Abstract
Aims
The Surgical Treatment for Ischemic Heart Failure (STICH) trial demonstrated no overall benefit when surgical ventricular reconstruction (SVR) was added to coronary artery bypass grafting (CABG) in patients with ischaemic cardiomyopathy. The present analysis was to determine whether, based on baseline left ventricular (LV) function parameters, any subgroups could be identified that benefited from SVR.
Methods and results
Among the 1000 patients enrolled, Core Lab measures of baseline LV function with adequate quality were obtained in 710 patients using echocardiography, in 352 using cardiovascular magnetic resonance, and in 344 using radionuclide imaging. The relationship between LV end-systolic volume index (ESVI), end-diastolic volume index, ejection fraction (EF), regional wall motion abnormalities, and outcome were first assessed only by echocardiographic measures, and then by 13 algorithms using a different hierarchy of imaging modalities and their quality. The median ESVI and EF were 78.0 (range: 22.8–283.8) mL/m2 and 28.0%, respectively. Hazard ratios comparing the randomized arms by subgroups of LVESVI and LVEF measured by echocardiography found that patients with smaller ventricles (LVESVI <60 mL/m2) and better LVEF (≥33%) may have benefitted by SVR, while those with larger ventricles (LVESVI >90 mL/m2) and lower LVEF (≤25%) did worse with SVR. Algorithms using all three imaging modalities found a weaker relationship between LV global function and the effects of SVR. The extent of regional wall motion abnormality did not influence the effects of SVR.
Conclusions
Subgroup analyses of the STICH trial suggest that patients with less dilated LV and better LVEF may benefit from SVR, while those with larger LV and poorer LVEF may do worse.
Clinical Trial Registration #: NCT00023595.
Keywords: STICH, Surgical ventricular reconstruction, Coronary disease, Heart failure
Introduction
Left ventricular (LV) dilatation resulting from LV remodelling after ischaemic myocardial injury is associated with a poor clinical outcome.1 Addition of surgical ventricular reconstruction (SVR) to coronary artery bypass grafting (CABG) for patients with anterior regional LV dysfunction has been proposed as a procedure that produces a smaller LV with a more natural ellipsoidal shape.2 The Surgical Treatment for Ischemic Heart Failure (STICH), an international multicentre randomized trial sponsored by the National Heart, Lung, and Blood Institute, tested the hypothesis that SVR improves survival free of subsequent hospitalization for a cardiac cause compared with CABG alone in patients who require CABG.3 The trial randomized 1000 patients in equal proportions to CABG or CABG + SVR. After a median follow-up of 4 years, the trial demonstrated no overall benefit from adding SVR to CABG.4 Since baseline LV function was assessed extensively by several imaging modalities, with echocardiogram (Echo) required for all patients as well as single-photon emission-computed tomography (SPECT) radionuclide (RN) imaging, and cardiovascular magnetic resonance (CMR) imaging when feasible, the present analysis focuses on whether any baseline LV function variables measured by imaging Core Laboratories (Core Labs) could identify a group of patients in whom adding SVR to CABG improved or impaired the clinical outcome.
Methods
Patients
The design and patient enrolment criteria of the STICH trial have been previously described.3,4 Briefly, between 12 September 2002 and 24 January 2006, 1000 patients with coronary artery disease amenable to CABG, with LVEF ≤35% and dominant anterior akinesia or dyskinesia amenable to SVR were randomized to CABG (499 patients with a median age of 62 years) or CABG + SVR (501 patients with a median age of 62 years) by 96 clinical sites in 23 countries. The requirement of LVEF ≤35% was based on the enroling clinical site's LV assessment performed within 3 months prior to randomization using any of the following modalities: Echo, CMR, RN, or invasive left ventriculography imaging. Surgical ventricular reconstruction eligibility was determined by a clinical decision, and no definite cut-off value for LV volumes was required for enrolment.
Imaging studies and their Core Labs
All imaging studies were sent to the respective Core Labs (University of Southern California for CMR, Mayo Clinic for Echo, and Northwestern University and Cedars-Sinai Medical Center for RN) to objectively measure of the LV function without knowledge of their randomized treatment assignment. A quality score was assigned to each study by each Core Lab using the following definitions: Excellent for textbook illustration quality, Good when all measurements were possible, Fair when most measurements were possible, Borderline when no quantitation was possible, and Unusable.
Each Core Lab was responsible for quality control and their data transfer to the STICH Statistical and Data Coordinating Center located at the Duke Clinical Research Institute.
Echocardiography Core Lab
Experienced sonographers and cardiologists used recommendations of the American Society of Echocardiography for analyses.5 An excellent or good quality study required clear LV border definition from two apical views, fair quality for studies with clear border definition from only one apical view. Left ventricular volumes were measured by Simpson's method and were averaged from three cardiac cycles in sinus rhythm, and three to five cardiac cycles for atrial fibrillation. Left ventricular regional wall motion analysis was performed visually using a 16-segment model that was converted into the 17-segment model6 to standardize LV zones applicable to all three imaging modalities. For this report, apical cap was given the average value from the four surrounding zones.
