Abstract
The cause of lung cancer is generally attributed to tobacco smoking. However lung cancer in never smokers accounts for 10 to 25% of all lung cancer cases. Arsenic, asbestos and radon are three prominent non-tobacco carcinogens strongly associated with lung cancer. Exposure to these agents can lead to genetic and epigenetic alterations in tumor genomes, impacting genes and pathways involved in lung cancer development. Moreover, these agents not only exhibit unique mechanisms in causing genomic alterations, but also exert deleterious effects through common mechanisms, such as oxidative stress, commonly associated with carcinogenesis. This article provides a comprehensive review of arsenic, asbestos, and radon induced molecular mechanisms responsible for the generation of genetic and epigenetic alterations in lung cancer. A better understanding of the mode of action of these carcinogens will facilitate the prevention and management of lung cancer related to such environmental hazards.
Background
Lung cancer is commonly associated with tobacco smoke exposure. However, lung cancer in never smokers accounts for 10 to 25% of all cases, ranking as the 7th most common cause of cancer-related death [1,2]. As lung cancer in never smokers is thought to develop through molecular pathways different from those induced by tobacco, the study of non-tobacco related carcinogens is fundamental to better understand the biology of lung tumors arising in never smokers [1-5].
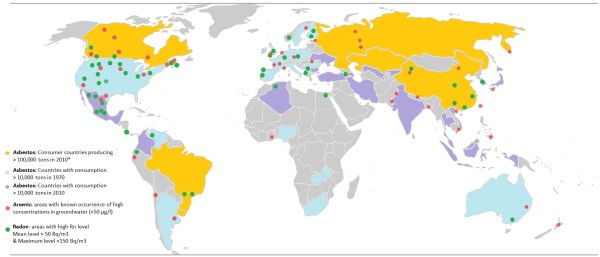
Arsenic, asbestos and radon are well known human carcinogens, based on evidence derived from human and animal studies [6,7]. These three agents have been strongly linked to lung cancer development, both in smoker and never smokers [5,8-19]. Due to its wide distribution on a global scale (Figure 1), chronic exposure to these agents poses a significant public health problem. Millions of people, including those who never smoke, are at risk of developing lung cancer induced by arsenic, asbestos and radon.
Figure 1.
Worldwide occurrence of asbestos, arsenic and radon. Regions known to be affected by contamination with asbestos are colored coded in yellow (production >100,000 tons in 2010), blue (consumption >10,000 tons in 1970) and purple (consumption >10,000 tons in 2010). The five largest producers of asbestos (yellow) in 2010 were Russia (1 million tons), China (0.35 million tons), Brazil (0.27 million tons), Kazakhstan (0.23 million tons) and Canada (0.1 million tons). *Zimbabwe produced 0.15 million tons in 2003 but its production was banned in 2004; however, a controversial production revival plan is expected. Countries with current high consumption of asbestos (purple) are distinguished from countries that had previously high consumption prior to the last decades (blue). Grey indicates consumption of less than 10,000 tons per year. Asbestos production and consumption trends (from 1900 through 2003 and for 2010) are provided by the US Geological Survey (USGS) [20,21]. Areas with known occurrence of arsenic in ground water at >50μg/L (red circles) are estimated using information retrieved from the International Groundwater Resources Assessment Centre (USGS) [22]. Areas reported (non-exhaustive) to have high radon levels are depicted by green circles. Radon occurrence was based on 238U detected in soil and country radon levels (UNSCEAR, WHO, USGS) [23,24]. Circle placement was determined by approximation; for detailed information, see references as information availability differed from country to country.
The carcinogenic effects due to exposure to these elements are well documented [5,8,9]. Table 1 summarizes different sources that provide scientific information linking exposure to these agents with lung cancer and other diseases. These lung carcinogens can induce a wide range of molecular alterations, including genetic (from specific point mutations to genome-wide aberrations) and epigenetic (including alterations in DNA methylation, and microRNA expression) [25]. Considering the relevance of this issue to public health, this article highlights the specific molecular events associated with exposure to arsenic, asbestos and radon as environmental carcinogens driving lung cancer.
Table 1.
