ABSTRACT
Combined hepatocellular-cholangiocarcinoma (cHCC-CC) is a rare type of primary liver cancer comprising histopathological features of both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC). Because of its rarity and controversial diagnostic criteria, it continues to be poorly understood with a lack of well-delineated treatment options for recurrent or metastatic disease. We report a case of cHCC-CC in a 31-year-old woman with no risk factors for HCC or CC with recurrent pulmonary metastasis treated with systemic chemotherapy.
Combined hepatocellular-cholangiocarcinoma (cHCC-CC), also known as mixed HCC-CC, is a rare form of primary liver cancer, showing histopathological features of both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC).1 Historically2,3 the incidence of this entity comprises less than 5% of primary liver cancer, though it has gained increasing recognition lately, partly because of the extensive sampling of explants and surgical resection specimens.4
Due to the rarity of this neoplasm, its diagnostic and prognostic features remain ill-defined, leaving clinicians little direction as to how to best manage cHCC-CC. Surgery has served as the only treatment option that offers chance of cure, provided that liver function will be fairly preserved postoperatively.5 In contrast, scarce literature data exist as for the value of systemic chemotherapy for recurrent or metastatic disease. Here we report a case of transitional-type cHCC-CC with complete follow-up, including 12-month duration of favorable response to combination chemotherapy with gemcitabine and cisplatin.
CASE REPORT
A 31-year-old Caucasian female, otherwise healthy, presented with persistent right-sided rib pain for 1 week after playing lacrosse. Her social history was noticeable for occasional alcohol drinking and history of marijuana and cocaine use. Family history was significant for colorectal cancer in her maternal grandfather, diagnosed in his 70s. Chest x-ray of the patient was negative for fracture, but a computed tomography (CT) scan of chest incidentally revealed a 10-cm, heterogeneous, right hepatic mass. Laboratory tests ruled out chronic hepatitis B, hepatitis C, or HIV infection. Additional liver function tests including prothrombin time and partial thromboplatin time were unremarkable, and tumor markers, such as cancer antigen 19-9, alpha-fetoprotein (AFP), and carcinoembryonic antigen (CEA) levels, were also within normal range.
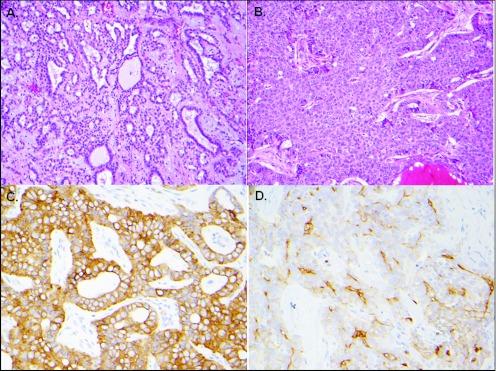
The patient underwent exploratory laparotomy with intraoperative ultrasound assistance and was found to have a bulky right-sided liver tumor extending into segment IV, in close apposition to the left portal vein. Consequently she received right hepatic lobectomy, cholecystectomy, and periportal lymph node dissection. Surgical pathology demonstrated a poorly differentiated carcinoma with a narrow surgical margin and 1 lymph node involved. Interestingly, the tumor was composed of malignant cells largely representing cholangiocellular differentiation with well-formed glandular structures (Figure 1). Immunohistochemistry (IHC) staining was strongly positive for cytokeratin 7 while negative for Hepar-1, AFP, monoclonal CEA, and cytokeratin 20. In addition, the tumor contained areas of solid growth pattern with focal positive canalicular staining with polyclonal CEA and CD10 suggestive of partial hepatocytic differentiation as well. She recovered well postoperatively, and CT scan of chest/abdomen/pelvis along with bone scintigraphy showed no evidence of disease. Therefore, she was diagnosed as AJCC stage IVA (pT1N1M0G3F0), transitional-type cHCC-CC.
Figure 1.
