Abstract
Martinez-Bello,Vladimir E., Fabian Sanchis-Gomar, Daniel Martinez-Bello, Gloria Olaso-Gonzalez, Mari Carmen Gomez-Cabrera, and Jose Viña. Vitamin C Supplementation Does Not Improve Hypoxia-Induced Erythropoiesis. High Alt Med Biol 13:269–274, 2012.—Hypoxia induces reactive oxygen species production. Supplements with antioxidant mixtures can compensate for the decline in red cell membrane stability following intermittent hypobaric hypoxia by decreasing protein and lipid oxidation. We aimed to determine whether supplementation with vitamin C is implicated in the regulation of erythropoiesis and in the oxygen-carrying capacity of the blood, and also whether antioxidant supplementation prevents the oxidative stress associated to intermittent hypoxia. Twenty-four male Wistar rats were randomly divided into four experimental groups: normoxia control (n=6), normoxia + vitamin C (n=6), hypoxia control (12 h pO2 12%/12 h pO2 21%) (n=6), and hypoxia + vitamin C (n=6). Animals were supplemented with vitamin C at a dose of 250 mg·kg−1·day−1 for 21 days. Red blood cell count, hemoglobin, hematocrit, reticulocytes, erythropoietin, and oxidative stress parameters such as malondialdehyde and protein oxidation in plasma were analyzed at two different time points: basal sample (day zero) and final sample (day 21). Similar RBC, Hb, Hct, and Epo increments were observed in both hypoxic groups regardless of the vitamin C supplementation. There was no change on MDA levels after intermittent hypoxic exposure in any experimental group. However, we found an increase in plasma protein oxidation in both hypoxic groups. Vitamin C does not affect erythropoiesis and protein oxidation in rats submitted to intermittent hypoxic exposure.
Key Words: hematocrit, hemoglobin, red blood cells, oxidative stress, antioxidants
Introduction
Intermittent hypoxia exposure (IHE) is one of the most important strategies used by athletes to increase performance since it induces endogenous erythropoietin (Epo) increase and, therefore, hematological adaptations (Garcia, 1957; Levine and Stray-Gundersen, 2005; Martinez-Bello et al., 2011). Hypoxia increases oxidative stress both in humans and in rodents (Radak et al., 1997; Asha Devi et al., 2005; Devi et al., 2007; Pialoux et al., 2009). It has been shown that supplements with antioxidant mixtures can compensate for the decline in membrane stability following intermittent hypobaric hypoxia as a consequence of decreased lipid peroxidation (LPO) and protein oxidation (Devi et al., 2007; Vani et al., 2010). The vast majority of endurance athletes, including elite athletes, take vitamin supplements such as vitamin C, often in large amounts (Sobal and Marquart, 1994., as an antioxidant and antianemic tool. In fact, it has been shown that dietary vitamin C deprivation leads to anemia (Maeda et al., 2000) and that vitamin C administration improves the response to recombinant human erythropoietin (rHuEpo) (Tarng, 2007). Keven et al., (2003) observed higher hemoglobin responses in normal iron status patients treated with vitamin C and recombinant human erythropoietin (rHuEpo).
The main aim of our study was to evaluate if vitamin C is implicated in the regulation of erythropoiesis in adult male Wistar rats subjected to oxidative stress induced by IHE (∼4000 m). For this purpose, we assessed the changes in hematological parameters, such as red blood cells count (RBC), hemoglobin (Hb), hematocrit (Hct), percentage of reticulocytes (ret%), and in erythropoietin levels (Epo). Moreover, we measured two oxidative stress parameters such as malondialdehyde (MDA) and the protein carbonyl groups to evaluate its induction by the hypoxic protocol, and the effect of vitamin C supplementation. We chose to examine the effect of vitamin C on erythropoiesis because it is often used as a recovery adjuvant, antioxidant, and antianaemic ergogenic aid by athletes. We report that vitamin C supplementation did not modify the hypoxia-induced erythropoiesis and plasma protein oxidation after 21 days of IHE.