Sources of information on environmental carcinogens associated with lung cancer
| Name | Website | Description |
|---|---|---|
| The IARC Monographs, International Agency for Research on Cancer (IARC) |
http://monographs.iarc.fr/ |
Compilation of reports about environmental factors that can increase the risk of human cancer: chemicals, complex mixtures, occupational exposures, physical agents, biological agents, and lifestyle factors |
| Carcinogens, American Cancer Society (ACS) |
http://www.cancer.org/Cancer/CancerCauses/OtherCarcinogens/index |
Information about environmental carcinogens that can be found at home, work, pollution, medical tests and treatments |
| Understanding Cancer Series, National Cancer Institute (NCI) |
http://www.cancer.gov/cancertopics/understandingcancer/environment |
Compilation of slides on environment and its association with cancer |
| Chemicals of Public Health Concern, World Health Organization (WHO) |
http://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/index.html |
Information on the 10 chemicals or groups of chemicals of major public health concern |
| Report on Carcinogens, National Toxicology Program (NTP) |
http://ntp.niehs.nih.gov/?objectid=72016262-BDB7-CEBA-FA60E922B18C2540 |
Congressionally mandated, science-based, public health reports that identify agents, substances, mixtures, or exposures in the environment that may potentially put people in the United States at increased risk for cancer |
| Science and Technology: Health, Environmental Protection Agency (EPA) |
http://www.epa.gov/gateway/science/humanhealth.html |
Information on human health impacts associated with environmental exposures |
| Work-Related Lung Disease (WoRLD) Surveillance System, National Institute for Occupational Safety and Health (NIOSH) |
http://www2.cdc.gov/drds/WorldReportData/ |
Contents on occupationally-related respiratory disease surveillance data. |
| U.S. Geological Survey (USGS) |
http://www.usgs.gov/ |
Organization that provides impartial information on the health of U.S. environment and the natural hazards |
| CARcinogen EXposure Canadian Surveillance Project (CAREX) | http://www.carexcanada.ca | Multi-institution research project that combines academic expertise and government resources to generate an evidence- based carcinogen surveillance program for Canada |
Environmental evidence for lung carcinogenesis induced by arsenic, asbestos and radon
Arsenic
Arsenic, a naturally occurring metalloid in earth’s crust, is a well-established human carcinogen [7]. Exposure occurs mainly through drinking water, but also via air and food [22,26]. Arsenic contamination has been considered the largest mass poisoning in mankind’s history, since ~160 million people live in regions with naturally elevated levels of arsenic in drinking water [27]. Health effects, including lung cancer, have been documented with chronic exposure at levels below the currently accepted threshold of 10μg/L [3,27-29] – and at such dosage several hundred million individuals would be affected.
Although skin cancer is the most common form of malignancy associated with arsenic exposure, lung, as well as bladder, liver, and kidneys, are other main targets of arsenic carcinogenesis [2,30]. Lung cancer is in fact the main cause of death following chronic arsenic ingestion, and this metalloid is considered as a risk factor for lung cancer in never smokers [2,5,26,31]. Augmented levels of arsenic in drinking water have been associated with an increase in the incidence of lung cancer. In the United States alone, an estimated 5,297 arsenic-related lung cancer cases per year are associated with arsenic exposure [32]. Moreover, arsenic exposure contributes synergistically with other risks factors such as tobacco smoke and history of lung disease [29,33]. The most frequent histological subtypes observed in arsenic-induced lung tumors are squamous cell carcinomas (SqCC) and small cell carcinomas (SCC), which are unusual in tumors arising in never smokers [29,34-37]. Lung SqCC associated with arsenic exposure exhibit unique patterns of genomic alterations, raising the possibility of arsenic-specific oncogenic pathways [37].
Asbestos
Asbestos are mineral fibers found naturally in rocks and widely used by industry. Exposure to asbestos fibers, such as chrysotile, amosite, anthophyllite and mixed fibers containing crocidolite, has resulted in a high incidence of lung cancer [6]. Like arsenic, asbestos can act independently or synergistically with tobacco smoke to induce lung cancer [6,38].
