Abstract
Aims
Patients with chronic heart failure (CHF) show impaired health-related quality of life (HRQoL), an important target for therapeutic intervention. Impaired iron homeostasis may be one mechanism underlying the poor physical condition of CHF patients. This detailed subanalysis of the previously published FAIR-HF study evaluated baseline HRQoL in iron-deficient patients with CHF and the effect of intravenous ferric carboxymaltose (FCM) on HRQoL.
Methods and results
FAIR-HF randomized 459 patients with reduced left ventricular ejection fraction and iron deficiency, with or without anaemia, to FCM or placebo (2:1). Health-related quality of life was assessed at baseline and after 4, 12, and 24 weeks of therapy using the generic EQ-5D questionnaire and disease-specific Kansas City Cardiomyopathy Questionnaire (KCCQ). Baseline mean Visual Analogue Scale (VAS) score was 54.3 ± 16.4 and KCCQ overall summary score was 52.4 ± 18.8. Ferric carboxymaltose significantly improved VAS and KCCQ (mean differences from baseline in KCCQ overall, clinical and total symptom scores, P< 0.001 vs. placebo) at all time points. At Week 24, significant improvement vs. placebo was observed in four of the five EQ-5D dimensions: mobility (P= 0.004), self-care (P< 0.001), pain/discomfort (P= 0.006), anxiety/depression (P= 0.012), and usual activity (P= 0.035). Ferric carboxymaltose improved all KCCQ domain mean scores from Week 4 onward (P≤ 0.05), except for self-efficacy and social limitation. Effects were present in both anaemic and non-anaemic patients.
Conclusions
HRQoL is impaired in iron-deficient patients with CHF. Intravenous FCM significantly improved HRQoL after 4 weeks, and throughout the remaining study period. The positive effects of FCM were independent of anaemia status.
Keywords: Anaemia, Chronic heart failure, Health-related quality of life, Iron deficiency
Introduction
Patients with chronic heart failure (CHF) suffer from a marked impairment of health-related quality of life (HRQoL) compared with the normal population and patients with other chronic conditions.1–3 Among the factors associated with a reduced HRQoL, dyspnoea and fatigue are major symptoms of CHF that persist in many patients despite optimal drug and device management.4 Limited options for treatment of underlying CHF pathology have caused the focus of therapeutic attention to shift towards patient-centred outcomes such as HRQoL, particularly in those already receiving evidence-based therapy and even more so in those with limited life expectancy.5,6 Currently, an improvement in patient-reported outcomes is considered one of the main goals of comprehensive CHF management.7–9 Moreover, despite their benefits in survival, some of the usual therapies used in CHF, such as beta-blockers, have failed to prove a positive effect in terms of HRQoL.10
Iron plays a vital role in energy-dependent physiological processes such as erythropoiesis and oxidative phosphorylation,11,12 and iron deficiency (ID) may be a frequent and significant comorbidity in CHF.13 Beyond anaemia, ID is a determinant of fatigue and impaired exercise capacity in otherwise healthy populations.14,15 In this context, there is recent evidence that ID in CHF patients is also associated with impaired exercise capacity, worse functional New York Heart Association (NYHA) class, and poorer outcomes, although this latter observation remains controversial.13,16,17 Iron deficiency is, therefore, a potential treatment target to improve HRQoL in CHF patients.11,18
Beyond small studies, there is little information about HRQoL in iron-deficient CHF patients and the potential benefits of iron repletion therapy. The FAIR-HF study (Ferric carboxymaltose Assessment in patients with IRon deficiency and chronic Heart Failure) was designed to determine the effects of intravenous (i.v.) ferric carboxymaltose (FCM) in iron-deficient CHF patients.18 Ferric carboxymaltose was well tolerated and significantly improved CHF symptoms, exercise capacity, and HRQoL.19 In the current study, we undertook a detailed subanalysis of FAIR-HF to gain specific insight into the effects of FCM on different domains of HRQoL in iron-deficient CHF patients.
Methods
Study design
The rationale and design of the FAIR-HF study has been previously published.18 Briefly, FAIR-HF was a randomized, double-blind, placebo-controlled, 24-week study of the effects of i.v. FCM [Ferinject®, Vifor (International), Inc., St Gallen, Switzerland] in iron-deficient patients with CHF due to systolic dysfunction, with or without anaemia. The main study inclusion criteria were NYHA class II/III, the left ventricular ejection fraction (LVEF) ≤40% (NYHA II) or ≤45% (NYHA III), haemoglobin (Hb) levels between 9.5 and 13.5 g/dL, and ID [defined as serum ferritin <100 µg/L, or as serum ferritin <300 µg/L if transferrin saturation (TSAT) was <20%].
After 24 weeks of therapy, the patients in the FCM group experienced a significant improvement in the Patient Global Assessment (PGA) score, NYHA class (co-primary endpoints) and 6-minute walking test distance (secondary endpoint) compared with patients receiving placebo.19
Health-related quality of life measurements
Two standard instruments were used: the disease-specific Kansas City Cardiomyopathy Questionnaire (KCCQ) and the generic EuroQoL EQ-5D questionnaire.