Material and Methods
Animals
Twenty-four male Wistar rats (3 months old, ∼250 g weight) were randomly divided into four experimental groups: normoxia control (NC) (n=6), normoxia + vitamin C (NVC) (n=6), hypoxia control (HC) (n=6), and hypoxia + vitamin C (HVC) (n=6). The experimental protocol was approved by the Committee on Ethics in Research of the Faculty of Medicine, University of Valencia. Our animals were fed a rodent maintenance diet of 2014 Harlan Teklad Global Diet®, with an iron content of 196.0 mg·kg−1.
Vitamin C administration
The dose of vitamin C was 250 mg·kg−1·day−1 administered orally to rats via the drinking water for 21 days. The dose of vitamin C was calculated by taking into account the animal's body surface area (BSA). BSA has been recommended as the main basis for drug dosage, as the rate of metabolism or redistribution of a drug is proportional to the metabolic rate, which in turn reflects heat losses that are generally proportional to the surface area (Lack and Stuart-Taylor, 1997; Reagan-Shaw et al., 2008). The dose administered to rats is equivalent to 0.12 mg/cm2 BSA. This dose is ∼2-fold to that used in humans. We gave this dose of vitamin C to the animals due to its similarity to the daily oral dose recommended in humans.
Hypoxic protocol
The animals were kept at 21% O2 12 h a day (9.00–1.00) and at 12% O2 (∼4000 m) 12 h a day (21.00–9.00) for 21 days. The hypoxic treatments were performed with the system Colorado Altitude Training chamber system (model: CAT-430™ Walk-In Tent).
Samples
Blood was collected from a common site for venepuncture in small animals (tail vein). Two tail blood samples (0.4 mL) were collected in EDTA-containing tubes at two different time points: day 0 and day 21. The samples were collected in the morning at the same time to limit effects of diurnal variations and were then centrifuged at 760 g for 20 min at room temperature. The plasma was collected and immediately stored at −20°C.
Whole blood analysis
RBC, Hb, Hct, and percentage of reticulocytes (Ret%) were analyzed within 3 hours of blood collection using a SYSMEX XT 2000i hematology analyzer (Roche Diagnostics).
Erythropoietin determination in plasma samples
The Quantikine® Mouse/Rat Epo Immunoassay was used to measure the Epo values in plasma samples.
Measurement of oxidative stress parameters in plasma samples
Lipid peroxidation was measured through accumulation of MDA, which was detected by HPLC as an MDA–thiobarbituric acid adduct (Wong et al., 1987). Oxidative modification of total proteins was assessed by immunoblot detection of protein carbonyl groups, using the ‘OxyBlot’ protein oxidation kit (Intergen) as previously described (Romagnoli et al., 2010). The procedure to quantify total protein carbonyls with the OxyBlot kit was densitometry of the oxyblot and of the Ponceau staining, followed by calculating the ratio between the total density given by the oxyblot and total density given by Ponceau staining.
Statistical analysis
For the statistical analysis of the results, the mean was taken as the measurement of the main tendency, and the standard deviation was taken as the dispersion measurement. A three-way analysis of variance (regimen x supplementation x time) with repeated measures in one factor (time) was used to determine the difference, after the experimental intervention (regimen and supplementation) on the hematological parameters. When an interaction effect was found, multiple comparisons using the Tukey post hoc test were performed. A two-way analysis of variance (regimen vs. supplementation) was used to analyze the Epo concentration, MDA, and protein oxidation levels at the end of the protocol. When appropriate, Tukey post hoc test was used to determine significant differences between groups. The alpha level for statistical significance was set at p<0.05.
Results
Body mass
No significant differences in body mass were found between groups. It remained unchanged until the end of the study in all the experimental groups (data not shown).
