ABSTRACT
BACKGROUND:
Randomized trials have shown an increase in survival with perioperative chemotherapy as well as with postoperative chemoradiation. It was hypothesized that combining induction chemotherapy with postoperative chemoradiation would be well tolerated and improve pathologic complete response.
METHODS:
Patients with resectable cancers of the stomach/gastroesophageal junction were eligible. Neoadjuvant chemotherapy consisted of 3 cycles of paclitaxel and cisplatin. Adjuvant therapy consisted of 1 cycle of 5-fluorouracil (FU) and leucovorin (LV) followed by chemoradiation (45 Gy with concurrent 5-FU/LV). Chemoradiation was followed by 2 additional cycles of 5-FU/LV. Response to neoadjuvant therapy was based on pathology.
RESULTS:
From 1999 to 2002, 38 eligible patients were enrolled; 35 completed induction chemotherapy, and 29 went on to surgery. Sixteen patients did not develop metastatic progression, 10 developed metastatic disease, and 12 were unevaluable. There were no pathologic complete responses after induction therapy. Twenty-five of 38 patients suffered grade 3-4 toxicities during induction paclitaxel/cisplatin. Six of the 7 patients who received postoperative therapy suffered grade 3–4 toxicities. Only 3 of 38 (7.9%) eligible patients completed all assigned treatment. The median overall survival was 1.6 years, and the 2-year survival was 40%.
CONCLUSIONS:
This regimen of neoadjuvant paclitaxel/cisplatin followed by postoperative 5-FU/LV-based chemoradiation did not have a high enough response rate and proved to be too toxic for further development.
Although the incidence of distal gastric cancers has been declining in the United States, the incidence of cancers of the cardia and gastroesophageal junction has been rapidly rising.1 Surgical resection remains the mainstay of potentially curative treatment; however, local-regional and distant recurrences are common, and survival remains poor at less than 20%.2
In 2001, a Phase III randomized trial showed a survival advantage from the addition of adjuvant chemoradiotherapy. INT0116 was a study that randomized patients to surgery alone or surgery followed by postoperative chemotherapy (5-fluorouracil [FU]/leucovorin [LV] × 1 cycle) followed by concurrent chemoradiation (45 Gy of radiation given over 5 ½ weeks with 5-FU/LV given during weeks 1 and 5). The 3-year overall survival (OS) was 50% with adjuvant chemoradiation therapy vs. 41% for surgery alone (P = .005).3 Despite such aggressive adjuvant treatment, the rates of distant metastases remained a significant cause of relapse (32% in surgery-only arm and 40% in chemoradiation arm). To improve on these results, Phase II studies were initiated that intensified the chemoradiation regimen used in INT0116.
Two different intensification strategies were developed. The first performed by the Radiation Therapy Oncology Group involved 2 cycles of induction fluorouracil, leucovorin, and cisplatin followed by neoadjuvant concurrent radiation and chemotherapy (infusional fluorouracil and weekly paclitaxel). Resection was attempted approximately 6 weeks after chemoradiotherapy was completed. This study achieved a pathologic complete response (pCR) rate of 20%. More patients who achieved a pCR (82%) were alive at 1 year than patients who were unable to achieve a pCR (69%).4
A second intensification strategy involved induction chemotherapy followed by surgery and postoperative chemoradiation. Our hypothesis was that preoperative chemotherapy combined with adjuvant chemoradiation could improve on the results of adjuvant chemoradiation alone by reducing the bulk of the primary cancer, thereby making both the surgery as well as the radiation more effective. With this goal in mind, the Eastern Cooperative Oncology Group (ECOG) launched E7296, which used preoperative chemotherapy (paclitaxel/cisplatin) followed by surgery and postoperative chemoradiation (5-FU and LV).
Preclinical studies had shown synergism between paclitaxel and cisplatin.5 Sixty-one patients with advanced, surgically unresectable, or metastatic squamous cell or adenocarcinoma of the esophagus were treated with paclitaxel, cisplatin, and 5-FU. Major responses were seen in 29/60 (48%) of patients, of which 7 were complete responses. Complete response was defined as complete disappearance of all evidence of tumor for at least 4 weeks and normal endoscopic biopsies.6 These encouraging results were the basis of testing neoadjuvant paclitaxel/cisplatin in the treatment of resectable gastric cancer.