Radionuclide Core Lab
Gated myocardial perfusion SPECT imaging was performed at clinical sites using a standard protocol. The gated raw projections were reconstructed by the RN Core Lab using automatic software (AutoSPECT). An algorithm was applied to correct any motion detected during image reconstruction from RN count data. Gated short-axis data were reviewed by the processing technologist to optimize the accuracy of LV contour boundaries. Manual adjustment addressed incorrect valve plane placement or contour deviations due to extra-cardiac radioactivity. The QGS software for LV function analysis7 produced values for the LV end-diastolic volume (EDV), the end-systolic volume (ESV), and the ejection fraction (EF), as well as a four-point grading scale for visually assessed LV wall motion.8
Cardiovascular magnetic resonance Core Lab
Resting CMR imaging was performed by clinical sites equipped with the specified CMR platform and software.9,10 At least two data sets of short-axis and two-chamber and four-chamber long-axis views were required to permit the Core Lab to select images with the highest quality. Short-axis images provided EF and volume data using software developed by the CMR Core Lab (USC Cardio). When data were corrupt or could not be downloaded from the FTP site or CD, the MARISA software (Chase Medical, Dallas, TX, USA) was used for the analysis. All gated short-axis data were displayed and reviewed by CMR Core Lab expert technologists to ensure that both external and internal LV boundaries were reliably demarcated. Manual adjustment was used to correct LV contours created by automatic border recognition software that appeared to be incorrect.
Utilization of Core Lab data and statistics
Since the number of studies performed by each imaging modality was different and their quality varied in addition to the variability in measurements among the three imaging modalities, several approaches were used to analyse the data. To assess measurement variability among imaging modalities, LV volumes from different imaging modalities were compared by examining the correlation (Pearson's linear correlation coefficient) of the volume information from different modalities among patients who had more than one imaging study. Regional LV function was described using standardized segmental nomenclature.6 Each Core Lab's motion data were converted to a consistent severity term for each segment based on a four-point regional function grading system: 1—normal, 2—hypokinetic, 3—akinetic, 4—dyskinetic or aneurysm. A wall motion score index was calculated as the average of the severity grades from all visible segments.
To analyse the relationship of baseline LV function parameters with clinical outcomes, we first used the data based on Echo Core Lab measures only, as Echo data were available in the largest number of patients. This choice thus eliminated inter-imaging modality variability. The baseline characteristics of patients with adequate Echo data were compared with those of patients without Echo data using the Wilcoxon rank-sum test for continuous or ordinal variables and the conventional Chi-square test for categorical variables. A second approach was to use the Echo data in all the patients where Echo information was available, but also include additional patients where LV function data were available from other modalities. Where the Echo data were not available, preference was given to CMR Core Lab data if available, then to RN Core Lab data, and last to site-reported data (referred to later as algorithm #11). Finally, we analysed the data based on LV volumes from an algorithm, which was found to have the strongest relationship of LVESVI with mortality (assessed using likelihood ratio Chi-square statistics from the Cox regression model), and provided the maximum number of patients with available LV volumes based on the quality of each study and the type of imaging modality (referred to later as algorithm #8) (Supplementary material online, Tables S1 and S2). Thirteen algorithms were considered: six algorithms were derived by giving preference to the quality of imaging study followed by the hierarchy of imaging modality within a given level of quality, six algorithms by giving preference to the imaging modality (of any adequate quality where available), and one algorithm based on choosing the average value when a patient had Core Lab measures from more than one modality.
Cox proportional hazards models were used to generate hazard ratios and 95% confidence intervals for comparing CABG + SVR vs. CABG with respect to death or cardiac hospitalization (primary endpoint) and overall mortality (secondary endpoint) for various patient subgroups defined either by Echo LV function data or data derived from three imaging modalities supplemented by clinical sites' data. The subgroups considered were defined using tertiles of the distribution the LV function variables, and additionally in the case of LVESVI, by other pre-specified clinically important cut-points (<60, 60–90, and >90 mL/m2). The presence of an interaction between the LV function parameters and the randomized treatment strategy with respect to these clinical outcomes was also tested using the Cox model. All analyses were performed using SAS version 8.2 statistical software.
Results
Quality and comparison of baseline cardiac imaging data
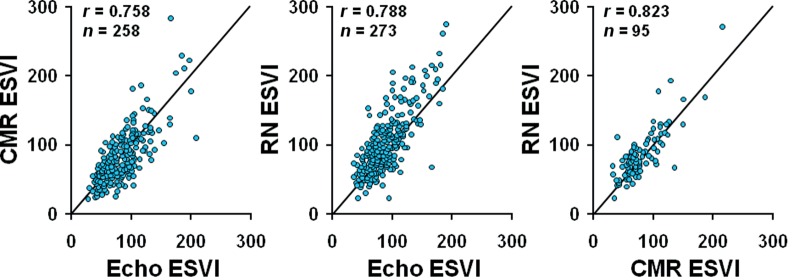
Baseline data processed by the three Core Labs included 950 Echo, 399 CMR, and 394 RN studies. A total of 1408 (81%) of those submitted studies were assigned an adequate (fair to excellent) quality for LV volume measurement by Core Labs: 710 Echo (75%), 352 CMR (88%), and 344 RN (87%). More than one set of Core Lab measurements were available in 457 patients. The correlation between different imaging modalities for ESVI is shown in Figure 1.