Effect of IHE and vitamin C supplementation on RBC, Hb, Hct, and Ret%
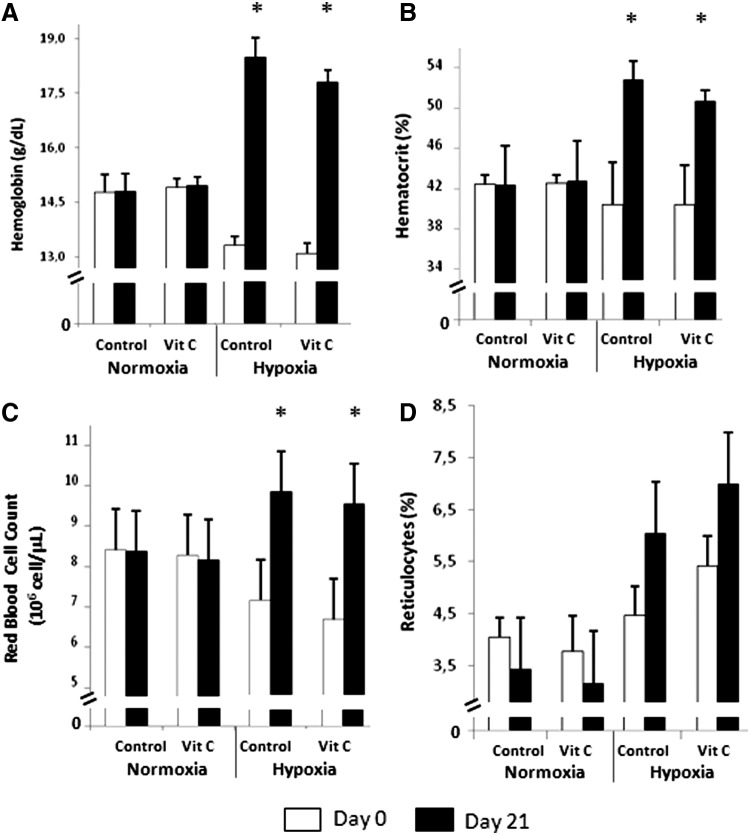
Figure 1A shows a significant increase in the Hb concentration in the animals subjected to IHE for 21 days, regardless of the vitamin C supplementation. Hb concentration increased from 13.4±0.5 to 18.5±0.5 g·dL−1 in the HC group, and from 13.1±0.3 to 17.8±0.3 g·dL−1 in the HVC group. No statistical significant changes were found before or after the experimental period in either the NC or NVC groups. Figure 1B shows a significant increase in the Hct concentration in the animals subjected to IHE. We did not find any effect after the supplementation with vitamin C. This parameter increased from 40.1±4.2% to 52.5±1.9% in the HC group, and from 40.1±3.9% to 50.4±1.0% in the HVC group. No changes were found before or after the experimental period in either the NC or the NVC groups. Figure 1C shows a significant increase in RBC in the animals subjected to IHE for 21 days, regardless of the vitamin C supplementation. This parameter increased from 7.2±0.8% to 9.8±0.3% in the HC group, and from 6.7±0.3% to 9.5±0.3% in the HVC group. No changes were found before or after the experimental period in either the NC or the NVC groups. Regarding the Ret%, we found an increase in this value after IHE although it was not statistically significant. Figure 1D shows that the Ret% increased from 4.5±0.6% to 6.0±0.8% in the HC group and from 5.4±0.6% to 7.0±2.8% in the HVC. No changes were found in the normoxic groups. Similarly to the other hematological parameters, we did not find any positive increment in the Ret% after administration of vitamin C.
FIG. 1.
Effect of IHE and vitamin C supplementation on hematological parameters. (A) Hemoglobin concentration, (B) hematocrit concentration, (C) red blood cells count, and (D) percentage of reticulocytes in hypoxic and normoxic groups supplemented or not with vitamin C during 21 days. Values are expressed as the mean±SD (n=6). A three-factor ANOVA with repeated measures in one factor (time) and post hoc Bonferroni's comparisons were used to identify significant differences. *indicates p<0.05 vs. Day 0.