The carcinoma showing features of both cholangiocarcinoma (A and B) and hepatocellular carcinoma (C and D). (A) An area with glandular formation (hematoxylin and eosin stain [H&E], 200× magnifications); (B) immunohistochemical stain demonstrating strong cytokeratin 7 expression (400× magnifications); (C) an area with solid growth pattern and no glandular formation (H&E stain, 200× magnifications); (D) stain for polyclonal carcinoembryonic antigen showing focal canicular pattern in areas with solid growth pattern (400× magnifications).
Admittedly, the close surgical margin and positive lymph node rendered this patient high risk for recurrence, yet no evidence has shown that upfront adjuvant chemotherapy could reduce this risk. As a result, the patient opted for close observation with regular follow-up. Five months later, a 2 cm periportal mass was detected and subsequently resected, with the pathology findings similar to the previous ones. Ultimately, 10 months after her initial surgery, a CT scan demonstrated a recurrent liver lesion and multiple lung nodules.
Chemotherapy consisting of gemcitabine 1,000 mg/m2 and cisplatin 25 mg/m2 (gem/cis) was initiated, both given on day 1 and day 8 of a 21-day cycle. She had remarkable response in her liver lesion and disappearance of her pulmonary nodules, sustained for 12 months. Upon suggestion of disease progression, her treatment was switched to leucovorin, fluorouracil, and oxaliplatin. Unfortunately, on this regimen she had clear evidence of progressive disease, so she opted to resume gem/cis, again resulting in disease stabilization. Unexpectedly, she developed transient infusion reaction to cisplatin with vomiting, dyspnea, and tachycardia. Shortly thereafter, she was lost to follow-up due to social issues.
Eight months later the patient returned to the clinic to pursue further treatment with massive hepatic progression. Gemcitabine with fluorouracil and leucovorin was planned but delayed and finally abandoned secondary to her sequential comorbidities, including bone fractures requiring surgery, obstructive jaundice requiring stent placement, serial bacterial and fungal infections, progressive ascites, and hepatic encephalopathy. Eventually she accepted hospice care and soon died, 41 months after her initial exploratory surgery.
DISCUSSION
Although this neoplasm was first described in 1949, little is fully understood in regard to its histopathogenesis,4,6,7 biological behavior, clinical features, and prognosis, in comparison to HCC or CC. Based on a large population study using the National Cancer Institute's registry database,2 the incidence rate of cHCC-CC was 1.3% among primary liver tumors. Interpretation of incidence data could be complicated by underdiagnosis of cases with inadequate tissue sampling or overdiagnosis of cases if pseudoglandular elements derived from hepatocyte-like tumor cells are mistakenly assumed to be pure cholangiocarcinoma.8 Histologically, 2 subclassification schemes have arisen. Classically Allen and Lisa9 segregated cHCC-CC into 3 subtypes: (1) separate tumors, each with single histopathology, (2) contiguous tumors with separate histopathologies, and (3) mixed histopathologies in individual tumors. Goodman et al10 also classified this neoplasm into 3 groups: Type I “collision tumors,” which had both components but no areas of transition, Type II “transitional tumors” with areas of intermediate differentiation and identifiable transition between 2 components, and Type III “fibrolamellar tumors,” which had features of both the fibrolamellar variant of HCC and cholangiocellular differentiation throughout without separate areas of one or the other. The Type III tumors were more likely to occur in younger patients without a background of cirrhosis and carried a better prognosis.
The aforementioned limitations have posed challenges regarding how to manage cHCC-CC appropriately. Surgery remains the only treatment option offering potential cure for localized disease, especially in noncirrhotic patients, although recurrence risk may be higher than those with pure HCC or CC.11–13 Since cHCC-CC tends to have portal and hepatic venous infiltration like HCC and lymph node metastasis like CC,14 hepatic resection with lymph node dissection is the proper treatment of choice for noncirrhotic patients. In addition, studies have shown that intrahepatic recurrence of CC can be managed by reresection alone.15 Correspondingly, our patient whose tumor contained a predominantly CC component received partial hepatectomy and lymphedenectomy initially, followed by resection of her peri-portal mass upon disease recurrence.