Figure 1.
Correlation between imaging modalities for measuring left ventricular end-systolic volume index by their Core Lab. CMR, cardiovascular magnetic resonance imaging; Echo, echocardiography; ESVI, end-systolic volume index; RN, radionuclide imaging.
Baseline left ventricular volumes and ejection fraction
A total of 924 patients had at least one Core Lab measure (in 866) or only site-reported measure (in 58) of LV volumes and EF. Regardless of which method was used, there was a wide range of EDVI and ESVI. As a representative example using algorithm #8 (the algorithm that produced the strongest relationship between ESVI and mortality), EDVI and ESVI values ranged from 39.0 to 306.2 mL/m2 and from 22.8 to 283.8 mL/m2, respectively. The median and mean (SD) values of LVEDVI, LVESVI, and EF were similar regardless of which method as shown in Table 1. The LVEF as measured by Echo Core Lab was >35% in 20.0% of the patients, and ESVI was <60 mL/m2 in 23.4% of patients.
Table 1.
Mean and median values of left ventricular end-systolic volume index, end-diastolic volume index, and ejection fraction using Echo, algorithm #11, or algorithm #8
| Mean ± SD |
Median |
|||||
|---|---|---|---|---|---|---|
| Echo | #11 | #8 | Echo | #11 | #8 | |
| No. of patients | 710 | 924 | 924 | 710 | 924 | 924 |
| ESVI (mL/m2) | 83.8 ± 31.2 | 83.3 ± 33.0 | 83.6 ± 34.6 | 79.0 | 77.9 | 78.0 |
| EDVI (mL/m2) | 117.0 ± 35.5 | 115.2 ± 37.1 | 114.4 ± 38.1 | 113.4 | 110.8 | 109.2 |
| LVEF (%) | 29.5 ± 8.1 | 29.0 ± 8.4 | 28.4 ± 8.9 | 29.0 | 29.0 | 28.0 |
Algorithm #11 used preferential imaging hierarchy of Echo, CMR, and RN in 710, 96, and 60 patients, respectively, as well as clinical site measures in 58 patients. Algorithm #8 used hierarchy of CMR, Echo, and RN in 352, 454, and 60 patients, respectively, as well as clinical site measures in 58 patients. (see Supplementary material online, Tables S1 and S2 for more details).
Baseline left ventricular global function and outcomes
Clinical outcome based on echo measurements
The clinical characteristics of patients who had evaluable Echo Core Lab measurements (n= 710) were similar to those without Echo (n= 290), except that the latter group was heavier (Table 2), and survival free of cardiac hospitalization was similar between CABG and CABG + SVR whether baseline Echo data were available or not. In the 710 patients with baseline Echo LV function data, the hazard ratio comparing CABG + SVR vs. CABG with respect to death or cardiac hospitalization was 0.99 (95% CI: 0.82–1.21; P= 0.95), whereas in patients without baseline Echo LV function data, the corresponding hazard ratio was 0.98 (95% CI: 0.73–1.33; P= 0.91). Based on the Cox model, the interaction between treatment as randomized and whether Echo Core Lab data were available was P= 0.95 for death or cardiac hospitalization and P= 0.82 for death, reflecting a high degree of consistency of the overall treatment comparisons in patients with vs. those without baseline Echo LV function data.
Table 2.
Characteristics of 1000 patients with and without an Echo Core Lab assessment of baseline left ventricular volumes
| With echo (n = 710) (%) | Without echo (n = 290) (%) | P-value | |
|---|---|---|---|
| Age (year) | 61.2 (54.0–68.3) | 63.9 (55.4–69.8) | 0.019 |
| Female sex | 15.1 | 13.8 | 0.605 |
| Weight (kg) | 78.0 (70.0–86.6) | 83.0 (73.0–95.0) | <0.0001 |
| BMI | 26.8 (24.3–29.6) | 28.1 (25.2–31.6) | <0.0001 |
| Hx MI | 86.6 | 88.6 | 0.390 |
| Hx stroke | 6.1 | 4.5 | 0.326 |
| Hx hypertension | 57.3 | 61.4 | 0.238 |
| Atrial fibrillation/flutter | 11.4 | 12.4 | 0.654 |
| Diabetes | 32.7 | 38.6 | 0.188 |
| Prior CABG | 2.1 | 3.1 | 0.353 |
| Prior PCI | 17.9 | 23.4 | 0.044 |
| Current NYHA | 0.499 | ||
| Class | |||
| I | 8.3 | 9.3 | |
| II | 43.0 | 42.8 | |
| III | 43.7 | 40.7 | |
| IV | 5.1 | 4.2 | |
| LVEF (site-reported) | 28.0 (23.0–31.0) | 28.5 (25.0–31.0) | 0.150 |
| Mitral regurgitation | 0.131 | ||
| None or trace | 35.6 | 39.2 | |
| Mild (≤2+) | 44.6 | 47.2 | |
| Moderate (3+) | 15.7 | 11.1 | |
| Severe (4+) | 4.1 | 2.4 | |
Entries for continuous variables in the table above are the median followed in parentheses by the 25th and 75th percentiles.