Asbestos fibers, with the exception of crocidolite, cause at least twice as many lung cancer deaths than asbestos-related mesothelioma, and these two malignancies combined are responsible for nearly 10,000 deaths per year in the United States [39,40]. The relative risk for developing lung cancer among individuals exposed to asbestos was more than 3 times higher than for non-exposed individuals after controlling for smoking, among other variables [41]. Asbestos-induced effects in the lungs are dose-dependent and are related to the type of fiber inhaled and its composition, such as iron-rich fibers, which are more redox reactive [38,42-45]. Other fibers, such as libby amphibole transition fibers and erionite, although not classified in the asbestos mineral group, have also been implicated in asbestos-associated diseases, suggesting that other thin mineral fibers may have carcinogenic properties similar to those found in asbestos [42]. Even though the current use and management of asbestos is under strict control in most countries, the high latency between exposure and asbestos-related disease development poses a significant public health threat [46]
Radon
Radon is a radioactive gas formed naturally by the breakdown of uranium from soils and rocks. Exposure to this gas is estimated to be associated with more than 20,000 lung cancer deaths per year in the United States [47-49]. Radon accounts for more than 50% of the annual effective dose of natural radioactivity exposure [50], affecting not only miners but also the general population as a ubiquitous contaminant of water and indoor environments [50,51].
The relationship between radon and lung cancer has mainly been established from epidemiologic studies of underground miners [52]. Specifically, non-smoking uranium miners in the southwestern United States experienced an increased incidence of lung cancer [53,54]. Further analysis established that up to 70% of lung cancer deaths among uranium miners can be attributed to radon exposure, and the risk of lung cancer among non-smoking miners was up to 3 times higher than in other occupations [48,50,55]. It has been estimated that up to 30% of lung cancer deaths among non-occupationally exposed never-smokers might be linked to indoor radon [48]. The maximum accepted level in most countries is currently 200 Bq/m3; however, studies have established an elevated lung cancer risk at radon levels as low as 100 Bq/m3[56,57].
Carcinogenic mechanisms induced by exposure to arsenic, asbestos, and radon
Arsenic
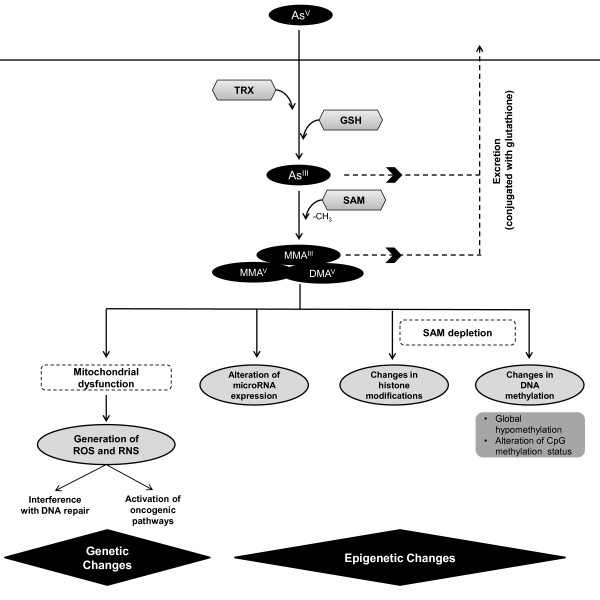
Carcinogenesis of arsenic is related to its biotransformation process. When arsenic enters the body, it induces a series of reduction, oxidation, and methylation reactions (Figure 2) [63]. Pentavalent arsenical species (AsV or arsenate) are reduced to trivalent species (AsIII or arsenite) in a glutathione (GSH)-dependent reaction [64], followed by oxidative methylation resulting in monomethylarsonous acid (MMAIII), methylarsonate (MMAV) or dimethylarsenate (DMAV) [65-67]. Some of the methylated metabolites generated in the detoxification process may in fact be more potent carcinogens than inorganic non-methylated species [58-60].
Figure 2.
Arsenic biotransformation drives carcinogenesis. Arsenic biotransformation occurs through a series of reduction, oxidation, and methylation reactions. Pentavalent arsenic (AsV) is reduced to arsenite (AsIII), using glutathione (GSH) and thioredoxin (TRX) as electron donors. In the excretion process of this compound, AsIII is methylated using S-Adenosyl methionine (SAM) as a source of methyl groups; however, this result in generation of arsenic species with higher carcinogenic potential [58-62]. Carcinogenic effects are mostly generated due to this biotransformation process, having effects at genetic and epigenetic levels. Genetic alterations are largely due to generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), partially derived from arsenic-induced mitochondrial dysfunction. Epigenetic effects, such as changes in DNA methylation patterns have been linked to deprivation of SAM. Changes in miRNA expression and histone modifications have also been reported.
