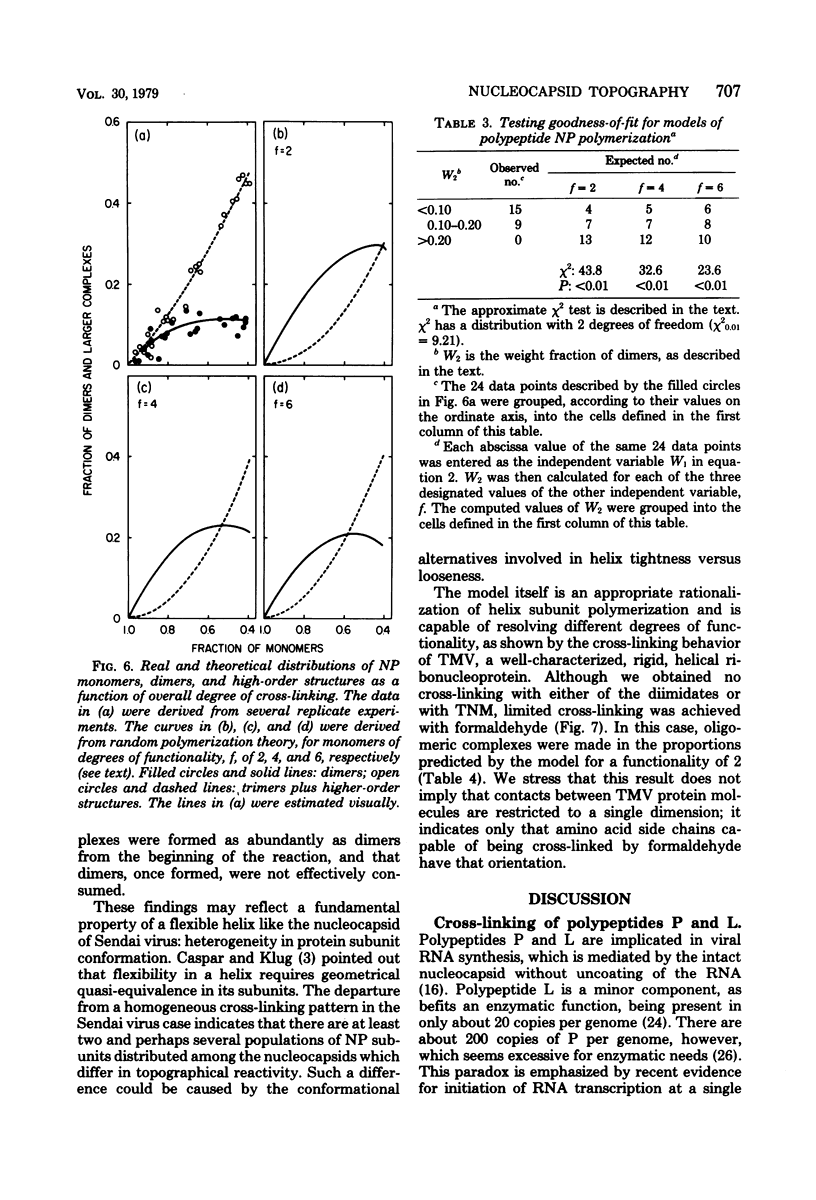
Abstract
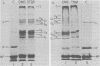
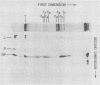
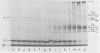
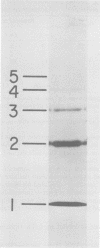
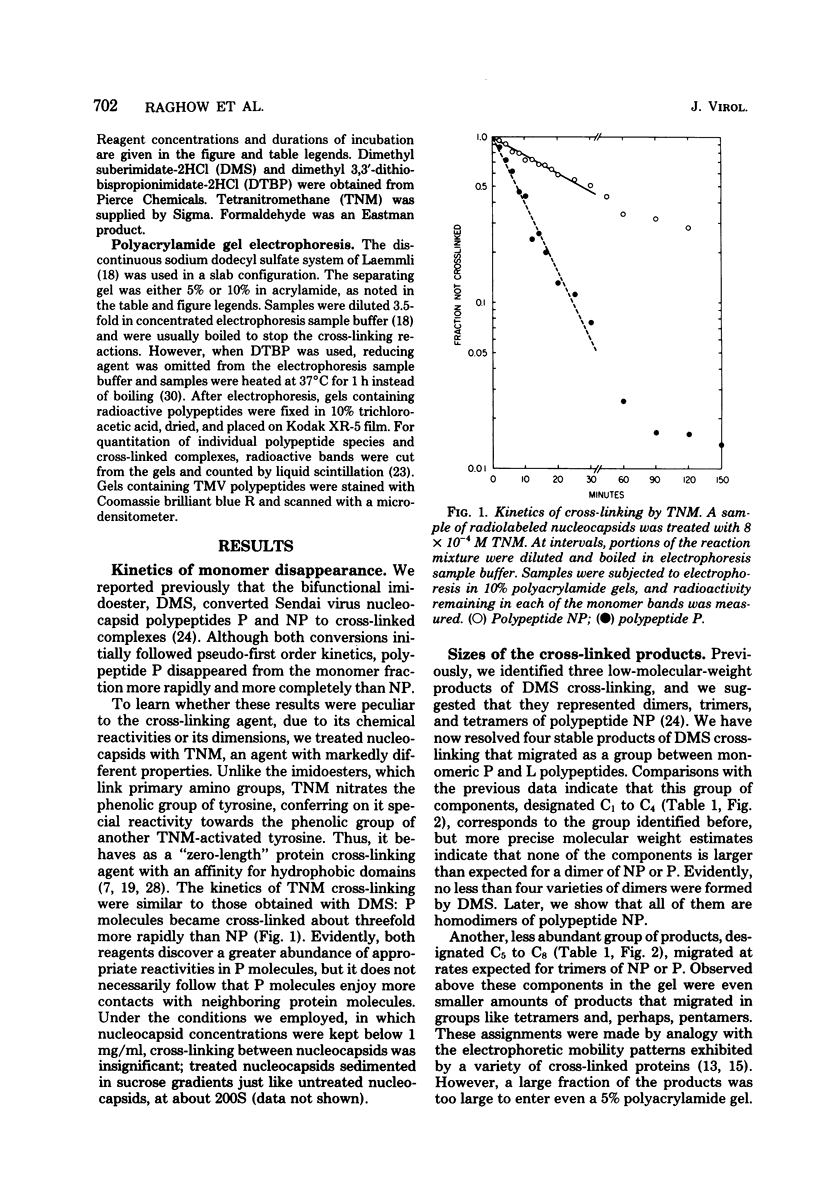
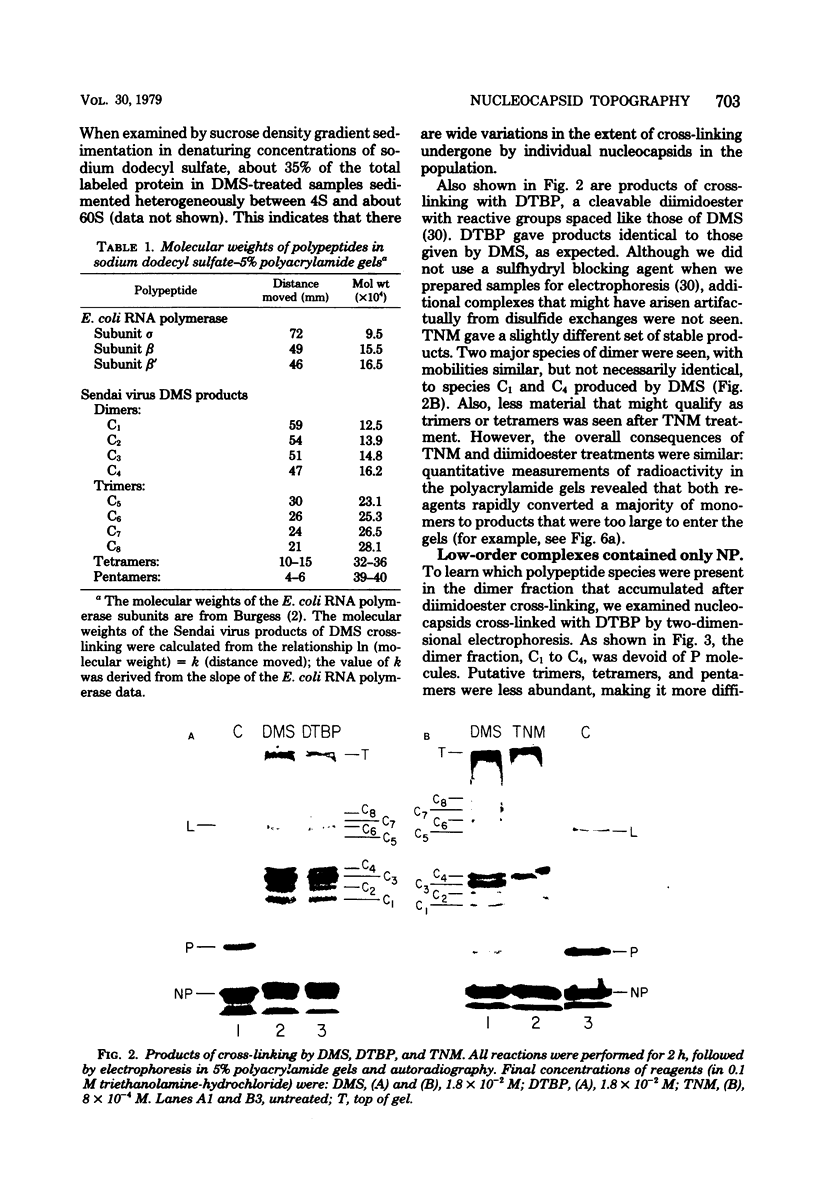
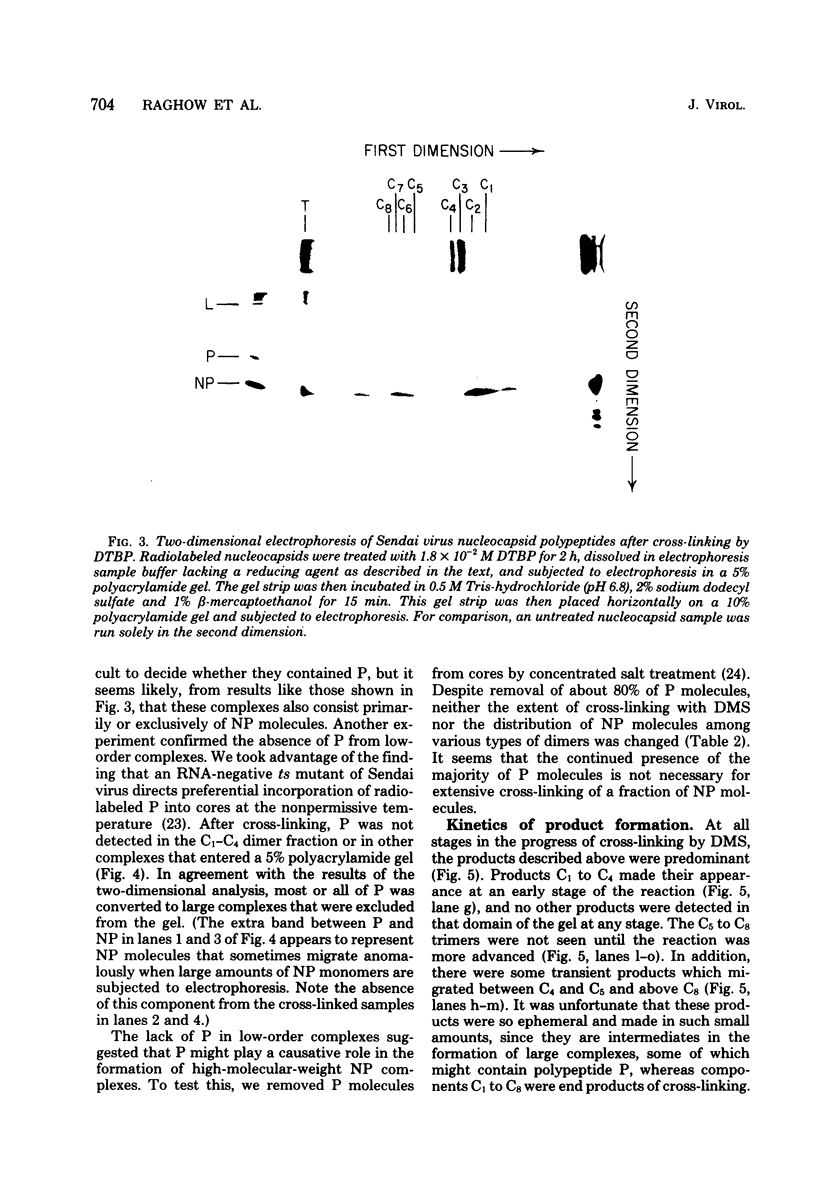
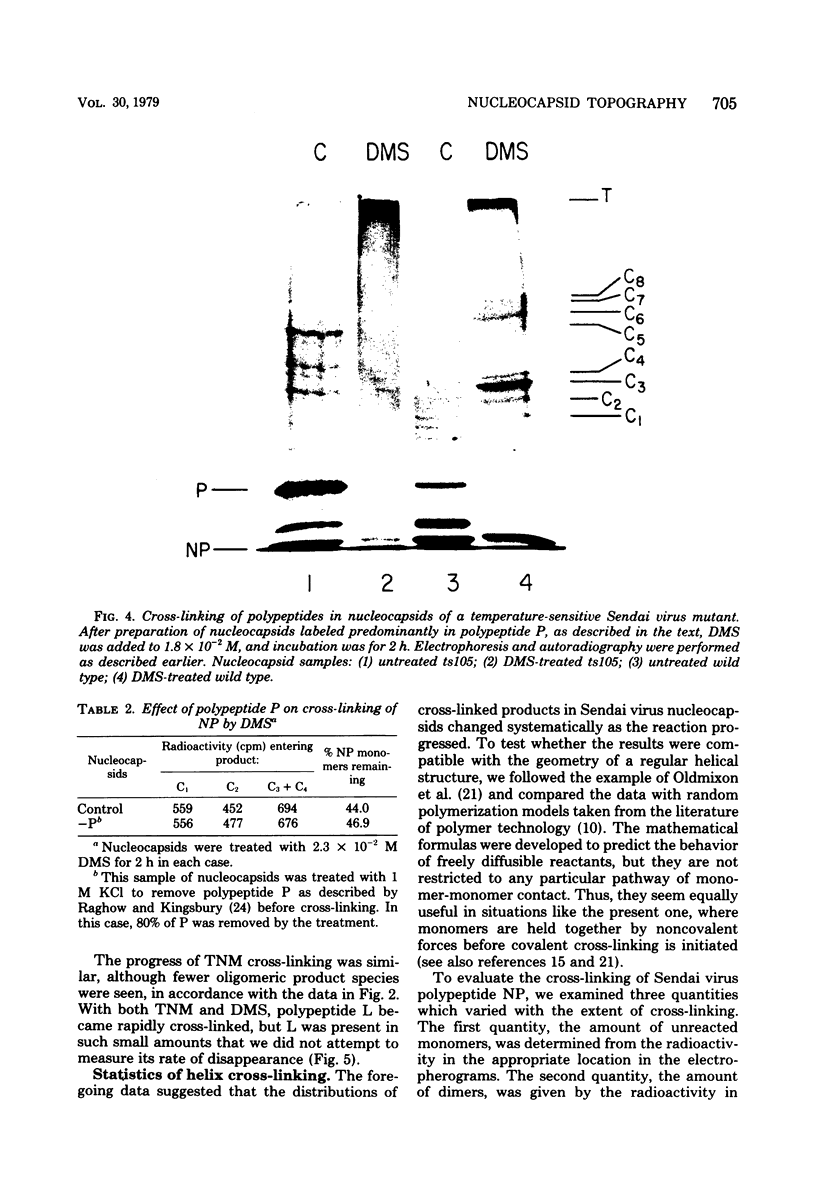
Contacts among the three polypeptide species in the flexible helical nucleocapsids of a paramyxovirus were examined with bifunctional protein cross-linking reagents. Polypeptides L and P, minor components of Sendai virus nucleocapsids implicated in viral RNA polymerase activity, were efficiently cross-linked into large complexes, indicating that they enjoy abundant contacts with neighboring protein molecules in the helix. Less reactivity was found in the case of the major structural polypeptide, NP; about half of all molecules of NP formed large cross-linked complexes, most of the rest remaining as monomers along