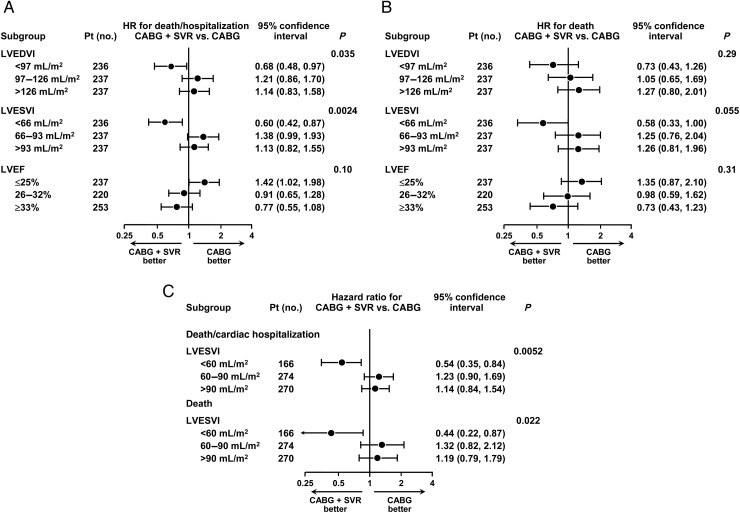
When these 710 patients were separated into tertile groups defined by LVEDVI, LVESVI, and EF, the treatment difference varied according to baseline LVESVI (interaction P= 0.0024 for death or hospitalization, and P= 0.055 for death) as shown in Figure 2. Although patients with lower baseline LVEF (≤25%) tended to do worse with CABG + SVR, and patients with higher baseline LVEF (≥33%) tended to do better, this interaction was not significant. If patients were separated into three groups according to whether their baseline LVESVI was <60, 60–90, or >90 mL/m2 using Echo measurements, the hazard ratios and treatment interaction favouring SVR in patients with smaller baseline LVESVI (<60 mL/m2), and favouring CABG alone in patients with larger LVESVI (≥60 mL/m2) were more significant (P= 0.0052 for death or cardiac hospitalization, and P= 0.022 for death) (Figure 2C).
Figure 2.
Hazard ratios and 95% confidence intervals for the primary outcome, death, or cardiac hospitalization (A), and for the secondary outcome, all-cause mortality (B), calculated for the tertile groups of left ventricular end-diastolic volume index, end-systolic volume index, and ejection fraction based on echo measurements only. (C) Hazard ratios and 95% confidence intervals for the primary outcome of the STICH trial, death or cardiac hospitalization, and for the secondary outcome, all-cause mortality, calculated for the three groups of left ventricular end-systolic volume index (≤60, 60–90, and ≥90 mL/m2) measured by echocardiography. The actual numbers of events in coronary artery bypass grafting and CABG + SVR groups are shown for the above groups of left ventricular end-diastolic volume index, end-systolic volume index, and ejection fraction based on echo Echo Core Lab measurements in Supplementary material online, Table S3.
Clinical outcome based on measurements by three different imaging modalities
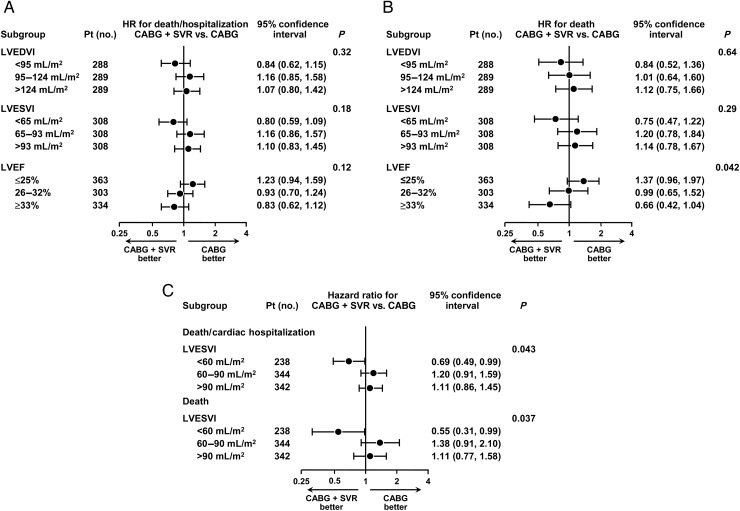
When the number of patients was augmented to 924 by first adding CMR (n= 96), then RN (n= 60), then, if Core Lab values were not available, clinical sites' measures of the LVESVI (n= 58) to the 710 patients with Echo Core Lab measures (algorithm #11), a similar but less significant result favouring SVR in patients with smaller baseline LVESVI and/or higher baseline LVEF, and favouring CABG alone in patients with larger baseline LVESVI and/or lower baseline LVEF was observed (Figure 3). When groups were defined using the LVESVI cut-offs of <60, 60–90, and >90 mL/m2, there was again a statistically significant treatment advantage favouring SVR in patients with LVESVI <60 mL/m2 (Figure 3C).