Arsenate interferes with phosphorylation reactions and competes with phosphate transport, while arsenite can react with the sulfhydryl groups of proteins, resulting in inhibition of many biochemical pathways [68]. It is also well established that free radicals are generated during the process of arsenic metabolism [69-73]. By interfering with enzymes that control redox status and glutathione production, arsenic compounds, especially trivalent species, inhibit the protection of cells against oxidative damage [74]. Moreover, arsenic induces a rapid depolarization of the mitochondrial membrane, together with mtDNA deletions and depletions which contribute to carcinogenicity in humans [75,76]. Under these conditions, mitochondria is considered to be the primary site of superoxide anion (·O2-) formation [69]. After formation of ·O2- in arsenic-induced oxidative stress, a cascade of secondary reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and hydroxyl radical (·OH) is generated [70]. The hydroxyl radical is one of the most impactful ROS and reacts with DNA to produce 8-Hydroxy-2’-deoxyguanosine, a major ROS-induced DNA base-modified product [71,77]. Furthermore, glutathione depletion induced by arsenic may increase its toxicity via ROS-related damage [71,78].
Asbestos
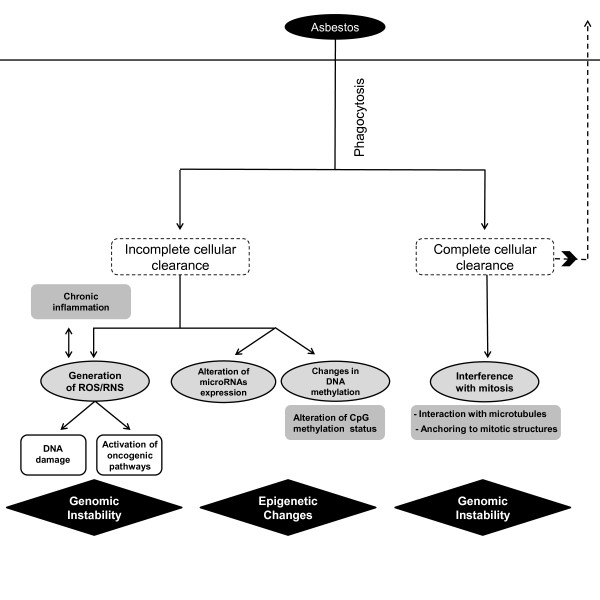
Inhaled asbestos fibers longer than 5μm are not efficiently eliminated by phagocytosis. This can induce a cascade of molecular events that lead to fibrosis, inflammation and carcinogenesis [79,80]. On the other hand, fully phagocytized fibers can interfere with mitosis, leading to chromosomal missegregation [81] (Figure 3). The induction of reactive oxygen and nitrogen species upon incomplete phagocytosis of fibers plays an important role in DNA damage [84]. Asbestos induces the release of ROS, including ·O2- and H2O2[79]. Such reactions can be catalyzed on the asbestos fiber surface, and asbestos fibers with high iron content, such as crocidolite and amosite, are capable of generating higher levels of ROS [85]. Similar to arsenic, asbestos also affects mitochondrial DNA and functional electron transport resulting in mitochondrial-derived ROS, which has been shown to induce base oxidation, single-strand breaks, micronuclei, and apoptosis in lung alveolar epithelial cells. [86,87]. Therefore, asbestos carcinogenesis is suspected to occur through creation of an environment of chronic inflammation, and especially through the induction of oxidative stress, a well-known inducer of DNA damage [88]. Lesions at sites of fiber deposition and alterations in gene expression are other relevant mechanisms in asbestos-induced neoplasia in lungs and other target organs [89]. Direct asbestos mutagenicity has also been proposed as a mode of action for asbestos, although this theory requires further study [89,90].
Figure 3.
Mechanisms of asbestos-induced carcinogenesis. Inhaled asbestos fibers can either be cleared by mucociliary movements and translocations, or undergo phagocytosis [44,82,83]. Fibers not efficiently eliminated by phagocytosis can generate reactive oxygen and nitrogen species (ROS and RNS, respectively) which can lead to generation of DNA single-strand breaks (SSBs) and cell signalling alterations, among other effects. Epigenetic changes, such as alterations in miRNA expression and DNA methylation are also a consequence of incomplete clearance of asbestos fibers. Alternately, fully phagocytized fibers can physically interfere with the mitotic process by interacting directly with microtubules or anchoring to mitotic structures.