E7296 was designed to (1) evaluate the tolerability and toxicity profile of neoadjuvant paclitaxel and cisplatin and postoperative chemoradiation therapy with 5-FU/LV in high-risk gastric patients, (2) determine the pathologic response rate of gastric tumors to neoadjuvant paclitaxel and cisplatin chemotherapy, and (3) estimate PFS and OS.
MATERIALS AND METHODS
Study population
Patients with localized, histologically confirmed gastric or gastroesophageal adenocarcinomas were eligible. Using the AJCC Manual for Staging of Cancer, 3rd ed,7 patients with T2 N1-2 M0 or T3-4 N1-2M0 were eligible, whereas patients with T1-2N0M0 tumors were not eligible. N3 nodes are defined in the 3rd edition of the AJCC as hepatic pedicle, retropancreatic, mesenteric root, lower thoracic, paraesophageal, and diaphragmatic. The presence of N3 nodes makes a gastric cancer unresectable, and therefore these patients were not eligible for study. Pretreatment staging evaluation included computed axial tomography of the abdomen/pelvis and chest x-ray. The study was completed before the widespread use of endoscopic ultrasound for gastric staging and therefore was not mandated by the protocol. Although initially laparoscopy for staging was mandated, because it was not common practice in the United States to perform laparoscopy only for staging, the study was amended to no longer require this in the study. The use of laparoscopic staging was left to the discretion of the treating physician. Patients had to be ≥18 years old and have an ECOG performance status ≤2. Eligible patients could not have had a prior history of cancer within the last 5 years. No prior chemotherapy or radiation was allowed. Patients were required to have adequate hematologic, liver, cardiac, and renal function. ECOG and individual institutional review boards approved the trial before patient entry. All patients signed written informed consent prior to enrollment.
Study Design
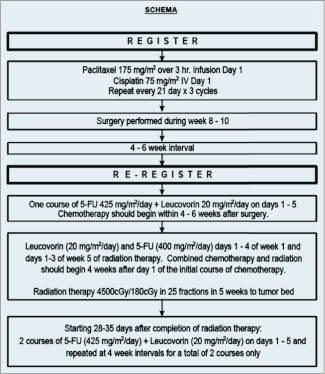
E7296 had a single-arm, 2-step Phase II study design. Step I consisted of 3 cycles of neoadjuvant chemotherapy (paclitaxel + cisplatin) followed by surgery. Surgery involved either a radical subtotal or total gastrectomy. Complete surgical resection was required to be reregistered to Step 2 of the protocol. Complete surgical resection included no microscopic residual disease or R0 resections.8 Patients with microscopically positive margins (R1 resections) were excluded. Adequate lymphatic staging was defined as removal of at least 15 lymph nodes in the surgical specimen. Evidence of metastatic disease at the time of reregistration made the patient ineligible for postoperative therapy. Figure 1 outlines the treatment schema.
Figure 1.
Treatment schema.
The primary objective of this study was to evaluate the tolerability and toxicity of the treatment regimen. The secondary objectives of the study were to assess the pathologic response of gastric cancer to neoadjuvant paclitaxel/cisplatin chemotherapy and to assess patterns of failure, DFS, and OS. A 2-year survival rate of 47% or median survival of 2.1 years would be considered promising.
Step 1: Neoadjuvant Chemotherapy
Neoadjuvant chemotherapy consisted of paclitaxel (175 mg/m2 administered as a 3 hour infusion) on day 1 followed by cisplatin (75 mg/m2 IV) on day 1. This regimen was repeated every 21 days for a total of 3 cycles. All eligible patients underwent surgery that included either a radical subtotal or total gastrectomy.
The type of surgery depended on the location and extent of the primary tumor. Patients were required to undergo a curative resection to be eligible for Step 2 of the trial. The following resections were considered curative: total gastrectomy, proximal gastrectomy, distal gastrectomy, or esophagogastrectomy. Patients who had only a wedge resection or segmental gastrectomies were ineligible to proceed to Step 2.
Step 2: Postoperative Therapy
Prior to initiating the postoperative component of the trial all patients were reregistered. Patients were deemed ineligible if they had developed metastatic disease or were unable to undergo a curative resection with adequate lymph node staging. Postoperative chemotherapy began 4–6 weeks following surgery. It consisted of 1 cycle of 5-FU (425 mg/m2/day) and LV (20 mg/m2/day), which was given on days 1–5. Both 5-FU and LV were administered as IV bolus, with 5-FU given immediately after LV.