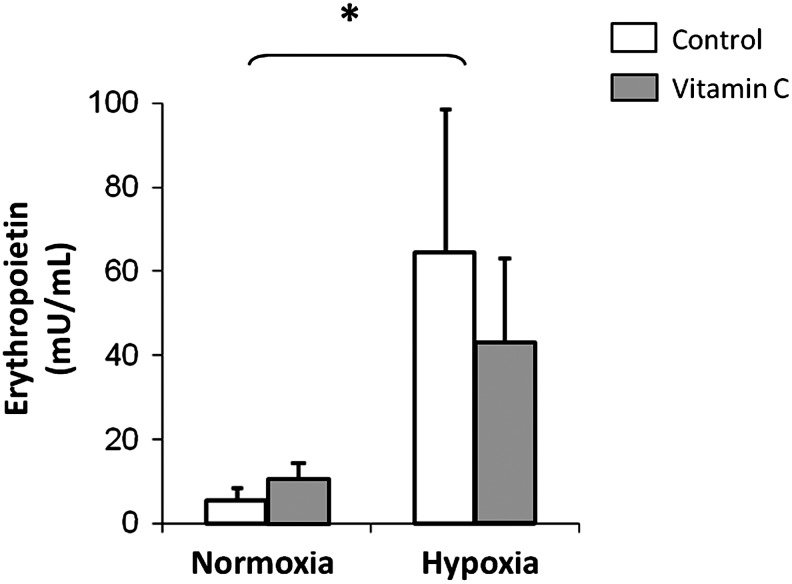
Effect of IHE and vitamin C supplementation on Epo levels
Figure 2 shows that the plasma Epo levels increased from 8.0±2.0 to 64.0±32.0 mU/mL in the HC group, and from 10.0±1.8 to 42.0±20.0 mU/mL in the HVC group. No significant changes were found between the HC and the HVC groups. As expected, the Epo values were significantly lower in the normoxic groups and unaffected by the antioxidant treatment.
FIG. 2.
Effect of IHE and vitamin C supplementation on Epo plasma levels (mU/mL). Values are expressed as mean±SD (n=6). A two-factor ANOVA and post hoc Bonferroni's comparisons were used to identify significant differences. *indicates p<0.05 vs. normoxia.
Effect of IHE and vitamin C supplementation on MDA and protein oxidation
Figure 3A shows plasma MDA levels as an index of lipid peroxidation. Although we found a trend to increase in the plasma MDA levels after IHE, it was not statistically significant. No changes were found in normoxic groups, neither in the NC nor in the NVC groups.
FIG. 3.
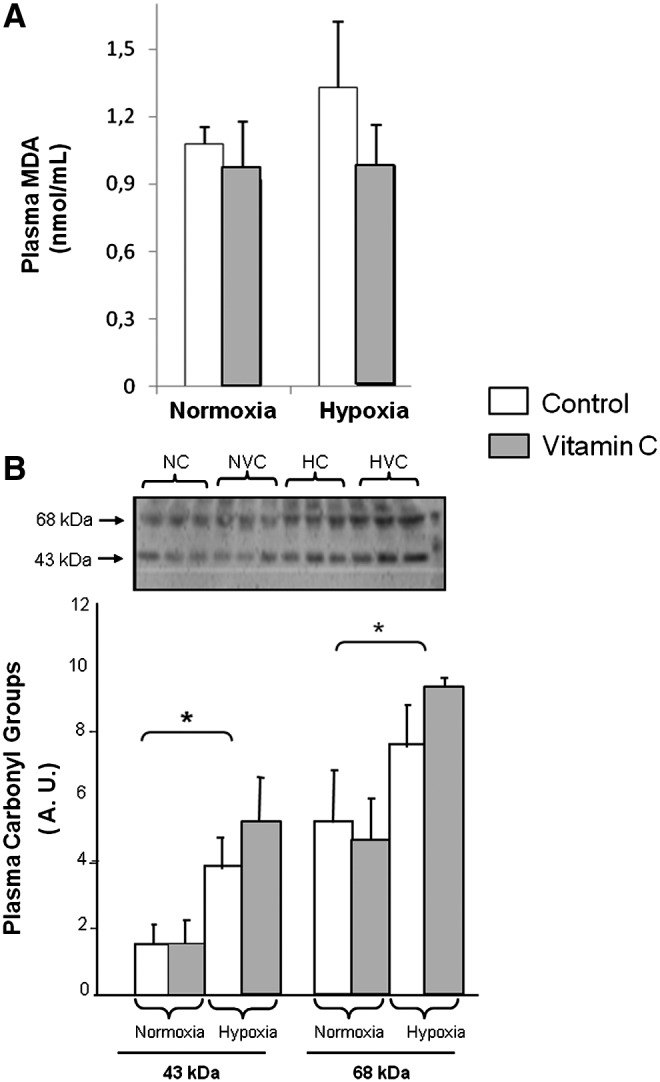
Effect of IHE and vitamin C supplementation on oxidative stress parameters. (A) MDA plasma levels, (B) plasma protein oxidation (Western blotting and densitometry analysis showing carbonylated proteins). A two-factor ANOVA and post hoc Bonferroni's comparisons were used to identify significant differences. *indicates p<0.05 vs normoxia.
As shown in Figure 3B, IHE caused a significant increase in the carbonylation of plasma proteins. Proteins with a molecular weight of 48 and 65 kDa were significantly carbonylated. Administration of vitamin C did not prevent plasma carbonylation induced by hypoxia. No changes were found in this oxidative stress parameter in the normoxic groups.
Discussion
The animals submitted to IHE showed significantly higher RBC, Hb, Hct, and Epo concentrations than the normoxic groups. These modifications have been also observed in previous studies after 21 days of IHE (Sanchis-Gomar et al., 2009).