The KCCQ20 is a disease-specific measure of HRQoL for heart failure comprising seven domains (physical limitation, symptom stability, symptom burden, symptom frequency, self-efficacy, quality of life, and social limitation) and three summary scores [total symptom score (TSS, comprising symptom frequency and symptom burden), overall summary score (OSS, comprising physical limitation, TSS, quality of life and social limitation), and clinical summary score (CSS, comprising physical limitation, TSS)]. Scores ranged between 0 and 100, where 100 is the best possible HRQoL status. A five-unit change in the OSS was defined as the minimally noticeable clinical difference in response to treatment.21
The EQ-5D instrument9 is a generic measure of HRQoL, comprising a Visual Analogue Scale (VAS) for self-rating of general health (a score of 100 is the best possible general health status) and five domains evaluating mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. In this instrument, 1 is the best possible general health status in each case; the dimensions may also be expressed as percentage of patients rating 1–3 (best to worst health status) or percentage of patients with ‘any’ complaint (scoring 2 or 3 in each domain). The results for the five domains can be converted into a single summary index score by weighting the individual values and deducting the weighted values from 1, the value for full health (i.e. state 11111). In this study, the index was multiplied by 100. The minimally important difference (MID) in the EQ-5D index score is defined as the smallest change in the index score that can be regarded as important and meaningful for health professionals, patients, and other stakeholders. Based on data from 11 patient groups in eight longitudinal studies, the MID has been estimated to be 0.074 (7.4%).9,22
During the FAIR-HF study, the EQ-5D questionnaire and the KCCQ were self-administered at baseline, and at Weeks 4, 12, and 24, before any other assessment or procedure.
Statistical analysis
Data analysis was performed using the full analysis set based on the intention-to-treat principle for each assigned study group. We used a two-tailed significance level of α = 0.05 for all assessments. We present data at baseline as mean ± SD or number (per cent). Health-related quality of life data are presented as mean ± SEM.
For the continuous variables, changes from the baseline value and values at Weeks 4, 12, and 24 were compared between the FCM group and the placebo group by means of repeated measures analysis of covariance. The statistical model included treatment group, visit and their interaction as categorical variables and baseline value as a continuous covariate. An unstructured covariance matrix was used.
For categorical endpoints, differences in the distribution of the two study groups were tested by means of ordered polytomous regression for repeated measures. The same explanatory variables were used as in the analyses of continuous endpoints, and an unstructured covariance matrix was used.
Missing HRQoL data at each time point were defined as the following: no EQ-5D VAS score, no EQ-5D index score, or no KCCQ OSS. Missing data for patients known to be alive and not hospitalized at the time point being analysed were imputed using the ‘last observation carried forward’ method, for which at least one previous follow-up value was required. If no preceding HRQoL values were available, the patient was excluded in that analysis. For hospitalized patients unable to complete the questionnaires, or if the patient had died between the last time point and the planned time point, the worst reply was used for the imputation (i.e 1 for the KCCQ individual domains, 3 for the EQ-5D sub-questions, and 0 for the EQ-5D VAS score).
The effects of baseline characteristics on baseline HRQoL were assessed by means of analysis of covariance models. Using simple backward elimination, non-significant variables (P> 0.10) were removed from the model one at a time. Tests for interaction (e.g. treatment-by-baseline NYHA) were performed by analysing the data by visit and adding the interaction term to the statistical models. All analyses were conducted with the SAS software, version 9.1 (SAS Institute, Inc., Cary, NC, USA: 2000–2004).
Results
The screening and inclusion process for eligible patients, baseline characteristics, and primary and secondary endpoint results have been published.19 Here, we present otherwise unpublished specific HRQoL analyses, and only show previously published data, appropriately cited, when they are required to understand the current analyses.
The two treatment groups were well matched for patient demographic, laboratory parameters, and treatment characteristics. At the start of the study, the mean (±SD) age was 67.8 ± 10.3 and 67.4 ± 11.1 years in the FCM and placebo groups, respectively. In the FCM group, 52.3% of patients were women, compared with 54.8% in the placebo group.19 All patients were in either NYHA class II or II, with 83% in the FCM group and 81% in the placebo group in NYHA class III.19 The FCM and placebo groups, respectively, were well matched for mean (±SD) % LVEF (32.5 ± 5 and 33 ± 6) and the Hb level (11.9 ± 1.3 and 11.9 ± 1.4 g/dL).19 In both groups, 48% of patients were anaemic (Hb ≤12 g/dL).19
Health-related quality of life data were available for over 90 per cent of patients at all time points. The two groups had similar HRQoL scores at baseline as measured by EQ-5D VAS score, index score, and domains, and by the KCCQ summary scores and domain scores (Table 1).
Table 1.
Baseline health-related quality of life
| FCM (n= 304) | Placebo (n= 155) | |
|---|---|---|
| HRQoL: EQ-5D | ||
| Visual analogue scale | ||
| Missing data, n (%) | 9 (3) | 3 (2) |
| Mean ± SEM | 54 ± 1 | 54 ± 1 |
| Index score (×100) | ||
| Missing data, n (%) | 6 (2) | 1 (1) |
| Mean ± SEM | 68 ± 1 | 69 ± 1 |
| Patients reporting any problem by domain | ||
| Mobility, n (%) | 245 (81) | 131 (85) |
| Self-care, n (%) | 146 (49) | 79 (51) |
| Usual activity, n (%) | 232 (77) | 124 (81) |
| Pain/discomfort, n (%) | 228 (76) | 117 (75) |
| Anxiety/depression, n (%) | 194 (65) | 97 (63) |
| HRQoL: KCCQ | ||
| Missing data, n (%) | 7 (2) | 4 (3) |
| Overall summary score, mean ± SEM | 52 ± 1 | 53 ± 1 |
| Clinical summary score, mean ± SEM | 55 ± 1 | 55 ± 1 |
| Total symptom score, mean ± SEM | 59 ± 1 | 59 ± 2 |
| Physical limitation, mean ± SEM | 52 ± 1 | 52 ± 2 |
| Symptom burden, mean ± SEM | 59 ± 1 | 60 ± 1 |
| Symptom frequency, mean ± SEM | 59 ± 1 | 58 ± 2 |
| Symptom stability, mean ± SEM | 54 ± 1 | 53 ± 1 |
| Self-efficacy, mean ± SEM | 64 ± 1 | 63 ± 2 |
| Quality of life, mean ± SEM | 47 ± 1 | 48 ± 2 |
| Social limitation, mean ± SEM | 51 ± 2 | 51 ± 2 |
Visual Analogue Scale and Kansas City Cardiomyopathy Questionnaire overall summary score data were previously published, and are included here for context.16
KCCQ, Kansas City Cardiomyopathy Questionnaire.