Even though the 5-year overall survival rate has exceeded 70% after liver transplantation (LT) for HCC patients,16,17 the corresponding rate for cHCC-CC patients is far from being satisfactory, according to 2 recent studies.18,19 Despite the discrepancies of radiographic findings and comparative prognosis between cHCC-CC and intrahepatic CC (I-CC), most centers exclude cHCC-CC and I-CC from LT, because of historically higher recurrence rate in shorter duration and poorer prognosis compared to HCC. This further emphasizes the significance of making an accurate diagnosis preoperatively among cHCC-CC, HCC, and I-CC, which poses a challenge for diagnostic radiology.20–23
Combined HCC-CCs are often less vascular and more fibrotic than HCC,5 which would likely limit the efficacy of transarterial chemoembolization (TACE), percutaneous ethanol injection, or sorafenib.24 A few case reports have described TACE among other treatment modalities with good response overall,25–27 yet the added value by TACE itself may need further evaluation. Other modalities such as radiofrequency ablation or cryoablation might also be of help in selected patients.
Little published data exist in respect to applying systemic chemotherapy for advanced cHCC-CCs, besides a few case reports (Table 1). Aside from one report26 where adjuvant chemoradiotherapy was given, most chose careful surveillance until unresectable disease recurrence. Platinum, gemcitabine, and fluorouracil appeared to be the chemo-agents frequently chosen, while doxorubicin has been used in 1 case, presumably for more HCC coverage.24 In our case the patient's tumor behaved aggressively with local recurrence in 5 months and distant recurrence in 10 months. A regimen of gemcitabine and cisplatin was selected because of predominance of the tumor's CC component and achieved greater than 12-month duration of disease control. Other newer chemo- regimens active in cholangiocarcinoma such as taxanes or irinotecan could have also been tried.28–33 However, her marked physical deterioration after an 8-month loss of follow-up deprived us of further chances for intervention.
Table 1.
Summary of case reports using systemic chemotherapy for cHCC-CC (n = 4)
| Author (year) | Age/gender | Risk factors | Metastasis | Chemotherapy | Result |
|---|---|---|---|---|---|
| Hayashi (2006)26 | 52/M | NA | NA | CDDP, 5-FU, RT | NED (42 mo) |
| Kim (2010)24 | 62/M | NA | Lung, bone | ACà5-FU | SD (18 mo) |
| Tani (2011)27 | 30/M | Hepatitis B | Lung | GEM+CBDCA+5FU | SD (TBD) |
| Chi (current) | 31/F | NA | Lung | GEM+CDDP | SD (12 mo) DOD (41 mo) |
*5-FU, flurouracil; AC, doxorubicin and cisplatin; CBDCA, carboplatin; CDDP, cisplatin; DOD, died of disease; GEM, gemcitabine; NA, not applicable; NED, no evidence of disease; RT, radiation therapy; SD, stable disease; TBD, to be determined.
In conclusion, systemic chemotherapy did result in clinical benefit in this patient with cHCC-CC and distant metastasis. Prospective evidence guiding therapeutic intervention for patients with advanced disease is clearly needed.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Gibson JB: Histological Typing of Tumours of the Liver, Biliary Tract and Pancreas. International Histological Classification of Tumours. Geneva, Switzerland, World Health Organization, 1978 [Google Scholar]
- 2. Wachtel MS, Zhang Y, Xu T, et al. : Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol 1:43–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edmondson HA, Peters RL: Neoplasms of the liver, in Schiff L, Schiff ER. (eds): Disease of the Liver (5th ed). Philadelphia, JB Lippincott, 1982 [Google Scholar]
- 4. Yeh MM: Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol 25:1485–1492, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Kassahun WT, Hauss J: Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract 62:1271–1278, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Fujii H, Zhu XG, Matsumoto T, et al. : Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol 31:1011–1017, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Theise ND, Yao JL, Harada K, et al. : Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology 43:263–271, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Edmondson HA: Tumors of the Liver and Intrahepatic Bile Ducts. Washington, DC, Armed Forces Institute of Pathology, 1958 [Google Scholar]
- 9. Allen RA, Lisa JR: Combined liver cell and bile duct carcinoma. Am J Pathol 25:647–655, 1949 [PMC free article] [PubMed] [Google Scholar]