Concurrent chemoradiation began 4 weeks after this initial course of postoperative chemotherapy. Concurrent 5-FU (400 mg/m2/day) and LV (20 mg/m2/day) were given days 1–4 of week 1 and week 5 of radiation.
Radiation fields were designed to include the tumor bed (preoperative location of the stomach plus the perigastric local tumor extension in T3 and T4 primary tumors) and nodal areas at risk. Nodal areas at risk included the gastric and gastroepiploic (usually resected with the primary), celiac, porta hepatis, subpyloric, gastroduodenal, splenic-suprapancreatic, and retropancreatico-duodenal nodes. These tumor volumes were based on pre- and postoperative diagnostic studies including upper GI barium studies, computerized axial tomography of the abdomen, surgical clip placement, and operative findings. For proximal T3 and T4 lesions, the medial ⅔–¾ of the left hemidiaphragm was included as target volume. For lesions involving the gastroesophageal junction, 5 cm margin of esophagus should be included in the cephalad field margin. All patients were treated using external beam radiation delivered by linear accelerators that delivered a dose of 45 Gy in 25 fractions of 1.8 Gy per fraction over the course of 5 weeks using at least 6 MV photons and a source-axis distance of at least 100 cm. Dose was prescribed to the isocenter. The ECOG Quality Assurance Review Center performed 2 separate reviews of radiation plans: a rapid review (at the initiation of radiation) and a final review (at the completion of radiation).
Following chemoradiation, 2 additional cycles of chemotherapy were given beginning 28–35 days after completion of radiation. This included 5-FU (425 mg/m2/day) and LV (20 mg/m2/day) on days 1–5 at 4 week intervals for a total of 2 courses.
Measurement of Tumor Response to Neoadjuvant Therapy
Tumor response was based on review of the pathologic specimen at time of definitive surgery. A patient achieved a pathologic complete response (pCR) if no gross or microscopic tumor was identified within the surgical specimen and nodal tissue. Progressive disease indicated metastatic spread, and stable disease was defined as those patients that did not achieve a complete response (pCR) or develop metastatic progression. A patient was considered unevaluable if the patient did not have surgery, the pathologist did not examine at least 15 lymph nodes, or the pathology report was not available.
Acute toxicities were evaluated by National Cancer Institute Common Toxicity Criteria, version 2.0,9 every 3 weeks during the preoperative chemotherapy and every 4 weeks postoperatively. Acute toxicity was assessed weekly during concurrent chemoradiation.
Following completion of therapy patients were followed at 1, 6, and 12 months, then every 6 months for 5 years and then annually until death.
Statistical Design and Analysis
The primary goal was to evaluate the toxicity profile of neoadjuvant paclitaxel and cisplatin and postoperative chemoradiation therapy with 5-FU and LV in high-risk gastric patients. An accrual of 42 patients was proposed because all patients were to be evaluable for toxicity of the preoperative regimen. Approximately 25–30% of patients were expected to be unevaluable for analysis of the postoperative component of therapy due to progressive disease before surgery, and 5–10% of patients were expected to be removed from the study due to metastatic or unresectable disease at the time of surgery. Thus, with an accrual goal of 42 patients, a total of 30 patients would be evaluable for toxicity of the full regimen. This sample size would provide a 90% confidence interval for toxicity that would be no wider than 33%. Secondary end points included the best confirmed response, patterns of failure, OS, and PFS. Response was based on pathology at time of surgery. Based on previous studies in this population, a 5-year survival rate of 20% would be considered promising. This translated into a median survival of 2.1 years and a 2-year survival rate of 47%.
Demographic and clinical characteristics of the patients were summarized with frequencies and percentages or means and standard deviations. OS and PFS were summarized using the method of Kaplan and Meier.10 OS was defined as the time from registration to death, where a subject was censored on the date of the last record that indicated that the patient was alive. PFS was defined as the overall time from initial registration until progression, recurrence, or death, whichever occurred first.
RESULTS
Patient Characteristics
From February 25, 1999, to March 18, 2002, 13 institutions participated in this study. Thirty-nine patients were registered to Step 1, all of whom started assigned therapy. One patient was later deemed ineligible after it was found on study entry that his disease was unresectable. Therefore, there were 38 eligible patients who completed treatment on Step 1. The mean age was 55 years (range 34–57 years).