Baseline health-related quality of life in chronic heart failure patients
At baseline, HRQoL was lower in patients with anaemia compared with non-anaemic patients (Table 2). Likewise, symptom severity (NYHA class III vs. II) was associated with incrementally worse HRQoL (Table 2).
Table 2.
Differences in baseline health-related quality of life according to anaemia status and NYHA class
| Anaemic (n= 220) | Non-anaemic (n= 221) | P (anaemic vs. non-anaemic) | NYHA III (n= 377) | NYHA II (n= 82) | P (NYHA III vs. NYHA II) | |
|---|---|---|---|---|---|---|
| HRQoL: EQ-5D | ||||||
| Visual Analogue Scale, mean ± SEM | 53.0 ± 1.1 | 55.6 ± 1.1 | 0.093 | 52.5 ± 0.8 | 62.4 ± 1.9 | <0.001 |
| Index score (×100), mean ± SEM) | 66.2 ± 1.0 | 70.7 ± 1.1 | 0.002 | 66.7 ± 0.8 | 76.5 ± 1.6 | <0.001 |
| HRQoL: KCCQ | ||||||
| Overall summary score, mean ± SEM | 49.3 ± 1.2 | 55.7 ± 1.3 | <0.001 | 49.4 ± 0.9 | 66.8 ± 1.9 | <0.001 |
| Clinical summary score, mean ± SEM | 52.4 ± 1.2 | 58.6 ± 1.3 | <0.001 | 52.0 ± 0.9 | 71.8 ± 1.8 | <0.001 |
| Total symptom score, mean ± SEM | 56.3 ± 1.3 | 61.6 ± 1.4 | <0.005 | 55.5 ± 1.0 | 75.2 ± 1.9 | <0.001 |
NYHA, New York Heart Association; EQ-5D, Euro QoL 5 Dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Age at baseline, gender, baseline NYHA, ischaemic aetiology of CHF, baseline serum iron, serum ferritin, TSAT, and Hb were associated with baseline QoL scores in the univariate analyses. We fitted an analysis of covariance model with a range of these possible predictors. Through backward elimination, non-significant variables were removed. In the final model, baseline NYHA class (P< 0.001), baseline Hb (P= 0.02), and baseline TSAT (P= 0.04) were associated with HRQoL (KCCQ OSS).
Effect of intravenous ferric carboxymaltose on health-related quality of life
As previously published, FCM significantly improved EQ-5D VAS and KCCQ OSS.19 In our analysis, we found that FCM also improved EQ-5D index scores, and other summary scores of the KCCQ, such as CSS and TSS consistently after 4 weeks of treatment initiation. These effects were maintained or increased throughout the study (FCM P< 0.001 vs. placebo at each time point, Tables 3 and 4). The changes from baseline in EQ-5D VAS and index scores ranged on average between 13 and 25% throughout the study, compared with <10% among the placebo group (Table 3).
Table 3.
Absolute and percentage change from baseline in generic quality of life measured using the EQ-5D instrument
| Week 4 |
Week 12 |
Week 24 |
||||
|---|---|---|---|---|---|---|
| FCM (n= 304) | Placebo (n= 155) | FCM (n= 304) | Placebo (n= 155) | FCM (n= 304) | Placebo (n= 155) | |
| EQ-5D Visual Analogue Scale | ||||||
| Missing data, n (%) | 30 (9.9) | 15 (9.7) | 21 (6.9) | 10 (6.5) | 19 (6.3) | 9 (5.8) |
| Mean ± SEM (% change from baseline) | 6.0 ± 0.7 (17.4 ± 2.1)*** | 0.8 ± 1.3 (4.8 ± 2.8) | 7.9 ± 0.8 (21.4 ± 2.3)*** | 2.4 ± 1.5 (7.9 ± 3.2) | 9.1 ± 1.0 (24.6 ± 2.7)*** | 3.4 ± 1.6 (9.9 ± 3.3) |
| EQ-5D index score | ||||||
| Missing data, n (%) | 29 (9.5) | 13 (8.4) | 17 (5.6) | 8 (5.2) | 16 (5.3) | 7 (4.5) |
| Mean ± SEM, ×100 (% change from baseline) | 5.9 ± 0.8 (12.6 ± 1.8)*** | 0.6 ± 1.5 (5.1 ± 2.8) | 6.9 ± 1.0 (15.1 ± 2.2)*** | −1.6 ± 1.7 (0.5 ± 3.3) | 6.6 ± 1.2 (14.4 ± 2.5)*** | −1.0 ± 1.8 (1.5 ± 3.2) |
| Mobility, n (%) reporting improvement | 46 (16.5) | 15 (10.4) | 55 (19.0)*** | 9 (6.1) | 59 (20.3)** | 17 (11.4) |
| Self-care, n (%) reporting improvement | 43 (15.5)** | 14 (9.8) | 54 (18.8)*** | 17 (11.6) | 61 (21.1)*** | 14 (9.5) |
| Usual activity, n (%) reporting improvement | 53 (18.9)* | 18 (12.7) | 62 (21.4) | 23 (15.6) | 70 (24.1)* | 23 (15.5) |
| Pain/discomfort, n (%) reporting improvement | 57 (20.3)* | 21 (14.7) | 77 (26.6)*** | 22 (14.9) | 82 (28.2)** | 25 (16.8) |
| Anxiety/depression, n (%) reporting improvement | 68 (24.3) | 30 (21.0) | 84 (29.1)* | 33 (22.3) | 94 (32.4)* | 37 (24.8) |
Visual Analogue Scale data were previously published, and are included here for context.16
*P< 0.05; **P< 0.01; ***P< 0.001 for ferric carboxymaltose vs. placebo.