Our results also indicate that vitamin C does not affect the stimulation of erythopoiesis and hence, the hematological parameters modified by IHE. It has been shown that vitamin C participates in the HIF-1α (hypoxia inducible factor) hydroxylation process (Li et al., 2010). HIF-1α is a transcription factor that respond to changes in available oxygen in the cellular environment, specifically, to decreases in oxygen, or hypoxia. HIF-1α activity is controlled by several independent steps. Prolyl hydroxylases (PHDs) are enzymes that hydroxylate HIF-1α. They use oxygen as a substrate for this purpose and require Fe2+ and 2-oxoglutarate as co-factors. In particular, vitamin C also participates in the HIF-1α hydroxylation process (Appelhoff et al., 2004). Administration of vitamin C can reduce significantly HIF-1α levels. Mechanistically, vitamin C is likely to repress HIF-1α by stimulating the enzymatic action of PHD2 (Li et al., 2010). Although we found a tendency to decrease in the plasma Epo levels in the group of animals treated with vitamin C and submitted to hypoxia (See Fig. 2), this decrement was not statistically significant. Moreover, no effect of the treatment with vitamin C was found in the other hematological parameters measured in the study (hemoglobin, hematocrit, red blood cell count, or reticulocytes). In accordance, Nytko and colleagues (2011) demonstrated that despite the lack of L-gulono-1,4-lactone oxidase (Gulo), a key enzyme for vitamin C synthesis, both the steady-state and the hypoxic induction of the hypoxic inducible factor (HIF) pathway appeared normal in vitamin C-deprived Gulo knockout mice. In addition, these mice showed similar circulating Epo levels regardless of whether their diet was either supplemented or not supplemented with vitamin C. These data suggest that, in our model, the ability of the PHDs to hydroxylate HIF-α, the prevalent regulator of circulating Epo levels, may not be affected by vitamin C status (Lappin and Masson, 2011).