- 10. Goodman ZD, Ishak KG, Langloss JM, et al. : Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 55:124–135, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Jarnagin WR, Weber S, Tickoo SK, et al. : Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 94:2040–2046, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Park H, Choi KH, Choi SB, et al. : Clinicopathological characteristics in combined hepatocellular-cholangiocarcinoma: a single center study in Korea. Yonsei Med J 52:753–760, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee JH, Chung GE, Yu SJ, et al. : Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol 45:69–75, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Uenishi T, Hirohashi K, Shuto T, et al. : Surgery for mixed hepatocellular and cholangiocellular carcinoma. Hepatogastroenterology 47:832–834, 2000 [PubMed] [Google Scholar]
- 15. Cherqui D, Tantawi B, Alon R, et al. : Intrahepatic cholangiocarcinoma: results of aggressive surgical management. Arch Surg 130:1073–1078, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Mazzaferro V, Regalia E, Doci R, et al. : Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334:693–699, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Yao FY, Xiao L, Bass NM, et al. : Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 7:2587–2596, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Panjala C, Senecal DL, Bridges MD, et al. : The diagnostic conundrum and liver transplantation outcome for combined hepatocellular-cholangiocarcinoma. Am J Transplant 10:1263–1267, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Sapisochin G, Fidelman N, Roberts JP, et al. : Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transplant 17:934–942, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Fukukura Y, Taguchi J, Nakashima O, et al. : Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr 21:52–58, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Anderson CD, Rice MH, Pinson CW, et al. : Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 8:90–97, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Sanada Y, Shiozaki S, Aoki H, et al. : A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res 32:185–195, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hwang J, Kim YK, Park MJ, et al. : Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imag 36:881–889, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Kim GM, Jeung HC, Kim D, et al. : A case of combined hepatocellular-cholangiocarcinoma with favorable response to systemic chemotherapy. Cancer Res Treat 42:235–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eguchi H, Nagano H, Sakon M, et al. : A successful resection and long-term survival of a patient with intrahepatic recurrences of combined hepatocellular-cholangiocarcinoma: report of a case. Surg Today 32:742–746, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hayashi H, Beppu T, Ishiko T, et al. : A 42-month disease free survival case of combined hepatocellular-cholangiocarcinoma with lymph node metastases treated with multimodal therapy. Gan To Kagaku Ryoho 33:1941–1943, 2006 [PubMed] [Google Scholar]
- 27. Tani S, Murata S, Tamura M, et al. : Effectiveness of systemic chemotherapy of GEM+CBDCA+5-FU/LV and hepatic arterial infusion of CDDP in a case of advanced, combined hepatocellular-cholangiocarcinoma with multiple lung metastases. Nihon Shokakibyo Gakkai Zasshi 108:1892–1901, 2011 [PubMed] [Google Scholar]
- 28. Kuhn R, Hribaschek A, Eichelmann K, et al. : Outpatient therapy with gemcitabine and docetaxel for gallbladder, biliary, and cholangio-carcinomas. Invest New Drugs 20:351–356, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Sakurai N, Okada T, Iizawa H: A case of recurrent cholangiocarcinoma responding to weekly paclitaxel. Gan To Kagaku Ryoho 37:1333–1335, 2010 [PubMed] [Google Scholar]
- 30. Adjei AA, Klein CE, Kastrissios H, et al. : Phase I and pharmacokinetic study of irinotecan and docetaxel in patients with advanced solid tumors: preliminary evidence of clinical activity. J Clin Oncol 18:1116–1123, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Romiti A, D'Antonio C, Zullo A, et al. : Chemotherapy for the biliary tract cancers: moving toward improved survival time. J Gastrointest Cancer 43:396–404, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Kuhlmann JB, Euringer W, Spangenberg HC, et al. : Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 24:437–443, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Karachaliou N, Polyzos A, Kentepozidis N, et al. : A multicenter phase II trial with irinotecan plus oxaliplatin as first-line treatment for inoperable/metastatic cancer of the biliary tract. Oncology 78:356–360, 2010 [DOI] [PubMed] [Google Scholar]