Table 4.
Absolute and percentage change from baseline in heart-failure-specific quality of life measured using the Kansas City Cardiomyopathy Questionnaire
| Week 4 |
Week 12 |
Week 24 |
||||
|---|---|---|---|---|---|---|
| FCM (n= 304) | Placebo (n= 155) | FCM (n= 304) | Placebo (n= 155) | FCM (n= 304) | Placebo (n= 155) | |
| HRQoL: KCCQ | ||||||
| Missing data, n (%) | 27 (8.9) | 15 (9.7) | 18 (5.9) | 11 (7.1) | 18 (5.9) | 10 (6.5) |
| Overall summary score, mean ± SEM (% change from baseline) | 9.4 ± 0.9 (33.6 ± 5.2)*** | 3.5 ± 1.2 (10.6 ± 3.3) | 12.2 ± 1.1 (41.9 ± 5.8)*** | 4.6 ± 1.4 (16.1 ± 5.4) | 12.8 ± 1.3 (43.9 ± 6.1)*** | 6.2 ± 1.5 (21.5 ± 6.7) |
| Clinical summary score, mean ± SEM (% change from baseline) | 9.3 ± 0.9 (29.9 ± 4.3)*** | 2.7 ± 1.2 (7.0 ± 2.5) | 11.6 ± 1.1 (36.2 ± 4.7)*** | 3.3 ± 1.4 (9.4 ± 3.5) | 11.4 ± 1.3 (36.8 ± 4.9)*** | 4.2 ± 1.5 (12.6 ± 3.7) |
| Total symptom score, mean ± SEM (% change from baseline) | 10.4 ± 1.0 (34.1 ± 5.1)*** | 3.1 ± 1.2 (7.5 ± 2.6) | 12.7 ± 1.2 (38.0 ± 4.4)*** | 3.3 ± 1.5 (8.2 ± 3.3) | 12.0 ± 1.3 (36.3 ± 4.5)*** | 3.9 ± 1.6 (11.5 ± 3.5) |
| Physical limitation, mean ± SEM (% change from baseline) | 8.2 ± 1.1 (31.7 ± 4.7)*** | 2.3 ± 1.4 (9.8 ± 3.7) | 10.5 ± 1.3 (39.5 ± 5.9)*** | 3.3 ± 1.6 (11.8 ± 4.2) | 10.7 ± 1.5 (45.5 ± 7.1)** | 4.5 ± 1.8 (13.5 ± 4.1) |
| Symptom frequency, mean ± SEM (% change from baseline) | 10.1 ± 1.1 (40.9 ± 8.8)*** | 4.1 ± 1.4 (12.2 ± 3.4) | 12.0 ± 1.2 (44.2 ± 7.9)*** | 3.2 ± 1.5 (10.2 ± 3.9) | 11.5 ± 1.4 (41.4 ± 8.1)*** | 4.6 ± 1.7 (17.6 ± 4.6) |
| Symptom stability, mean ± SEM (% change from baseline) | 12.7 ± 1.4 (32.3 ± 3.3)*** | 1.6 ± 1.9 (8.9 ± 4.4) | 14.2 ± 1.7 (37.6 ± 3.9)*** | 1.7 ± 2.1 (11.6 ± 5.4) | 13.2 ± 1.7 (33.8 ± 3.7)*** | 4.5 ± 2.1 (17.0 ± 5.4) |
| Symptom burden, mean ± SEM (% change from baseline) | 10.8 ± 1.1 (35.2 ± 5.2)*** | 2.1 ± 1.3 (6.1 ± 2.7) | 13.3 ± 1.2 (40.8 ± 5.1)*** | 3.3 ± 1.6 (9.2 ± 3.6) | 12.5 ± 1.4 (39.0 ± 5.1)*** | 3.3 ± 1.7 (10.2 ± 3.6) |
| Self-efficacy, mean ± SEM (% change from baseline) | 6.9 ± 1.3 (23.5 ± 4.3) | 5.7 ± 1.9 (23.0 ± 7.4) | 8.9 ± 1.3 (28.8 ± 4.8)* | 4.3 ± 2.4 (21.3 ± 7.4) | 9.7 ± 1.5 (30.0 ± 5.0) | 6.0 ± 2.4 (26.3 ± 7.8) |
| Quality of life, mean ± SEM (% change from baseline) | 8.7 ± 1.1 (36.8 ± 5.8)** | 3.6 ± 1.6 (19.8 ± 7.1) | 13.3 ± 1.3 (54.9 ± 7.0)*** | 4.5 ± 1.6 (21.7 ± 6.9) | 14.8 ± 1.4 (59.1 ± 7.3)*** | 6.8 ± 1.8 (31.8 ± 8.8) |
| Social limitation, mean ± SEM (% change from baseline) | 10.2 ± 1.4 (46.6 ± 8.5)** | 4.1 ± 1.7 (15.0 ± 5.3) | 12.1 ± 1.6 (55.2 ± 11.0)* | 7.2 ± 2.1 (25.4 ± 7.1) | 13.1 ± 1.7 (54.4 ± 9.7) | 8.5 ± 2.2 (27.5 ± 6.9) |
KCCQ overall summary score data were previously published, and are included here for context.16
*P< 0.05; **P< 0.01; ***P< 0.001 for ferric carboxymaltose vs. placebo.