Figure 3.
Based on algorithm #11, hazard ratios and 95% confidence intervals for the primary outcome, death or cardiac hospitalization (A), and for the secondary outcome, all-cause mortality (B), calculated for the tertile groups of left ventricular end-diastolic volume index, end-systolic volume index, and ejection fraction. (C) Hazard ratios and 95% confidence intervals for the primary outcome of the STICH trial, death or cardiac hospitalization, and for the secondary outcome, all-cause mortality, calculated for the three groups of left ventricular end-systolic volume index (≤60, 60–90, and ≥90 mL/m2). The actual numbers of events in coronary artery bypass grafting and CABG + SVR groups are shown for the above groups of left ventricular end-diastolic volume index, end-systolic volume index, and ejection fraction based on algorithm # 11 in Supplementary material online, Table S3.
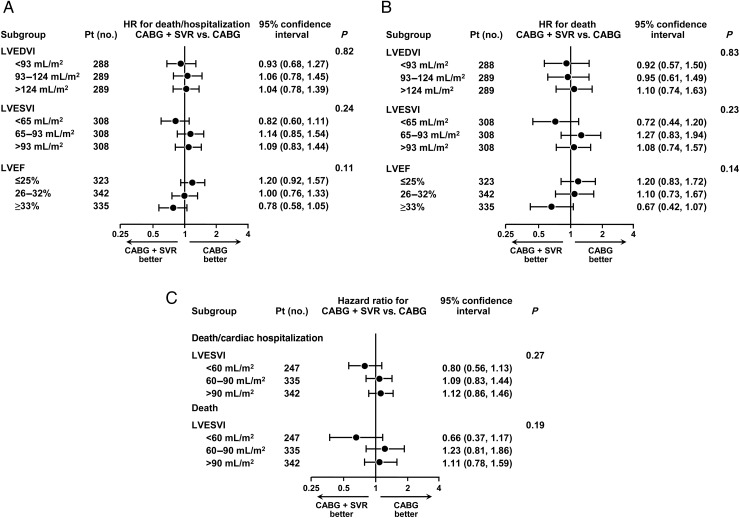
The prognostic relationship between the LVESVI and the LVEF and outcome was directionally similar regardless of the algorithm used. However, when the algorithm with the strongest relationship with death (algorithm #8, n= 924 patients, where CMR values were used first in 352, followed by Echo in 454 with no CMR, by RN in 60 with no CMR or Echo, and then by clinical sites' measurements in 58 patients with no Labs measures), the relationship between baseline LVESVI and LVEF, and treatment effect was less pronounced, with no interaction approaching significance (Figure 4). The results presented for these different algorithms are representative and cover the range of findings observed with the remainder of the 13 algorithms.
Figure 4.
Based on algorithm # 8, hazard ratios and 95% confidence intervals for the primary outcome, death or cardiac hospitalization (A), and for the secondary outcome, all-cause mortality (B), calculated for the tertile groups of left ventricular end-diastolic volume index, end-systolic volume index, and ejection fraction. (C) Hazard ratios and 95% confidence intervals for the primary outcome of the STICH trial, death or cardiac hospitalization, and for the secondary outcome, all-cause mortality, calculated for the three groups of LVESVI (≤60, 60–90, and ≥90 mL/m2). The actual numbers of events in coronary artery bypass grafting and CABG + SVR groups are shown for the above groups of left ventricular end-diastolic volume index, end-systolic volume index, and ejection fraction based on algorithm #8 in Supplementary material online, Table S3.
Baseline left ventricular regional function and outcomes
Wall motion abnormality for each of the 17 segments was assessed among the 815 patients with available wall motion analysis from one of the three imaging modalities using algorithm #11. The segments usually targeted by SVR had the greatest regional wall motion abnormalities with >80% of patients having akinesia or dyskinesia in the apical anterior, septal, and inferior walls as well as in the apical tip. (Supplementary material online, Figure S1).
There was no statistically significant interaction between treatment strategy and regional LV function, and no subgroup based on regional wall motion abnormalities could be identified that benefitted from adding SVR to CABG (Supplementary material online, Figure S2).
Discussion
The present analyses of the LV imaging characteristics of the patients enrolled in the STICH study suggest that SVR may benefit patients with an early stage of LV remodelling when the LV is not markedly dilated and that SVR may be detrimental in patients with more dilated ventricles. The possible interaction found between baseline LVESVI and treatment modality is not consistent with the premise upon which the STICH study was founded, or according to current thinking. These findings were most striking when only Echo Core Lab measurements were used, but, although directionally similar, were less impressive when measurements from CMR and RN as well as clinical sites were combined with those from Echo.
