearts were removed and mounted as previously described. Imaging and analysis were identical to lysotracker protocols. A minimum of 10 fly hearts were analyzed for each genotype/treatment. Baseline stains (before exercise) were performed in both male and female flies and exhibited similar cardiac Oil Red O phenotypes compared to wild-type controls. All exercise experiments were performed on males.
Statistical analysis
Data for triglycerides, total protein, feeding rate, glycogen, free FA, fluorescent stains, and single-time-point cardiac analyses were analyzed by Student’s t-test. Survival analyses were conducted using log-rank test. Negative geotaxis, cardiac, and metabolic analyses traced over time were analyzed by two-way anova. Bonferroni pos t-test s were used to compare significantly different values. For exercise treatment, multivariate regression was used to assess treatment by age. All statistics were generated using GraphPad Prism software version 5 (La Jolla, CA, USA).
Acknowledgments
We acknowledge Michael Hayes, Gretchen Oleson, and Suraj Rajeha for preparation and maintenance of fly lines, Greg Miller for assistance with oxidative stress protocols, and Scott Pletcher for providing software analysis tools. We also acknowledge Rolf Bodmer, in whose laboratory the cardiac phenotype of dFatp was originally identified by RW. We also thank anonymous reviewers and editors for thoughtful comments that greatly improved this manuscript.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1 dFatpK10307 heterozygotes exhibit specific reduction of dFatp expression.
Fig. S2 Triglycerides are increased in dFatp heterozygotes in multiple wild-type backgrounds.
Fig. S3 Reduction of dFatp expression extends lifespan in two of three genetic backgrounds.
Fig. S4 Age-specific mortality plots from lifespan experiments in Figure 2.
Fig. S5 Age-specific mortality plots from lifespan experiments in Figure 3.
Fig. S6 Age-specific mortality plots from lifespan experiments in Figures 5 and S3.
Data S1. Methods.
References
- Bartke A. Single-gene mutations and healthy ageing in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:28–34. doi: 10.1098/rstb.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim. Biophys. Acta. 2007;1771:286–298. doi: 10.1016/j.bbalip.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Chabowski A, Zmijewska M, Gorski J, Bonen A, Kamisk K, Winnicka MM. Effect of il-6 deficiency on myocardial expression of fatty acid transporters and intracelular lipid deposits. J. Physiol. Pharmacol. 2007;58:73–82. [PubMed] [Google Scholar]
- Chen C-Y, Hsu H-C, Lee B-C, Lin H-J, Chen Y-H, Huang H-C, Ho Y-L, Chen M-F. Exercise training improves cardiac function in infarcted rabbits: involvement of autophagic function and fatty acid utilization. Eur. J. Heart Fail. 2010a;12:323–330. doi: 10.1093/eurjhf/hfq028. [DOI] [PubMed] [Google Scholar]
- Chen JH, Cottrell EC, Ozanne SE. Early growth and ageing. Nestle Nutr. Workshop Ser. Pediatr. Program. 2010b;65:41–50. doi: 10.1159/000281144. discussion 50–44. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37:737–763. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- Cook-Wiens E, Grotewiel MS. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp. Gerontol. 2002;37:1347–1357. doi: 10.1016/s0531-5565(02)00096-7. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Tsuchiya T, Kim HJ, Mote PL, Spindler SR. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Fullekrug J, Pohl J, Ring A, Herrmann T, Stremmel W. Translocation of long chain fatty acids across the plasma membrane--lipid rafts and fatty acid transport proteins. Mol. Cell. Biochem. 2006;284:135–140. doi: 10.1007/s11010-005-9034-1. [DOI] [PubMed] [Google Scholar]
- Ford JH. Saturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism aging. Age (Dordr.) 2010;32:231–237. doi: 10.1007/s11357-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatikos SI, Subbaiah PV, Victor TA, Miller WM. Diverse effects of essential (n-6 and n-3) fatty acids on cultured cells. Cytotechnology. 1994;15:31–50. doi: 10.1007/BF00762377. [DOI] [PubMed] [Google Scholar]