with a small proportion of homodimers and low-order oligomers. Marked heterogeneity in the cross-linking reactivity of NP molecules, which may reflect the conformational quasi-equivalence inherent in a flexible helix, was indicated by the production of several conformers of homodimers and other low-order oligomers of NP, and by failure of the kinetics of NP cross-linking to conform to a simple statistical model of random polmerization. The validity of the statistical model was shown by cross-linking experiments with the rigid helical virus, tobacco mosaic virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Champness J. N., Bloomer A. C., Bricogne G., Butler P. G., Klug A. The structure of the protein disk of tobacco mosaic virus to 5A resolution. Nature. 1976 Jan 1;259(5538):20–24. doi: 10.1038/259020a0. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Bello J., Roholt O. A. Probable protein crosslinking with tetranitromethane. Biochim Biophys Acta. 1968 Jun 26;160(2):274–276. doi: 10.1016/0005-2795(68)90103-7. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Gibbs A. J. Observations on the structure of the nucleocapsids of some paramyxoviruses. J Gen Virol. 1970 Jan;6(1):141–150. doi: 10.1099/0022-1317-6-1-141. [DOI] [PubMed] [Google Scholar]
- Garoff H. Cross-linking of the spike glycoproteins in Semliki Forest virus with dimethylsuberimidate. Virology. 1974 Dec;62(2):385–392. doi: 10.1016/0042-6822(74)90400-0. [DOI] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu J., Bartha F., Friedrich P. Crosslinking with bifunctional reagents as a means for studying the symmetry of oligomeric proteins. Eur J Biochem. 1976 Sep 15;68(2):373–383. doi: 10.1111/j.1432-1033.1976.tb10824.x. [DOI] [PubMed] [Google Scholar]
- Hucho F., Müllner H., Sund H. Investigation of the symmetry of oligomeric enzymes with bifunctional reagents. Eur J Biochem. 1975 Nov 1;59(1):79–87. doi: 10.1111/j.1432-1033.1975.