It has been shown that hypoxia exposure increases oxidative stress, increasing ROS production (Radak et al., 1997; Pialoux et al., 2009; Pialoux et al., 2010). Furthermore, it has been suggested that ROS are involved in Epo-mediated erythroid differentiation and that antioxidants could attenuate erythropoiesis (Nagata et al., 2007). However, it has been also reported that hemolysis is directly proportional to LPO and protein oxidation (Vani et al., 2010). Therefore, antioxidant supplementation could act as a double-edge sword. Zembron-Lacny et al. (2010) observed that a daily dose of 1,200 mg of N-acetylcysteine (NAC) affects hematological parameters in healthy men. NAC increased the plasma levels of Epo, Hb, and Hct at rest and after exercise (Zembron-Lacny et al. 2010). On the other hand, the authors showed that LPO was reduced by NAC by more than 30% (Zembron-Lacny et al. 2010). However, we did not find any significant modification in any hematological parameter or a reduction in the plasma protein and lipid oxidation after vitamin C supplementation in animals submitted to our IHE protocol. In accordance with our results, Vani et al. (2010) reported that rats exposed to intermittent hypobaric hypoxia (simulated altitudes of 5100 m and 6700 m), and supplemented with antioxidant mixtures, showed significant elevated hemoglobin concentrations, demonstrating that intermittent hypoxia was effective in order to induce hematological increments, regardless of the antioxidant supplementation. However, these authors also reported a reduction in the plasma LPO and in protein oxidation in the antioxidant-supplemented animals exposed to high altitude (Vani et al. 2010). On the contrary, Esteva et al. (2010) did not find significant differences in the oxidative stress parameters in animals submitted to intermittent hypobaric hypoxia (5000 m). Although we found a significant increment in plasma protein oxidation after 21 days of hypoxic exposure, administration of vitamin C did not prevent it. Thus, the lack of a significant effect in the reduction of the protein oxidation by vitamin C supplementation does not affect the hematological adaptations induced by intermittent hypoxic exposure.
It has been previously shown that oxidative markers do not respond in the same manner during hypoxia (Chao et al., 1999). In fact, in 1997 Radak and co-workers reported that 4 weeks of exercise at an altitude of 4000 m increased reactive carbonyl derivatives but not lipid peroxidation in skeletal muscle of rats (Radak et al., 1997). Although in our study we determined the oxidative stress parameters in plasma, we also found an increase in reactive carbonyl derivatives with hypoxia but no significant modifications in the MDA levels. These results indicate that amino acids are more sensitive to oxidative modification than polyunsaturated fatty acids and/or that the underlying mechanism of these two processes is different.
When supplementing with vitamin C there is the possibility that it may act as a pro-oxidant. These pro-oxidative reactions of vitamin C readily occur in vitro, and recently it has been demonstrated that they might also have relevance in vivo (Childs et al., 2001). High intake of iron along with ascorbic acid could increase lipid peroxidation of LDL and therefore the risk of atherosclerosis (Berger et al., 1997). However, in our study, we did not find any indication of in vivo pro-oxidant effect of vitamin C in any of the experimental groups.
Conclusions
Our study investigated whether vitamin C is implicated in the regulation of erythropoiesis and in the oxygen carrying capacity of the blood. We have shown that vitamin C supplementation did not modify the hypoxia-induced erythropoiesis and plasma protein oxidation after 21 days of IHE.
Acknowledgments
This work was supported by Grants SAF2010-19498 from the Spanish Ministry of Education and Science (MEC); ISCIII2006-RED13-027 from the “Red Temática de investigación cooperativa en envejecimiento y fragilidad (RETICEF), PROMETEO2010/074 and EU Funded COSTB35. This study has been co-financed by FEDER funds from the European Union. VE Martínez-Bello was recipient of a research fellowship from the Research and Scientific Policy Department of the University of Valencia, Spain, and EPICA (Empresa de Productos de Investigación y Ciencias Aplicadas, Spain). F Sanchis-Gomar was recipient of a research fellowship from the Conselleria d’ Educació, Generalitat Valenciana, “VALi+d program”.