There was a greater improvement in the percentage of patients reporting any complaint in each EQ-5D domain in the FCM group compared with the placebo group (Table 3). Throughout the study, significantly more (P< 0.02) patients reported an MID in the FCM group (37–45%) compared with the placebo group (22–29%; Figure 1). In addition to overall improvement, FCM treatment was associated with lower rates of deterioration in HRQoL reported throughout the study (Figure 2).
Figure 1.
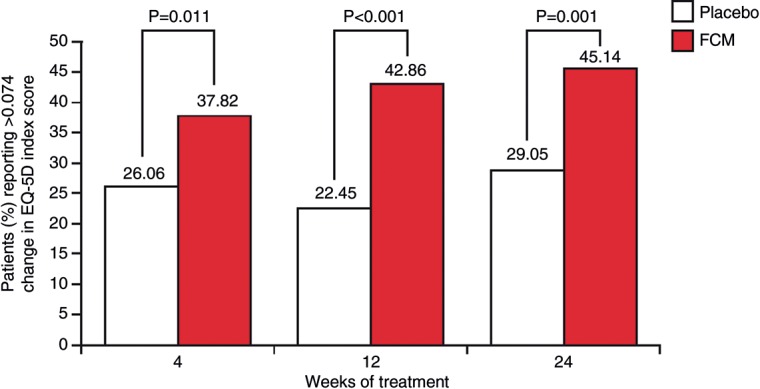
Percentage of iron-deficient chronic heart failure patients treated with ferric carboxymaltose or placebo reporting at least a minimally important difference in EQ-5D index score at each study time point [minimally important difference is the smallest index score change meaningful for health professionals, patients and other stakeholders, and is 0.074 (7.4%) for the EQ-5D index score].1,22
Figure 2.
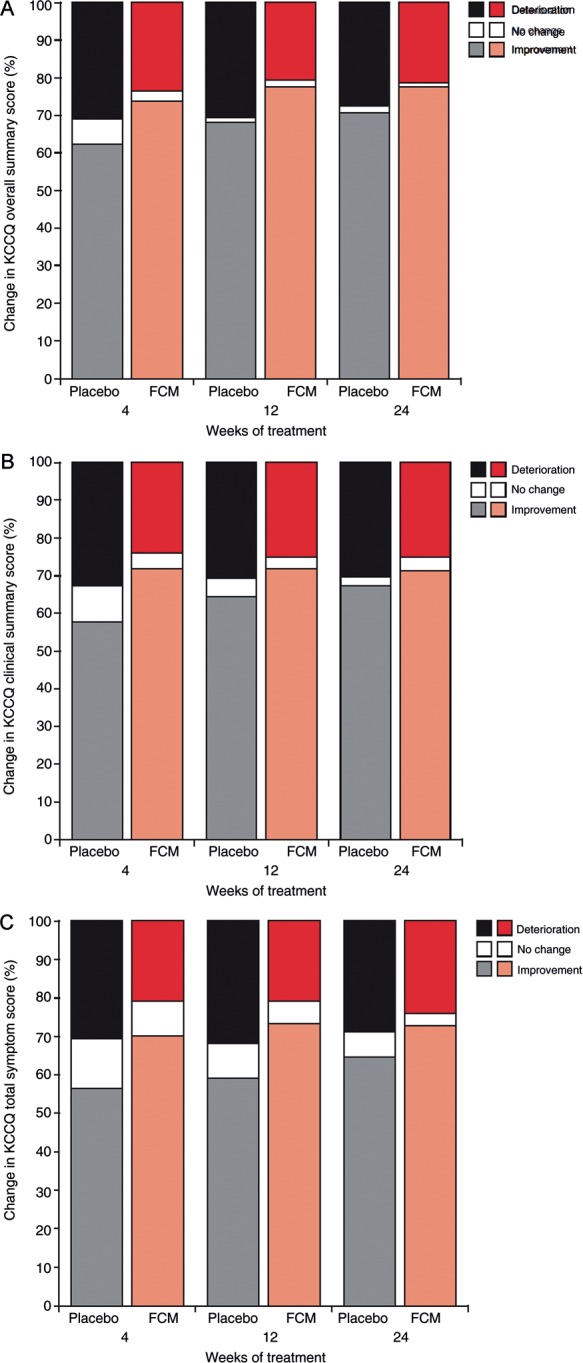
Percentage of iron-deficient chronic heart failure patients treated with ferric carboxymaltose or placebo reporting improvement or deterioration in health-related quality of life at study Week 24 as measured using the Kansas City Cardiomyopathy Questionnaire (A) overall summary score, (B) overall clinical score, and (C) total symptom score.
The proportion of patients experiencing a clinically significant five-point change in KCCQ OSS at Week 24 was significantly higher in the FCM group compared with placebo patients (61 vs. 51%; P= 0.04). The individual percentage changes in KCCQ CSS and TSS (Table 4) were at least three times greater among patients receiving FCM (30–37%) than those receiving placebo (7.0–12.6%), and OSS changes were two or three times greater among the iron-treated group. Among the individual domains, the greatest differences in percentage change between FCM and placebo groups were seen in symptom-related domains, where improvements were mostly three or four times greater in the FCM group than the placebo group. Likewise, iron treatment resulted in an approximately three-fold improvement in physical limitation compared with placebo. However, there were no major differences between groups in self-efficacy scores.