Table 1 summarizes the patient characteristics at study entry for the 38 eligible patients treated on Step 1. All 38 patients were evaluable for toxicity. The gastroesophageal junction was the most common primary site (21/38; 55%). Thirty-six patients (95%) had adenocarcinoma as the histologic type, and 2 patients (5%) had squamous cell. These 2 patients were included in the toxicity assessment but were considered unevaluable for response.
Table 1.
Patient characteristics
| N = 38 (eligible and treated on step 1) | %* | |
|---|---|---|
| Gender | ||
| Male | 25 | 66 |
| Female | 13 | 34 |
| Race | ||
| White | 30 | 79 |
| Black | 3 | 8 |
| Asian | 1 | 3 |
| Hispanic | 1 | 3 |
| Missing/unknown/pre-NCI | 3 | 8 |
| ECOG performance status | ||
| 0 | 17 | 45 |
| 1 | 18 | 47 |
| 2 | 3 | 8 |
| Primary site of cancer | ||
| GE junction | 21 | 55 |
| Cardia | 4 | 11 |
| Pyloris or antrum | 4 | 11 |
| Body, greater curvature | 2 | 5 |
| Fundus | 2 | 5 |
| Diffuse | 2 | 5 |
| Multicentric | 1 | 3 |
| Body, lesser curvature | 1 | 3 |
| Unknown | 1 | 3 |
| Histologic type | ||
| Adenocarcinoma | 36 | 95 |
| Squamous cell | 2 | 5 |
| Estimated weight loss in previous 6 months | ||
| <5% of body weight | 12 | 32 |
| 5%–10% of body weight | 9 | 24 |
| 10%–20% of body weight | 11 | 29 |
| Unknown | 6 | 16 |
| TNM staging for stomach cancer | ||
| T2N1M0 | 2 | 5 |
| T2N2M0 | 2 | 5 |
| T3NXM0 | 2 | 5 |
| T3N1MX | 1 | 3 |
| T3N1M0 | 13 | 34 |
| T3N2M0 | 3 | 8 |
| T3N0M0 | 12 | 32 |
| T4N1M0 | 2 | 5 |
| T4N0M0 | 1 | 3 |
Percentages may not add to 100 because of rounding error.
Siewert's classification is based on anatomical criteria and is divided into Type I (distal esophagus to GE junction), Type II (true cardia arising immediately at the GE junction, and Type III (subcarinal).11 The large majority of patients on this study were Siewert's Type I (n = 21), with only 2 patients being Type II and the remaining (n = 12) being Type III. The Lauren classification divides gastric cancer into 2 major histologic types, intestinal or diffuse.12 Only 2 of the 38 patients had the diffuse histology.
Neoadjuvant Chemotherapy
Of the 38 eligible and treated patients on Step 1, 35 completed 3 cycles of neoadjuvant chemotherapy. Three patients received only 2 of the 3 planned cycles of chemotherapy. One patient developed a duodenal ulcer; a second patient developed severe back pain 15 min following the first and second infusion. A third patient stopped mid-cycle 2 due to toxicity, opting instead for an off-study gastrectomy followed by 5-FU, LV, and radiation therapy.
Surgery
Of the 38 eligible and treated patients on Step 1, 29/38 (76%) went on to definitive surgical resection, including 1 patient who went to surgery despite finishing only 2 cycles of chemotherapy. Nine of the 38 patients did not have potentially curative surgery. Three of the 9 patients were unresectable at surgery, 2 were unevaluable and went off study, 2 progressed before surgery, and 2 withdrew from study after cycle 2 of neoadjuvant chemotherapy.
Postoperative Chemotherapy Followed by Concurrent Chemoradiation
Of the 29 patients who had surgery, 26 (90%) had R0 resections and 3 (10%) had R1 resection. Only 10 patients were reregistered to Step 2. The reason that 19 of the 29 patients were ineligible at time of reregistration varied. This included fewer than 15 lymph nodes examined (n = 6), positive margins (n = 3), both fewer than 15 nodes examined and positive margins (n = 3), developed postoperative complications (n = 2), progressed prior to surgery (n = 2), and had N3 disease (n = 2). No reason was documented for 1 patient. For the 9 patients for whom pathologic data was available, the median number of nodes examined was 19 (range, 15–43) and the median number of nodes that were positive was 8 (range, 1–17).