- Greer ER, Perez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008;8:118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc. Natl. Acad. Sci. USA. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annu. Rev. Entomol. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, Ehsani A, Gropler RJ. Impact of Aging on substrate metabolism by the human heart. J. Am. Coll. Cardiol. 2003;41:393–399. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Wisloff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol. (Oxf.) 2010;199:425–439. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- King VL, Hatch NW, Chan HW, de Beer MC, de Beer FC, Tannock LR. A murine model of obesity with accelerated atherosclerosis. Obesity (Silver Spring) 2010;18:35–41. doi: 10.1038/oby.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lapierre LR, Gelino S, Melendez A, Hansen M. Autophagy and Lipid Metabolism Coordinately Modulate Life Span in Germline-less C. elegans. Curr. Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee JM, Brooks CC, Stanley RW. Amino acid and fatty acid composition of cane molasses. J. Sci. Food Agric. 1979;30:429–432. doi: 10.1002/jsfa.2740300413. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Coleman CM, Eadie S, Goodwin SF. Compartmentalization of neuronal and peripheral serotonin synthesis in Drosophila melanogaster. Genes Brain Behav. 2007;6:756–769. doi: 10.1111/j.1601-183X.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- Partridge L, Alic N, Bjedov I, Piper MD. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 2011;46:376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J. Nutr. 2004;134:2444S–2449S. doi: 10.1093/jn/134.9.2444S. [DOI] [PubMed] [Google Scholar]
- Piazza N, Wessells RJ. Drosophila models of cardiac disease. Prog. Mol. Biol. Transl. Sci. 2011;100:155–210. doi: 10.1016/B978-0-12-384878-9.00005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE. 2009;4:e5886. doi: 10.1371/journal.pone.0005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MR, Harp JD, Ory DS, Schaffer JE. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J. Lipid Res. 2006;47:665–672. doi: 10.1194/jlr.M500514-JLR200. [DOI] [PubMed] [Google Scholar]
- Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:149–154. doi: 10.1016/j.plefa.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Short KR, Blackett PR, Gardner AW, Copeland KC. Vascular health in children and adolescents: effects of obesity and diabetes. Vasc. Health Risk Manag. 2009;5:973–990. doi: 10.2147/vhrm.s7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. Autophagy and regulation of lipid metabolism. Results Probl. Cell Differ. 2010;52:35–46. doi: 10.1007/978-3-642-14426-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res. Rev. 2006;5:144–164. doi: 10.1016/j.arr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Soh JW, Sijana H, Arking R. Dietary restriction in Drosophila is dependent on mitochondrial efficiency and constrained by pre-existing extended longevity. Mech Ageing Dev. 2007;128:581–593. doi: 10.1016/j.mad.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Stahl A. A current review of fatty acid transport proteins (SLC27) Pflugers Arch. 2004;447:722–727. doi: 10.1007/s00424-003-1106-z. [DOI] [PubMed] [Google Scholar]
- Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol. Metab. 2001;12:266–273. doi: 10.1016/s1043-2760(01)00427-1. [DOI] [PubMed] [Google Scholar]
- Teleman AA. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2010;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Caloric restriction, gene expression, and aging. Alzheimer Dis. Assoc. Disord. 2003;17(Suppl 2):S58–S59. doi: 10.1097/00002093-200304002-00008. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Bodmer R. Screening assays for heart function mutants in Drosophila. Biotechniques. 2004;37:58–60. doi: 10.2144/04371ST01. 62, 64 passim. [DOI] [PubMed] [Google Scholar]
- Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, DiRusso CC, Ctrnacta V, Black PN. Fatty acid transport in Saccharomyces cerevisiae. Directed mutagenesis of FAT1 distinguishes the biochemical activities associated with Fat1p. J. Biol. Chem. 2002;277:31062–31071. doi: 10.1074/jbc.M205034200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