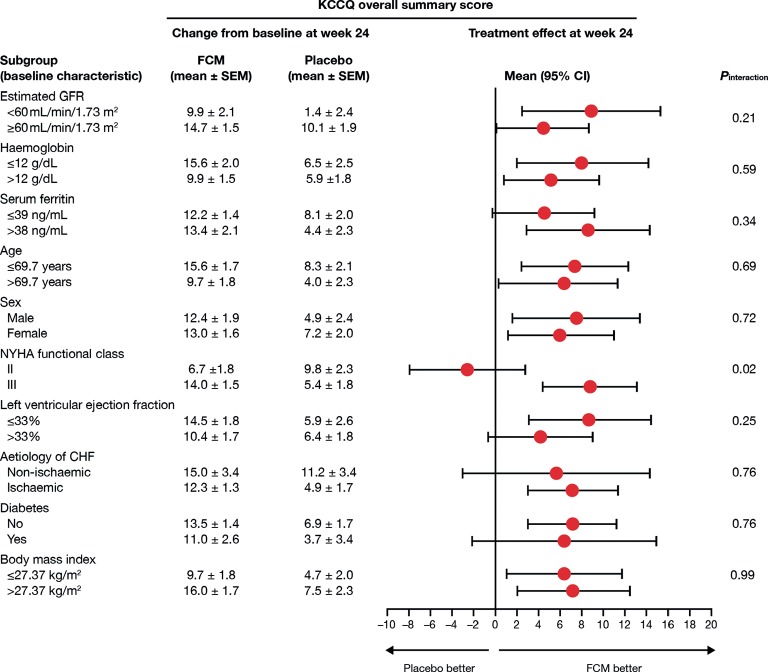
As shown in Figures 3 and 4, the effects of FCM on HRQoL as measured by KCCQ were independent of a range of pre-specified variables, including baseline anaemia, renal function, age, CHF aetiology, and diabetes. The efficacy of FCM at Week 24 appeared to be affected by baseline CHF severity as indicated by NYHA class (Figure 3), an effect also observed in the KCCQ CSS (PInteraction= 0.04), TSS (PInteraction= 0.03), QoL score (PInteraction= 0.01), and symptom burden score (PInteraction= 0.02).
Figure 3.
Effect of ferric carboxymaltose on Kansas City Cardiomyopathy Questionnaire overall summary score among pre-specified subgroups in the FAIR-HF study population. CHF, chronic heart failure; FCM, ferric carboxymaltose; GFR, glomerular filtration rate; HRQoL, health-related quality of life; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association.
Figure 4.
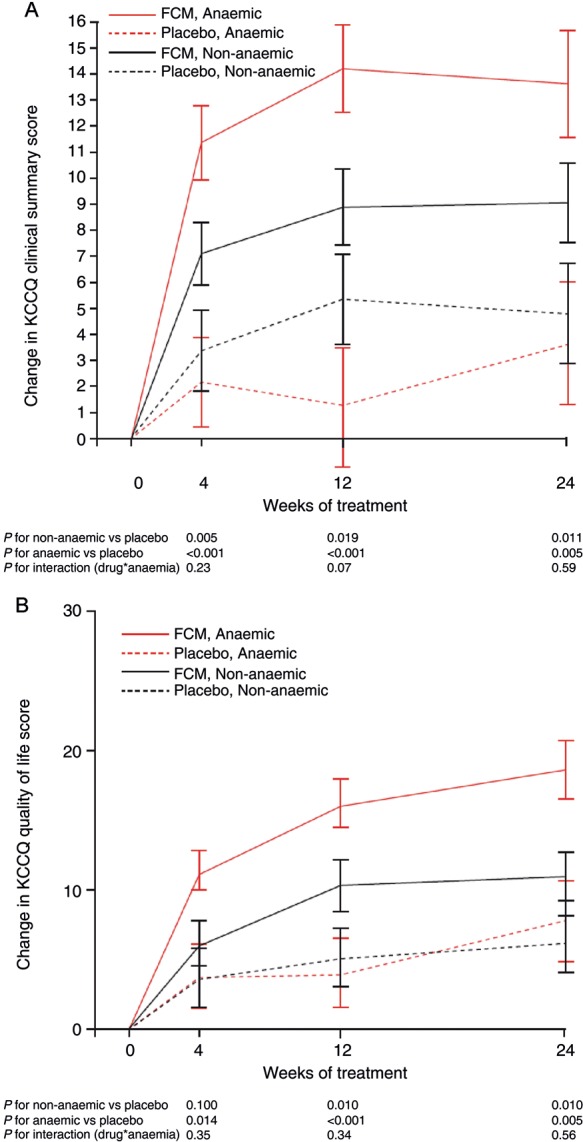
Effect (mean ± SE) of ferric carboxymaltose on (A) Kansas City Cardiomyopathy Questionnaire clinical summary score (CSS; integrates total symptom score and physical limitation domain) and (B) Kansas City Cardiomyopathy Questionnaire quality of life domain among anaemic and non-anaemic subjects in the FAIR-HF study population.