Author Disclosure Statement
None of the authors had any conflicts of interest with the funding agencies or professional relationships with companies or manufacturers who may benefit from the results of the present study.
References
- Appelhoff RJ. Tian YM. Raval RR. Turley H. Harris AL. Pugh CW. Ratcliffe PJ. Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Asha Devi S. Subramanyam MV. Vani R. Jeevaratnam K. Adaptations of the antioxidant system in erythrocytes of trained adult rats: Impact of intermittent hypobaric-hypoxia at two altitudes. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:59–67. doi: 10.1016/j.cca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Berger TM. Polidori MC. Dabbagh A. Evans PJ. Halliwell B. Morrow JD. Roberts LJ., 2nd Frei B. Antioxidant activity of vitamin C in iron-overloaded human plasma. J Biol Chem. 1997;272:15656–15660. doi: 10.1074/jbc.272.25.15656. [DOI] [PubMed] [Google Scholar]
- Chao WH. Askew EW. Roberts DE. Wood SM. Perkins JB. Oxidative stress in humans during work at moderate altitude. J Nutr. 1999;129:2009–2012. doi: 10.1093/jn/129.11.2009. [DOI] [PubMed] [Google Scholar]
- Childs A. Jacobs C. Kaminski T. Halliwell B. Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- Devi SA. Vani R. Subramanyam MV. Reddy SS. Jeevaratnam K. Intermittent hypobaric hypoxia-induced oxidative stress in rat erythrocytes: Protective effects of vitamin E, vitamin C, and carnitine. Cell Biochem Funct. 2007;25:221–231. doi: 10.1002/cbf.1344. [DOI] [PubMed] [Google Scholar]
- Esteva S. Pedret R. Fort N. Torrella JR. Pages T. Viscor G. Oxidative stress status in rats after intermittent exposure to hypobaric hypoxia. Wilderness Environ Med. 2010;21:325–331. doi: 10.1016/j.wem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Garcia JF. Erythropoietic response to hypoxia as a function of age in the normal male rat. Am J Physiol. 1957;190:25–30. doi: 10.1152/ajplegacy.1957.190.1.25. [DOI] [PubMed] [Google Scholar]
- Keven K. Kutlay S. Nergizoglu G. Erturk S. Randomized, crossover study of the effect of vitamin C on EPO response in hemodialysis patients. Am J Kidney Dis. 2003;41:1233–1239. doi: 10.1016/s0272-6386(03)00356-1. [DOI] [PubMed] [Google Scholar]
- Lack JA. Stuart-Taylor ME. Calculation of drug dosage and body surface area of children. Br J Anaesth. 1997;78:601–605. doi: 10.1093/bja/78.5.601. [DOI] [PubMed] [Google Scholar]
- Lappin T. Masson N. Two antioxidants are better than one. Blood. 2011;117:5276–5277. doi: 10.1182/blood-2011-03-340414. [DOI] [PubMed] [Google Scholar]
- Levine BD. Stray-Gundersen J. Point: Positive effects of intermittent hypoxia (live high:train low) on exercise performance are mediated primarily by augmented red cell volume. J Appl Physiol. 2005;99:2053–2055. doi: 10.1152/japplphysiol.00877.2005. [DOI] [PubMed] [Google Scholar]
- Li SH. Ryu JH. Park SE. Cho YS. Park JW. Lee WJ. Chun YS. Vitamin C supplementation prevents testosterone-induced hyperplasia of rat prostate by down-regulating HIF-1alpha. J Nutr Biochem. 2010;21:801–808. doi: 10.1016/j.jnutbio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Maeda N. Hagihara H. Nakata Y. Hiller S. Wilder J. Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000;97:841–846. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Bello VE. Sanchis-Gomar F. Nascimento AL. Pallardo FV. Ibanez-Sania S. Olaso-Gonzalez G. Calbet JA. Gomez-Cabrera MC. Vina J. Living at high altitude in combination with sea-level sprint training increases hematological parameters but does not improve performance in rats. Eur J Appl Physiol. 2011;111:1147–1156. doi: 10.1007/s00421-010-1740-z. [DOI] [PubMed] [Google Scholar]