Left ventricular remodelling and surgical ventricular reconstruction
After initial remodelling of the necrotic area with fibrosis and thinning, the non-infarcted zone dilates with hypertrophic myocyte elongation and interstitial fibrosis.11,12 Although LV dilatation is initially an adaptive process, increased LV volume is strongly predictive of a poor clinical outcome.1 Hence, the current management strategy for ischaemic cardiomyopathy has been geared towards reversing the remodelling process by medical and/or device therapy. The SVR procedure has been performed to restore the remodelled LV to a smaller size and a more natural shape by eliminating thinned and scarred parts of the LV, thereby achieving more drastic and immediate mechanical reverse remodelling. Although the initial SVR procedure was for removal of LV aneurysm,13 many centres have performed SVR in patients with akinetic and/or dyskinetic areas without a frank aneurysm and reported a good surgical outcome.2,14–19 However, those studies were not randomized to compare CABG + SVR with CABG alone. The RESTORE Group reported early and late survival data in a registry of 1198 patients with heart failure after anterior myocardial infarction.2 Concomitant CABG was performed in 95%, and the overall 30-day mortality was 5.3%, similar to that of the STICH trial. Their mean baseline LVEF and LVESVI were 29.6 ± 11% and 80.4 ± 51.4 mL/m2, respectively, with a 5-year survival of 68.6 ± 2.8%, which were again similar to those in STICH SVR patients. Factors increasing the risk of death were LVEF ≤30%, LVESVI ≥80 mL/m2, advanced New York Heart Association functional class, and age ≥75 years. The largest single-centre experience in SVR comes from Menicanti's group that reported a total of 1161 consecutive patients.18 Their average baseline LV end-systolic volume (not indexed) was 145 ± 64 mL and the LVEF was 33%. Long-term survival in the overall population was 63% at 5 years, but SVR outcome was not compared with that of the patients who had CABG alone. At least moderate mitral regurgitation, New York Heart Association class greater than II, and diastolic dysfunction were predictors of operative mortality.
Initially, the STICH trial design excluded patients with an LVESVI <60 mL/m2, as conventional wisdom suggested that these patients would not benefit from SVR. As the STICH study evolved, due to the empirical nature of the entry criteria, it was decided to liberalize inclusion criteria to include patients amenable to SVR surgery in the opinion of the investigators. At the time of this change in inclusion criteria, only 31 patients were enrolled. This led to the inclusion of a significant number of patients with an LVESVI <60 mL/m2; those that appeared in the STICH trial to have, if anything, benefitted the most from SVR. Patients thought to most likely benefit from SVR, those with 60–90 mL/m2, clearly showed no benefit, while those with the largest ventricles (>90 mL/m2), and thought more likely to improve with SVR, may have, if anything, responded more poorly to SVR. These results question our current thinking regarding the criteria used to identify patients for SVR, and again underscore the importance of performing randomized clinical trials to best describe the use of an intervention.
Influence of baseline left ventricular regional function on the results of surgical ventricular reconstruction
The amount of akinesia and/or dyskinesia in the SVR zone, as well as the regionality of remote zones, were analysed in tertiles to determine the influence of regional function on the outcome of SVR. There was no characteristic regionality distribution favouring SVR. Di Donato et al. attempted to assess the influence of LV shape abnormalities on clinical and cardiac status after SVR.20 They classified LV shapes into three types based on the number of systolic borders between thickening and non-thickening myocardium: true aneurysm with two borders, intermediate type with one border, and global hypokinesis type with no border. There was no statistically significant difference in mortality among the three types although there was a tendency for a higher mortality in intermediate and global hypokinesis groups in the above study. These groups also had larger LV volumes and lower LVEF. Therefore, it is difficult to determine whether LV shape or LV volume is responsible for the observed tendency.
Why may surgical ventricular reconstruction benefit patients with ischaemic cardiomyopathy and mild left ventricular dilatation?
It is plausible that patients with a less dilated LV are at an earlier stage of remodelling in ischaemic cardiomyopathy and have a larger proportion of healthy muscle that can compensate for the loss of cardiac mass at the site of SVR. It is also possible that elimination of a dyssynergic apical segment when the LV is not too dilated can prevent further LV remodelling and other associated complications such as progressive fibrosis and dysfunction of the remaining non-infarcted portions of the heart. It appears that abrupt surgical reverse remodelling may be different from that induced by heart failure drug therapies or cardiac resynchronization therapy. Although LV dilatation is a marker of a poor clinical outcome, it is also a compensatory mechanism for myocardial injury. Removing this compensatory mechanism by mechanical or surgical resection may disturb this balance in some patients by abruptly affecting diastolic distensibility. Indeed, Ferrazzi et al. demonstrated that elasticity of the acutely enlarged and dysfunctional heart was important in augmenting myocardial relaxation.21 Tulner et al. found increased diastolic chamber stiffness immediately after SVR,22 but it was thought to be transient and associated with postoperative inflammation. More recently, the diastolic stiffness constant was found to be still elevated at 6 months after SVR.23 Another possibility is that non-viable segments of the dilated LV continue to remodel even after CABG and SVR, and LV dilatation more likely occurs along the short-axis of the LV rather than the long-axis in patients who undergo SVR. This discordant remodelling process would result in a more globular shape of the LV, contrary to what was expected from SVR.