Of the 10 patients who reregistered to Step 2, 1 patient progressed before starting Step 2 therapy, 1 patient was ineligible, and 1 died of sepsis. Therefore, 7 eligible patients were treated on Step 2. Of the 7 patients, 4 patients received 5 cycles of adjuvant chemotherapy. One patient withdrew due to excessive complications. Two patients refused treatment after 1 cycle of adjuvant chemotherapy and withdrew from the study due to excessive complications. One patient had 1 cycle, but then discontinued treatment due to progressive disease. Only 3 patients completed chemoradiation as planned.
Toxicity
Treatment-related toxicities were evaluated for all treated patients (n = 38). Following neoadjuvant paclitaxel/cisplatin common grade 3 and 4 toxicities included vomiting (grade 3: n = 1) and neutropenia (grade 3: n = 10; grade 4: n = 8). The worst grade 3 and 4 toxicities occurred in 66% (25/38) of patients, with 13 patients suffering worst grade 3, and 12 patients had worst grade 4 toxicity. There were no treatment-related deaths (Table 2).
Table 2.
Neoadjuvant paclitaxel/cisplatin: treatment-related toxicities (n = 38)
| Grade |
||
|---|---|---|
| 3 | 4 | |
| Toxicity type | (n) | (n) |
| Allergic reaction | 1 | — |
| Leukocytes | 4 | — |
| Neutrophils | 10 | 8 |
| Platelets | 1 | — |
| Ventricular arrhythmia | 1 | — |
| Hypotension | 1 | — |
| Anorexia | — | 1 |
| Constipation | 1 | — |
| Dehydration | 3 | — |
| Nausea | 1 | — |
| Vomiting | 1 | — |
| Alkaline phosphatase | 1 | — |
| Hyperkalemia | 1 | — |
| Hypocalcemia | 2 | — |
| Hypokalemia | 1 | 2 |
| Hypomagnesemia | 1 | 1 |
| Confusion | 1 | — |
| Dizziness/lightheaded | 1 | — |
| Syncope | 1 | — |
| Arthralgia | — | 1 |
| Myalgia | 1 | 1 |
| Creatinine | 1 | — |
| Worst degree | 13 | 12 |
Treatment-related toxicities were evaluated for the 7 treated and evaluable patients who were eligible to continue to Step 2 (Table 3). Eighty-six percent (6 of 7) of the patients suffered worst grade 3 (n = 2) or grade 4 (n = 4) toxicity.
Table 3.
Adjuvant FU/LV → concurrent chemoradiation → FU/LV: treatment-related toxicities (n = 7)
| Grade |
||
|---|---|---|
| 3 | 4 | |
| (n) | (n) | |
| Leukocytes | 2 | 1 |
| Neutrophils | 3 | 2 |
| Platelets | 1 | — |
| Weight loss | 1 | — |
| Anorexia | — | 2 |
| Dehydration | 2 | — |
| Dysphagia | 1 | 1 |
| Nausea | 2 | — |
| Stomatitis | — | 1 |
| Vomiting | 1 | 2 |
| Diarrhea | 2 | — |
| Hypoalbuminemia | 1 | — |
| Catheter-related infection | 1 | — |
| Febrile neutropenia | 2 | — |
| Infection with grade 3 or 4 neutropenia | 1 | — |
| Hyponatremia | 1 | — |
| Hypophosphatemia | 1 | — |
| Depressed level of consciousness | — | 1 |
| Depression | — | 1 |
| Worst degree | 2 | 4 |
Best Confirmed Response
The best confirmed response was based on pathology at the time of surgery and was assessed on all evaluable and treated patients (n = 38) in Step 1 (Table 4). Sixteen patients did not develop metastatic disease (42%), and 10 patients developed metastatic disease (26%). Twelve patients had unevaluable disease (32%). Eight of the 12 were unevaluable due to inadequate staging at time of surgery (less than 15 nodes examined), 3 went off study, and 1 patient had no pathologic information despite multiple queries. There were no pathologic complete responses following Step 1.
Table 4.
Step 1 best confirmed response
| Response | N | % |
|---|---|---|
| Stable disease | 16 | 42 |
| Progression | 10 | 26 |
| Unevaluable | 12 | 32 |
| Complete response | 0 | 0 |
Stable disease = neither complete pathologic response nor distant metastatic progression. Progression = development of distant metastatic disease. Unevaluable = inadequate nodal staging, went off study, no pathologic data available.