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Hsu C. H., Murti K. G. Intracellular metabolism of sendai virus nucleocapside. Virology. 1978 Nov;91(1):86–94. doi: 10.1016/0042-6822(78)90357-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., McCarthy B. J. Histone-histone interactions within chromatin. Preliminary characterization of presumptive H2B-H2A and H2B-H4 binding. Biochemistry. 1976 Sep 7;15(18):4126–4131. doi: 10.1021/bi00663a033. [DOI] [PubMed] [Google Scholar]
- Oldmixon E. H., Dezélée P., Ziskin M. C., Shockman G. D. Monomer addition as a mechanism of forming peptide cross-links in the cell-wall peptidoglycan of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1976 Sep;68(1):271–280. doi: 10.1111/j.1432-1033.1976.tb10786.x. [DOI] [PubMed] [Google Scholar]
- Portner A. Association of nucleocapsid polypeptides with defective RNA synthesis in a temperature-sensitive mutant of Sendai virus. Virology. 1977 Apr;77(2):481–489. doi: 10.1016/0042-6822(77)90473-1. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Regulatory events in the synthesis of Sendai virus polypeptides and their assembly into virions. Virology. 1976 Aug;73(1):79–88. doi: 10.1016/0042-6822(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Identification of neighboring protein pairs in the Escherichia coli 30 S ribosomal subunit by crosslinking with methyl-4-mercaptobutyrimidate. J Mol Biol. 1976 Oct 5;106(4):995–1015. doi: 10.1016/0022-2836(76)90348-x. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Kingsbury D. W., Darlington R. W. Sendai virus-induced transcriptase from infected cells: polypeptides in the transcriptive complex. J Virol. 1972 Nov;10(5):1037–1043. doi: 10.1128/jvi.10.5.1037-1043.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M., Delaage M. On the use of tetranitromethane as a nitration reagent. The reaction of phenol side-chains in bovine and porcine trypsinogens and trypsins. Eur J Biochem. 1970 Feb;12(2):250–257. doi: 10.1111/j.1432-1033.1970.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]