Although anaemia was associated with lower baseline HRQoL in patients with ID compared with ID without anaemia, there were no significant differences between FCM treatment effects observed in anaemic and non-anaemic patients at the end of the study (after adjustment for baseline differences) for the KCCQ OSS (Figure 3). Values at Week 24 in the FCM group [adjusted for baseline (± SEM) in the anaemic and non-anaemic subgroups, respectively] were: EQ-5D index score 74.7 ± 1.6 vs. 75.7 ± 1.6 (P= 0.66); EQ-5D VAS 63.1 ± 1.3 vs. 63.8 ± 1.3 (P= 0.74); KCCQ OSS 66.1 ± 1.6 vs. 64.9 ± 1.6 (P= 0.61). The absence of differences in treatment effects according to anaemia status was also observed for other relevant domains and summary scores of the KCCQ that specifically capture information about symptoms and physical limitation such as KCCQ CSS, and about HRQoL, such as the KCCQ QoL domain (Figure 4).
Discussion
The primary analysis of the FAIR-HF study showed that FCM treatment improved overall measures of HRQoL (EQ-5D VAS and index scores, and KCCQ OSS) in patients with CHF and ID after 4 weeks of treatment,19 confirming the findings of several smaller studies.23–25 Here, we report detailed HRQoL data for baseline status and the impact of comorbidities on the efficacy of FCM treatment.
As expected for patients with CHF, cross-study comparisons revealed a markedly reduced baseline HRQoL in the FAIR-HF patients relative to age-matched normal populations.1,26 Health-related quality of life impairment was strongly related to domains most influenced by physical limitation, such as mobility and usual activities, although many patients also reported problems in pain/discomfort and anxiety/depression domains, highlighting the multidimensional nature of HRQoL.
The effect of FCM on HRQoL was significant throughout the study, with more patients reporting clinically meaningful changes in the FCM treatment arm. Consistent with these findings, the proportion of patients reporting any problems in the EQ-5D dimensions was significantly reduced at the end of the study only in the FCM group, and fewer patients in the FCM group experienced deteriorating HRQoL during the study, which may be important given the progressive nature of the disease.
The positive effect of FCM in HRQoL was consistent among all pre-specified subgroups, except for NYHA class. Although this latter observation may be, in part, attributable to the low sample size and the apparent improvements seen in the placebo arm among patients in NYHA class II, it may also suggest less improvement in HRQoL in patients with in a lower NYHA class. New York Heart Association class II patients have better QoL than patients in higher classes2 and therefore hypothetically there is less room for improvement. This interaction between NYHA class and HRQoL was observed consistently in the specific domains and the summary scores; however, there was no interaction between NYHA class and the 6MWT, an objective measure of functional capacity.19 This apparent discrepancy gives support to the evidence that the correlation between objective functional capacity and HRQoL is not perfect and that HRQoL measures may provide additional information not captured by more conventional ways of assessing physical limitation in patients with CHF.27
Ferric carboxymaltose was equally effective in anaemic and non-anaemic patients and the effect was independent of baseline renal function. In the FCM group, the change was more apparent in anaemic patients, but this could mostly be accounted for by their lower baseline HRQoL.
The physiological mechanisms behind the favourable change in HRQoL due to FCM treatment have not been completely elucidated. Transferrin saturation, a marker of iron availability and probably ID at the tissue level,28 was independently associated with abnormalities in HRQoL at baseline, and further work is needed to determine the relevance of this. There is some evidence that poor iron homeostasis is one of the mechanisms underlying exercise intolerance in CHF,13,17,19 partly due to impaired physical function as a result of ID in skeletal and myocardial muscle cells.14,15 In this context, recent studies have shown that patients with CHF and anaemia commonly exhibit tissue ID at the myocyte level.29 Furthermore, treatment with i.v. iron may have favourable effects in cardiac structure and remodelling.30 We can therefore hypothesise that ID impairs physical function and promotes symptoms such as fatigue due to impaired energy metabolism, a common feature in CHF, as well as due to abnormal erythropoiesis where anaemia is present.11,13,17,23 These mechanisms may explain why FCM administration significantly improved HRQoL in both anaemic and non-anaemic patients, and why this benefit was seen early after initiation of the therapy, after just 4 weeks, despite only a modest increase in the Hb level.
Health-related quality of life instruments capture self-perceived health and are a useful measure of the efficacy of any therapeutic intervention from the perspective of the patient and the treating professional. This is particularly relevant in elderly patients with CHF, where trading longevity for improvement in physical, emotional and social well-being may be the preference.1,27 Also, patient-reported outcomes have been found to be independent predictors of outcome in HF patients; therefore, HRQoL may give important prognostic information.27,31
There are two main limitations to our subanalysis of the FAIR-HF study. First, the study was not originally powered for significant differences in terms of the various HRQoL summary scores and domains, although the findings are consistent with the changes observed in the two symptom-based primary endpoints of the FAIR-HF study (PGA and NYHA).19 Second, the selection criteria could limit the generalization of the results to the CHF population at large, although the positive effects of FCM were observed in a wide range of different patient subgroups.
In our study, i.v. iron treatment was considered to be well tolerated.19 However, it must be noted that long-term safety data for treatment with this drug in the setting of CHF are currently lacking. Studies evaluating longer periods of treatment, such as CONFIRM-HF (ClinicalTrials.gov identifier: NCT01453608),32 and evaluation of the effect on mortality and morbidity would be desirable.
In conclusion, we have shown that iron repletion with FCM over a 24-week period improves generic and disease-specific measures of HRQoL in stable, symptomatic, and ambulatory CHF patients with ID and systolic dysfunction. The beneficial effects of FCM on patient-centred outcomes were observed 4 weeks after initiation of therapy, and were independent of baseline anaemia status and renal function. In addition to improvements, we observed less deterioration in HRQoL in patients treated with FCM. Further research is required to confirm these findings and to determine the underlying mechanisms of the efficacy of i.v. iron in patients with ID and CHF.