Radon
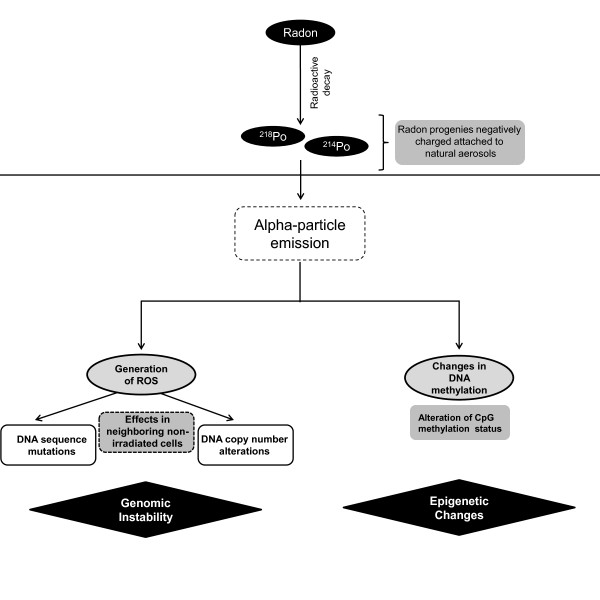
Although chemically inert, radon decays into active progenies that are electrically charged and can be inhaled when attached to natural aerosols, eventually reaching lung epithelial cells. Once in the lung tissue, deposited radon progeny decays to generate alpha-particles, which damage DNA both directly and through generation of free radicals (Figure 4) [51]. Decay of alpha-particles results in the ejection of electrons from water, generating several reactive species leading to cellular damage by hydroxyl radical attack [92,93]. Cellular damage can also occur in nearby, non-irradiated bystander cells through the release of chemical byproducts by irradiated cells [94]. This ‘bystander’ effect can result in non-linear dose–response damage and underestimation of radon exposure risks [95]. In fact, it has been proposed that lung tissue cellular injury from alpha particles is predominantly due to chromosomal damage in neighboring non-irradiated cells [96]. Moreover, after exposure to alpha-particle radiation, observable levels of cytokines are detected in the supernatants of exposed cells, implying a possible effect of cytokines in radon-induced carcinogenesis [97].
Figure 4.
Radon induces reactive oxygen species through emission of alpha-particles. Radon decays to radioactive progenies (218Po and 214Po) that can be inhaled when attached to natural particles in aerosol [50,91]. Once inside the lungs, radon progenies emit alpha-particles that can lead to generation of reactive oxygen species (ROS), eventually resulting in DNA damage not only in the irradiated cell itself, but also affecting neighboring non-irradiated cells [51]. Additionally, changes in DNA methylation are also observed in radon-induced lung tumors.
Genetic and epigenetic consequences in lung tumor genomes due to arsenic, asbestos and radon exposure
The mechanisms discussed above lead to specific genetic alterations including mutations and chromosomal rearrangements, as well as epigenetic changes, meaning mechanisms of gene expression disruption that do not modify the DNA sequence itself, such as DNA methylation, histone modifications and microRNA (miRNA) regulation [98]. Although there have been several reports of specific genetic alterations in lung cancer in never smokers, only a few studies have directly linked such alterations with specific environmental carcinogens.
Molecular and genetic alterations
Arsenic
Moderate levels of arsenic activate the EGFR pathway in human lungs and other target organs of arsenic-carcinogenesis, such as the liver [68,99]. Moreover, arsenic-induced ROS activate the Wnt/β-catenin signaling pathway (which has been shown to promote lung cancer), and stimulate angiogenesis through AKT and ERK1/2 [100-102]. In murine lung tissue, arsenic interferes with expression and protein levels of components of DNA repair machinery, such as APE1, LIG1, OGG1, PARP1 [103].