Measurement of left ventricular volumes in ischaemic cardiomyopathy
Left ventricular volume is one of the most important parameters for managing patients with ischaemic cardiomyopathy and can be measured by different imaging modalities. Although the relationship between baseline LVESVI and clinical benefit of adding SVR persists regardless of which imaging modality is used, the significance of the relationship differs depending on which imaging modality was utilized in our assessment. When Echo, CMR, and RN were compared (Figure 1) in patients who had more than one imaging study, LV volume was measured higher by RN than by Echo and CMR. Echocardiogram is most widely available in clinical practice and was obtained in 950 patients since it was required for all study patients. Echo Core Lab LV volume measurement was possible, however, in only 710 patients. Therefore, we used three different approaches (Echo alone in 710 patients, algorithms #8 and #11 utilizing three imaging modalities in 924 patients). The less impressive association between baseline LVESVI and outcomes when CMR and RN were used may be related to the fact that the number of patients who had evaluable CMR or RN studies was about half the number of patients with evaluable Echo data. It is also possible that mixing of different imaging modalities in LV volume measurements was responsible. There may be unmeasured characteristics of the patients or clinical sites related to the use of cardiac imaging modality, which might have confounded the relationship between the ESVI source and its relationship to later death. From our exhaustive analyses of LV volumes by different algorithms as well as by a single imaging modality, we learned that there is clinically significant variation among imaging modalities in measuring LV volumes and how we determine LV volumes can potentially affect the outcome of a clinical trial. The intent of our analysis was not to claim an intrinsic superiority for any specific imaging test. We are not able to recommend a specific imaging method to determine optimal LV volume measurement since the accuracy and reliability of an imaging test depends on many variables such as the patient's body habitus, examiner's experience, and the type of equipment. In clinical practice, it will not be practical or possible to perform more than one imaging modality for making a clinical decision.
Limitations
There were several limitations in this study. First of all, a single Core Lab assessment of LV global and regional function measures was not available in every patient. However, 96.6% of patients had at least one imaging study reviewed by a blinded Core Lab and 86.6% had an adequate quality study for Core Lab volume measures. For those patients with more than one baseline imaging study, we used all three modalities to assign the best available LV volume to each patient. This approach to obtain LV volumes and the EF using three imaging modalities has the limitation of measurement variability among imaging modalities while providing values for a larger number of patients. We, therefore, analysed the data based only on Echo in a smaller but still 71% of study population, to avoid the variability of measurements among three imaging modalities.
Secondly, the impact of the amount of LV volume reduction by SVR on the clinical outcome was not assessed in this manuscript. In STICH, 4-month and 2-year follow-up imaging studies were obtained, and other manuscripts incorporating these data are forthcoming.
Finally, the results presented are based on LV function subgroup analyses of the overall study population. The perils of subgroup analysis are well documented, and thus cautious interpretation is required. It is especially important to exercise caution in interpreting a ‘positive’ subgroup result (i.e. a subgroup where there appears to be a beneficial treatment effect) when the overall results of the trial show no benefit.
Conclusions
The addition of SVR to CABG in patients with CAD amenable to CABG, an LVEF ≤35%, and a dominant anterior region of myocardial akinesia or dyskinesia was shown to have no benefit in the 1000 patients enrolled in STICH. Although by no means conclusive, subgroup analyses in patients with baseline assessments of LV geometry suggest that a relationship may exist between the LV volume as well as the LVEF and the impact of SVR on outcome, with patients with a smaller LVESVI, especially those with a LVESVI <60 mL/m2, and EF ≥33% doing better and patients with larger LVESVI or low LVEF doing worse with the addition of SVR.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants 5U01-HL69015, 5U01-HL69013, 5U01-HL69010, 5U01-HL69012, and 5U01-HL72683 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors acknowledge the support of Vanessa Moore and Jayne Roth in the preparation of this article.