Overall Survival and Progression-Free Survival
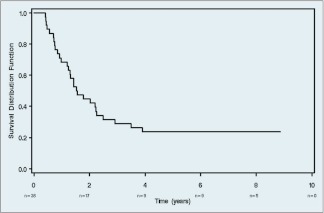
Twenty-nine (76.3%) of the 38 eligible and treated patients died as of the time of final analysis (August 2010). The median survival was 1.6 years (90% confidence interval [CI]: 1.24 years, 2.23 years). Figure 2 shows the Kaplan-Meier plot for OS for these patients. Thirty (79%) of the 38 eligible and treated patients were known to have had a recurrence, progression, or death. The median PFS was 0.68 years (90% CI: 0.35 years, 1.43 years).
Figure 2.
Overall survival.
DISCUSSION
This study was a Phase II, single-arm, 2-step design evaluating the tolerability and toxicity of neoadjuvant paclitaxel and cisplatin chemotherapy, surgery, and postoperative 5-FU/LV-based chemoradiation in the treatment of localized, resectable, high-risk adenocarcinoma of the stomach or GE junction. Only 7 of the 38 (18%) patients were able to complete all planned treatment. This compares poorly with other trials where at least 30–40% of all patients who enrolled on study were able to complete the study.3,13 The reasons for this are manifold but primarily due to the stringent criteria used to define patients eligible to continue on protocol therapy following completion of neoadjuvant chemoradiation and surgery. In the INT-0,116 study although a D2 resection was recommended, only 10% had this done. A critique of this study was that the benefit of adjuvant chemoradiation seen in this trial was a function of inadequate lymph node dissection.3 To determine the value of adjuvant chemoradiation, surgery has to be standardized for all patients. This protocol attempted to control for this by requiring an R0 resection or no residual tumor at time of surgery. Although D2 lymphadenectomy was recommended, it was not required. To control for inadequate lymph node staging, a minimum of 15 nodes were required at time of surgery. Unfortunately these stringent requirements eliminated a large number of patients from going on to receive postoperative therapy. Despite retrospective studies that suggest that adjuvant chemoradiation is beneficial even in patients with adequate lymph node staging,14 a randomized study has failed to show a benefit to the addition of postoperative chemoradiation to postoperative chemotherapy alone.15
As a Phase III study, INT0116 clearly showed a survival advantage to the use of postoperative chemoradiation. Intensification with the addition of induction chemotherapy with more active systemic agents was postulated to improve survival. Paclitaxel is one of the most active single agents in the treatment of cancers of the gastroesophageal junction. A 3-hour infusion schedule was used in this study as this was considered to be less myelotoxic and therefore would allow for full doses of cisplatin. This combination, however, proved to be too toxic. Worst grade 3 or 4 toxicities occurred in 66% (25/38) of patients with 13 patients suffering worst grade 3, and 12 patients had worst grade 4 toxicity. In a Phase II study utilizing a similar 3 hour paclitaxel infusion almost one-half the patients suffered severe toxicity requiring dose attenuation and half of the patients were hospitalized for toxicity.6
Although preoperative cisplatin and paclitaxel as outlined in this study proved to be too toxic, a Phase III randomized trial in patient with cancers of the esophagus and GE junction using preoperative concurrent weekly carboplatin, paclitaxel and radiation has shown improved survival when compared to surgery alone.16 In a study of perioperative chemotherapy (epirubicin, cisplatin, and 5-fluorouracil) alone, the British Medical Research Council Adjuvant Gastric Cancer Infusional Chemotherapy (MAGIC) trial suggests that the most valuable component of the protocol might be the neoadjuvant chemotherapy, because only 40% of patients actually received the postoperative chemotherapy. Patients randomized to perioperative chemotherapy had a 34% improvement in PFS and a 25% improvement in OS.13
Other trials have also attempted to combine perioperative chemotherapy with postoperative chemoradiation using a more active systemic combination of epirubicin, cisplatin, and 5-FU (ECF) to 5-FU-based chemoradiation. In a Phase III trial (CALGB 80,101) the addition of ECF before and after 5-FU/radiation did not improve survival when compared to bolus 5-FU/LV before and after 5-FU/radiation.17
Adjuvant chemotherapy alone given after D2 resection has shown promise in 2 separate randomized studies. The CLASSIC study (capecitabine and oxaliplatin adjuvant study in stomach cancer) showed improved results with the capecitabine and oxaliplatin over surgery alone.18 In another randomized study the use of adjuvant S-1, an oral fluorpyrimidine, in the ACTS-GC trial (Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer) found improved 3-year survival over surgery alone.19