- Nagata M. Arimitsu N. Ito T. Sekimizu K. Antioxidant N-acetyl-L-cysteine inhibits erythropoietin-induced differentiation of erythroid progenitors derived from mouse fetal liver. Cell Biol Int. 2007;31:252–256. doi: 10.1016/j.cellbi.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Nytko KJ. Maeda N. Schlafli P. Spielmann P. Wenger RH. Stiehl DP. Vitamin C is dispensable for oxygen sensing in vivo. Blood. 2011;117:5485–5493. doi: 10.1182/blood-2010-09-307637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux V. Brugniaux JV. Rock E. Mazur A. Schmitt L. Richalet JP. Robach P. Clottes E. Coudert J. Fellmann N. Mounier R. Antioxidant status of elite athletes remains impaired 2 weeks after a simulated altitude training camp. Eur J Nutr. 2010;49:285–292. doi: 10.1007/s00394-009-0085-z. [DOI] [PubMed] [Google Scholar]
- Pialoux V. Mounier R. Rock E. Mazur A. Schmitt L. Richalet JP. Robach P. Brugniaux J. Coudert J. Fellmann N. Effects of the 'live high-train low' method on prooxidant/antioxidant balance on elite athletes. Eur J Clin Nutr. 2009;63:756–762. doi: 10.1038/ejcn.2008.30. [DOI] [PubMed] [Google Scholar]
- Radak Z. Asano K. Lee KC. Ohno H. Nakamura A. Nakamoto H. Goto S. High altitude training increases reactive carbonyl derivatives but not lipid peroxidation in skeletal muscle of rats. Free Radic Biol Med. 1997;22:1109–1114. doi: 10.1016/s0891-5849(96)00350-4. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S. Nihal M. Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Romagnoli M. Gomez-Cabrera MC. Perrelli MG. Biasi F. Pallardo FV. Sastre J. Poli G. Vina J. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med. 2010;49:171–177. doi: 10.1016/j.freeradbiomed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F. Martinez-Bello VE. Domenech E. Nascimento AL. Pallardo FV. Gomez-Cabrera MC. Vina J. Effect of intermittent hypoxia on hematological parameters after recombinant human erythropoietin administration. Eur J Appl Physiol. 2009;107:429–436. doi: 10.1007/s00421-009-1141-3. [DOI] [PubMed] [Google Scholar]
- Sobal J. Marquart LF. Vitamin/mineral supplement use among athletes: A review of the literature. Int J Sport Nutr. 1994;4:320–334. doi: 10.1123/ijsn.4.4.320. [DOI] [PubMed] [Google Scholar]
- Tarng DC. Novel aspects of vitamin C in epoetin response. J Chin Med Assoc. 2007;70:357–360. doi: 10.1016/S1726-4901(08)70020-0. [DOI] [PubMed] [Google Scholar]
- Vani R. Reddy CS. Asha Devi S. Oxidative stress in erythrocytes: A study on the effect of antioxidant mixtures during intermittent exposures to high altitude. Int J Biometeorol. 2010;54:553–562. doi: 10.1007/s00484-010-0304-6. [DOI] [PubMed] [Google Scholar]
- Wong SH. Knight JA. Hopfer SM. Zaharia O. Leach CN., Jr. Sunderman FW., Jr. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin Chem. 1987;33:214–220. [PubMed] [Google Scholar]
- Zembron-Lacny A. Slowinska-Lisowska M. Szygula Z. Witkowski Z. Szyszka K. Modulatory effect of N-acetylcysteine on pro-antioxidant status and haematological response in healthy men. J Physiol Biochem. 2010;66:15–21. doi: 10.1007/s13105-010-0002-1. [DOI] [PubMed] [Google Scholar]