References
- 1.White H, Norris R, Brown M, Brandt P, Whitlock R, Wild C. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Athanasuleas C, Stanley A, Buckberg G, Dor V, Di Donato M, Blackstone E. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. J Am Coll Cardiol. 2001;37:1199–1209. doi: 10.1016/s0735-1097(01)01119-6. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez E, Lee K, O'Connor C, Oh J, Bonow R, Pohost G, Feldman A, Mark D, Panza J, Sopko G, Rouleau J, Jones R. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones R, Velazquez E, Michler R, Sopko G, Oh J, O'Connor C, Hill J, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau J, Lee K. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang R, Bierig M, Devereux R, Flachskampf F, Foster E, Pellikka P, Picard M, Roman M, Seward J, Shanewise J, Solomon S, Spencer K, Sutton M, Stewart W. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Cerqueira M, Weissman N, Dilsizian V, Jacobs A, Kaul S, Laskey W, Pennell D, Rumberger J, Ryan T, Verani M. Standardized myocardial segmental and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 7.Germano G, Kiat H, Kavanagh P, Moriel M, Mazzanti M, Su H, Van Train K, Berman D. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138–2147. [PubMed] [Google Scholar]
- 8.Sharir T, Berman D, Waechter P, Areeda J, Kavanagh P, Gerlach J, Kang X, Germano G. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: Normal heterogeneity and criteria for abnormality. J Nucl Med. 2001;42:1630–1638. [PubMed] [Google Scholar]
- 9.Lawson M, Blackwell G, Davis N, Roney M, Dell'Italia L, Pohost G. Accuracy of biplane long-axis left ventricular volume determined by cine magnetic resonance imaging in patients with regional and global dysfunction. Am J Cardiol. 1996;77:1098–1104. doi: 10.1016/s0002-9149(96)00140-3. [DOI] [PubMed] [Google Scholar]
- 10.Pohost G, Hung L, Doyle M. Clinical use of cardiovascular magnetic resonance imaging. Circulation. 2003;108:647–653. doi: 10.1161/01.CIR.0000083233.86078.3E. [DOI] [PubMed] [Google Scholar]
- 11.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovascular imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Buckley O, Di Carli M. Predicting benefit from revascularization in patients with ischemic heart failure: imaging of myocardial ischemia and viability. Circulation. 2011;123:444–450. doi: 10.1161/CIRCULATIONAHA.109.903369. [DOI] [PubMed] [Google Scholar]
- 13.Dor V, Saab M, Coste P, Kornaszewska M, Montiglio F. Left ventricular aneurysm: A new surgical approach. Thorac Cardiovasc Surg. 1989;37:11–19. doi: 10.1055/s-2007-1013899. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo M, Amaducci A, Brunelli F, Tomba M, Parrella P, Tasca G, Troise G, Quaini E. Determinants of postinfarction remodeling affect outcome and left ventricular geometry after surgical treatment of ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2004;127:1648–1656. doi: 10.1016/j.jtcvs.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 15.Di Donato M, Castelvecchio S, Brankovic J, Santambrogio C, Montericcio V, Menicanti L. Effectiveness of surgical ventricular restoration in patients with dilated ischemic cardiomyopathy and unrepaired mild mitral regurgitation. J Thorac Cardiovasc Surg. 2007;134:548–553. doi: 10.1016/j.jtcvs.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Dzemali O, Risteski P, Bakhtiary F, Singer E, Zierer A, Kleine P, Moritz A. Surgical left ventricular remodeling leads to better long-term survival and exercise tolerance than coronary artery bypass grafting alone in patients with moderate ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2009;138:663–668. doi: 10.1016/j.jtcvs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Ferrazzi P, Matteucci M, Merlo M, Iacovoni A, Rescigno G, Bottai M, Parrella P, Lorini L, Senni M, Gavazzi A. Surgical ventricular reverse modeling in severe ischemic dilated cardiomyopathy: The relevance of the left ventricular equator as a prognostic factor. J Thorac Cardiovasc Surg. 2006;131:357–363. doi: 10.1016/j.jtcvs.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Menicanti L, Castelvecchio S, Ranucci M, Frigiola A, Santambrogio C, de Vincentiis C, Brankovic J, Di Donato M. Surgical therapy for ischemic heart failure: Single-center experience with surgical anterior ventricular restoration. J Thorac Cardiovasc Surg. 2007;134:433–441. doi: 10.1016/j.jtcvs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Mickleborough L, Merchant N, Ivanov J, Rao V, Carson S. Left ventricular reconstruction: early and late results. J Thorac Cardiovasc Surg. 2004;128:27–37. doi: 10.1016/j.jtcvs.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Di Donato M, Castelvecchio S, Kukulski T, Bussadori C, Giacomazzi F, Frigiola A, Menicanti L. Surgical ventricular restoration: left ventricular shape influence on cardiac function, clinical status, and survival. The Ann Thorac Surg. 2009;87:455–461. doi: 10.1016/j.athoracsur.2008.10.071. [DOI] [PubMed] [Google Scholar]
- 21.Ferrazzi P, Senni M, Iascone M, Merlo M, Triggiani M, Lorusso R, Herijgers P, Schreuder J, Pentiricci S, Iacovoni A, Quaini E. Implantation of an elastic ring at equator of the left ventricle influences cardiac mechanics in experimental acute ventricular dysfunction. J Am Coll Cardiol. 2007;50:1791–1798. doi: 10.1016/j.jacc.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 22.Tulner S, Steendijk P, Klautz R, Bax J, Schalij M, van der Wall E, Dion R. Surgical ventricular restoration in patients with ischemic dilated cardiomyopathy: evaluation of systolic and diastolic ventricular function, wall stress, dyssynchrony, and mechanical efficiency by pressure-volume loops. J Thorac Cardiovasc Surg. 2006;132:610–620. doi: 10.1016/j.jtcvs.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 23.ten Brinke E, Klautz R, Tulner S, Verwey H, Bax J, Schalij M, van der Wall E, Versteegh M, Dion R, Steendijk P. Long-term effects of surgical ventricular restoration with additional restrictive mitral annuloplasty and/or coronary artery bypass grafting on left ventricular funtion: Six-month follow-up by pressure-volume loops. J Thorac Cardiovasc Surg. 2010;140:1338–1344. doi: 10.1016/j.jtcvs.2010.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.