Although the role of adjuvant therapy has been clearly established in the management of cancers of the stomach and gastroesophageal junction, the debate continues regarding whether to use postoperative chemoradiation as is common in North America, perioperative chemotherapy alone as is used in Europe, or postoperative chemotherapy alone as is common in Asia. An ongoing trial is attempting to answer this question. The CRITICS study (ChemoRadiotherapy after Induction chemotherapy In Cancer of the Stomach) is enrolling patients with Stage IB–IV (M0) resectable adenocarcinoma of the stomach who will be treated with preoperative chemotherapy consisting of epirubicin, cisplatin, capecitabine (ECC) times 3 cycles, followed by surgery, followed by concurrent chemoradiation (45 Gy with cisplatin and capecitabine) or preoperative ECC times 3 cycles, followed by surgery, followed by postoperative ECC times 3 cycles, without radiation.20
E7296 was a Phase II, single-arm, 2-step design study evaluating the tolerability and toxicity of neoadjuvant paclitaxel and cisplatin chemotherapy, surgery, and postoperative 5-FU/LV-based chemoradiation in the treatment of localized, resectable, high-risk adenocarcinoma of the stomach or GE junction. Despite encouraging preclinical data, the preoperative chemotherapy regimen in this trial did not have a high enough response rate and proved to be too toxic. No conclusions can be drawn regarding the impact of the postoperative treatment in this trial, because only 7.9% of all patients were able to complete all assigned therapy. Neoadjuvant chemotherapy using newer combinations of systemic agents followed by adjuvant chemoradiation is a strategy that warrants further evaluation.
Footnotes
This study was conducted by ECOG (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA49957, CA15488, CA73590, CA27525, CA13650, CA21076, CA17145 and by the NCI, NIH, and Department of HHS. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI.
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Blot WJ, Devesa SS, Kneller RW, et al. : Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265:1287–1289, 1991 [PubMed] [Google Scholar]
- 2. Songun I, Putter H, Kranenbarg EM, et al. : Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11:439–449, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Macdonald JS, Smalley SR, Benedetti J, et al. : Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Ajani JA, Winter K, Okawara GS, et al. : Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 24:3953–3958, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Rowinsky EK, Gilbert MR, McGuire WP, et al. : Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol 9:1692–1703, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Ilson DH, Ajani J, Bhalla K, et al. : Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 16:1826–1834, 1998 [DOI] [PubMed] [Google Scholar]
- 7. American Joint Committee on Cancer Staging Manual (3rd ed). Philadelphia: Lippincott, 1988 [Google Scholar]
- 8. Hermanek P, Wittekind C: Residual tumor (R) classification and prognosis. Semin Surg Oncol 10:12–20, 1994 [DOI] [PubMed] [Google Scholar]
- 9. National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 2.0, 1999. Available at: http://ctep.cancer.gov/reporting/ctc.html Accessed November 2, 2012
- 10. Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481, 1958 [Google Scholar]
- 11. Siewert JR, Stein HJ: Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85:1457–1459, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Lauren P: The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathologica et Microbiologica Scandinavica 64:31–49, 1965 [DOI] [PubMed] [Google Scholar]
- 13. Cunningham D, Allum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Snyder RA, Castaldo ET, Bailey CE, et al. : Survival benefit of adjuvant radiation therapy for gastric cancer following gastrectomy and extended lymphadenectomy. Int J Surg Oncol 2012:11–307670, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J, Lim do H, Kim S, et al. : Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30:268–273, 2012 [DOI] [PubMed] [Google Scholar]
- 16. van Hagen P, Hulshof MC, van Lanschot JJ, et al. : Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084, 2012 [DOI] [PubMed] [Google Scholar]
- 17. Fuchs CS, Tepper JE, Niedzwiecki D, et al. : Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction (GEJ) adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (CRT) compared with bolus 5-FU/LV before and after CRT: intergroup trial CALGB 80101. J Clin Oncol 29:2074–4003, 2011 [Google Scholar]
- 18. Bang YJ, Kim YW, Yang HK, et al. : Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379:315–321, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Sakuramoto S, Sasako M, Yamaguchi T, et al. : Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. : Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 11:329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]