DMAV has been able to induce lung-specific DNA strand breaks by arsenic-mediated production of ROS in mice [104]. Arsenic has been shown to increase the frequency of micronuclei in cultivated cells from human small airways [105]. Compared to tumors of non-exposed individuals, DNA losses at chromosomal locus 1q21.1 and gains at 19q13.31 and 19q13.33 have been observed lung squamous cell carcinoma (SqCC) in arsenic exposed never smokers [37]. In human small airway epithelial cells arsenic increases expression of cancer related genes and protein levels, such as C-MYC, C-HA-RAS, and C-FOS and decreases β4 integrin protein expression compared with non-exposed cells [105].
Asbestos
In lung epithelial cells, asbestos-induced glutathione depletion results in phosphorylation and activation of EGFR, overexpression of different AP-1 proto-oncogenes, and AP-1 transactivation [106]. In human epithelial bronchial cell lines (Beas-2B), asbestos exposure induces a range of gene expression alterations, affecting MAP4K3, CEBPZ, QPCT, FANCG, IGFBPL1, CCL19, MELK, FANCM, and CDKL1 [107]. Asbestos inhalation also causes up-regulation of mRNA levels of matrix metalloproteinase family members in rat lungs, suggesting induction of extracellular matrix remodeling [108]. Mutations in the KRAS and TP53 genes have been detected in animal models human tumors linked to asbestos exposure, although these alterations have not been conclusively associated with asbestos exposure Nelson, 1999 #387, [109,110].
A higher frequency of deletions affecting the P16/CDKN2A locus has been identified in asbestos-exposed non-small cell lung cancer cases compared to unexposed cases, which represent a main gene inactivation mechanism, although no differences were reported linked to smoking status [111]. Changes at chromosomes 5, 8 and 19 have been detected in HBECs transformed by chrysotile asbestos [112]. Additionally, asbestos interferes with chromosomal segregation by interacting directly with microtubules and chromosomes [113-116].
Radon
Inhaled particles of radon generate alpha-emissions that cause DNA damage primarily through double-strand breaks and large chromosomal aberrations, mainly deletions and, to a lesser extent, point mutations [117,118]. Specific mutational events have been described in radon-induced lung cancer. Vahakangas et al. detected both P53 mutations and deletions in lung tumors from uranium miners. Although the contribution of tobacco smoke cannot be completely ruled out, the most frequent base substitutions associated with tobacco smoking (G:C to T:A transversions), were not identified in that study [119].
Table 2 summarizes the most known genetic alterations observed in lung cancer associated with exposure to these three carcinogens. Specific genetic alterations appear to be prevalent to the exposure to a given carcinogen, for example, DNA losses at chromosomal locus 1q21.1 and gains at 19q13.31 and 19q13.33 are associated with arsenic exposure in never smokers [37].
Table 2.
Genetic alterations occurring in environmentally induced lung cancer
| CNA* at Locus | Carcinogen | References |
|---|---|---|
| 1q21.1 |
Arsenic |
[37] |
| 2p21-p16 |
Asbestos |
[120] S,
[121,122] |
| Ch.5 |
Asbestos |
[112] CL |
| 5q35 |
Asbestos |
[120] S,
[122] |
| Ch.8 |
Asbestos |
[112] CL |
| 9p21.3 (CDKN2A) |
Radon, Asbestos |
[123] R,
[111] |
| 12p12.1 (KRAS**) |
Asbestos |
[124] |
| 16p13.3 |
Asbestos |
[122] |
| 17p13.1 (TP53**) |
Asbestos |
[110] S |
| Ch.19 |
Asbestos |
[112] CL |
| 19p13.3-13.1 |
Asbestos |
[120] S,
[122] |
| 19q13.31 |
Arsenic |
[37] |
| 19q13.33 (SPIB, NR1H2, POLD1) |
Arsenic |
[37] |
| 22q12.3-q13.1 |
Asbestos |
[122] |
| Xq28 | Asbestos | [120] |
* CNA = copy number alteration.
** Sequence mutation.
References include both smokers and non smokers except if indicated (S: smokers only, CL: Cell Lines, R: rat).
Epigenetic alterations
Specific alterations at the epigenetic level, such as modifications in DNA methylation and microRNA (miRNA) expression patterns, have been associated with arsenic, asbestos and radon exposure. Aberrant methylation of CpG islands in the promoter region of tumor suppressor genes (TSGs) are linked to gene silencing, while deregulation of miRNAs − small, noncoding RNAs species that regulate gene expression − is implicated in diverse human pathologies, including lung cancer (reviewed in [125-128]).