Funding
The FAIR-HF study was sponsored by Vifor Pharma Ltd. Funding to pay the statistical support, the editorial assistance and the Open Access publication charges for this article was provided by Vifor Pharma Ltd.
Conflict of interest: S.D.A., P.P., J.C.C., G.F., R.W., K.D., and T.L. are members of the FAIR-HF steering committee. S.D.A. and R.W. are consultants and have received honoraria for speaking for Vifor Pharma Ltd and Amgen, Inc. S.D.A. has received honoraria for speaking for Roche Pharma and Teva. J.C.C., G.F., and T.L. have received honoraria for speaking for Vifor Pharma Ltd. P.P. is a consultant and has received honoraria for speaking from Vifor Pharma Ltd. C.M. and P.J. are employees of Vifor Pharma Ltd., Switzerland and hold stock in Galenica Ltd. M.L. received the Heart Failure Association Research Fellowship.
Acknowledgements
The FAIR-HF was sponsored by Vifor-Pharma Ltd, Switzerland. Janne Harjunpää provided statistical support under the direction of Dr Comin-Colet. Dr David Floyd (formerly Vifor Pharma Ltd., Glattbrugg, Switzerland) and Dr Linn Hjortsberg (Vifor Pharma Ltd, Glattbrugg, Switzerland) provided editorial assistance in the preparation of this manuscript.
References
- 1.Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–241. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002;23:1867–1876. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 4.Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis. 2007;49:366–384. doi: 10.1016/j.pcad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Gravely-Witte S, Jurgens CY, Tamim H, Grace SL. Length of delay in seeking medical care by patients with heart failure symptoms and the role of symptom-related factors: a narrative review. Eur J Heart Fail. 2010;12:1122–1129. doi: 10.1093/eurjhf/hfq122. [DOI] [PubMed] [Google Scholar]
- 6.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:433–443. doi: 10.1093/eurjhf/hfp041. [DOI] [PubMed] [Google Scholar]
- 7.Lainscak M, Blue L, Clark AL, Dahlstrom U, Dickstein K, Ekman I, McDonagh T, McMurray JJ, Ryder M, Stewart S, Stromberg A, Jaarsma T. Self-care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:115–126. doi: 10.1093/eurjhf/hfq219. [DOI] [PubMed] [Google Scholar]
- 8.McDonagh TA, Blue L, Clark AL, Dahlstrom U, Ekman I, Lainscak M, McDonald K, Ryder M, Stromberg A, Jaarsma T. European Society of Cardiology Heart Failure Association Standards for delivering heart failure care. Eur J Heart Fail. 2011;13:235–241. doi: 10.1093/eurjhf/hfq221. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Calvert MJ, Verboven Y, Freemantle N. Effects of cardiac resynchronization therapy on long-term quality of life: an analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J. 2009;157:457–466. doi: 10.1016/j.ahj.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Dobre D, van Jaarsveld CH, deJongste MJ, Haaijer Ruskamp FM, Ranchor AV. The effect of beta-blocker therapy on quality of life in heart failure patients: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2007;16:152–159. doi: 10.1002/pds.1234. [DOI] [PubMed] [Google Scholar]
- 11.Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(2):676S–688S. doi: 10.1093/jn/131.2.676S. discussion 688S-690S. [DOI] [PubMed] [Google Scholar]
- 12.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2):568S–579S. doi: 10.1093/jn/131.2.568S. discussion 580S. [DOI] [PubMed] [Google Scholar]
- 13.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, McMurray JJ, Anker SD, Ponikowski P. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158. [DOI] [PubMed] [Google Scholar]
- 14.Brownlie T, IV, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–443. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 15.Verdon F, Burnand B, Stubi CL, Bonard C, Graff M, Michaud A, Bischoff T, de Vevey M, Studer JP, Herzig L, Chapuis C, Tissot J, Pecoud A, Favrat B. Iron supplementation for unexplained fatigue in non-anaemic women: double blind randomised placebo controlled trial. BMJ. 2003;326:1124. doi: 10.1136/bmj.326.7399.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. 2011;4:599–606. doi: 10.1161/CIRCHEARTFAILURE.111.960906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. doi: 10.1016/j.cardfail.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Mori C, von Eisenhart Rothe B, Pocock S, Poole-Wilson PA, Ponikowski P. Rationale and design of Ferinject assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study: a randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail. 2009;11:1084–1091. doi: 10.1093/eurjhf/hfp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 20.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 21.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 23.Bolger AP, Bartlett FR, Penston HS, O'Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–1665. doi: 10.1016/j.jacc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan MD, Levy WC, Russo JE, Crane B, Spertus JA. Summary health status measures in advanced heart failure: relationship to clinical variables and outcome. J Card Fail. 2007;13:560–568. doi: 10.1016/j.cardfail.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057. [PubMed] [Google Scholar]
- 29.Maeder MT, Khammy O, Dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–480. doi: 10.1016/j.jacc.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008;21:236–242. [PubMed] [Google Scholar]
- 31.Farkas J, Nabb S, Zaletel-Kragelj L, Cleland JG, Lainscak M. Self-rated health and mortality in patients with chronic heart failure. Eur J Heart Fail. 2009;11:518–524. doi: 10.1093/eurjhf/hfp038. [DOI] [PubMed] [Google Scholar]
- 32.A Study to Compare the Use of Ferric Carboxymaltose with Placebo in Patients with Chronic Heart Failure and Iron Deficiency (CONFIRM-HF) http://clinicaltrials.gov/ct2/show/NCT01453608. (23 January 2011)