Arsenic
Arsenic induces promoter hypermethylation and subsequent transcriptional silencing of tumor suppressors genes, such as P53, CDKN2A and RASSF1A in animal models [129,130]. Chronic arsenic exposure depleted miR-200 levels in human bronchial epithelial cells (HBECs) through increased promoter methylation, and interestingly, re-established expression of miR-200b alone was capable of entirely reversing and preventing arsenic-induced EMT and malignant transformation [131].
Asbestos
Epigenetic inactivation of tumor-suppressor genes, such as RASSF1A and CDKN2A (p16) has been observed in lung cancer patients exposed to asbestos [132]. Interestingly, p16 has been found to be inactivated in NSCLC tumors from nonsmokers only through promoter hypermethylation [133]. A recent study has identified an asbestos-associated miRNA signature in lung cancer, where miR-148b, miR-374a, miR-24-1*, let-7d, Let-7e, miR-199b-5p, miR-331-3p, and miR-96, were found to be over-expressed, while miR-939, miR-671-5p, miR-605, miR-1224-5p and miR-202 were under-expressed [134].
Radon
Long-term radon exposure has been associated with increased CDKN2A and MGMT promoter methylation among Chinese miners [135]. The locus containing the CDKN2A gene is in fact frequently affected by DNA losses in radon-induced lung tumors in rats [123]. Interestingly, exposure to plutonium, which similar to radon exerts its effects through alpha particles, can induce CDKN2A gene inactivation by promoter methylation [136].
Table 3 summarizes epigenetic changes observed in lung tumors associated with exposure to these three agents.
Table 3.
Epigenetic alterations occurring in environmentally induced lung cancer
| Type of alteration | Carcinogen | Gene | References |
|---|---|---|---|
| Hypermethylation |
Radon, Asbestos |
CDKN2A |
[132,135,136] |
| Hypermethylation |
Arsenic |
TP53 |
[129,137] |
| Hypermethylation |
Arsenic, Asbestos |
RASSF1A |
[130] |
| Histone Methylation |
Arsenic |
H3K4, H3K9, H3K27 |
[138-140] |
| Histone Hypoacetylation |
Arsenic |
H4K16 |
[138,140,141] |
| Global DNA Hypomethylation |
Arsenic |
N.A. |
[137,142] |
| miR Downregulation |
Arsenic |
miR-200 |
[131] CL |
| miR Overexpression |
Asbestos |
miR-148b, miR-374a, miR-24-1*, Let-7d, Let- 7e, miR-199b-5p, miR- 331-3p, miR-96, miR- 17-92 |
[134] S |
| miR Downregulation | Asbestos | miR-939, miR-671-5p, miR-605, miR-1224-5p, miR-202 | [134] S |
Studies concern both smokers and non smokers except if indicated (S: smokers only, CL: Cell Lines).
Management strategies for radon, arsenic and asbestos exposure
Geological carcinogen mapping plays an essential role in risk management. Examples of geological maps for radon and arsenic can be found on websites from U.S. Environmental Protection Agency as well as from the U.S. Geological Survey (Table 1). Similar maps for Canada are available from the CAREX (CARcinogen EXposure) Canadian surveillance project website ( http://www.carexcanada.ca). Mapping these carcinogens will help to determine occurrence of co-exposure its health consequences, and the urgency for specific management strategies.
Methods for arsenic removal from water include oxidation, precipitation, coagulation, adsorption, nanofiltration, reverse osmosis, and even bioremediation [143]. A cost-effective technique is based on Arsenic Removal Using Bottom Ash (ARUBA) whereby particles of coal bottom ash (a waste material from coal fired power plants), coated with iron hydroxide react with and immobilize arsenic by adsorption and/or co-precipitation. In Bangladesh, ARUBA has been shown to reduce arsenic concentrations in contaminated groundwater to below the Bangladesh safety threshold [144]. Non-viable fungal biomasses of Aspergillus niger coated with iron oxide have also been shown to remove approximately 95% of As(V) and 75% of As(III) from aqueous solutions [145].
The issue of asbestos is more amendable to control since the release of asbestos into the environment originates from human activity. Most developed countries have well documented and regulated management strategies, such as the Italian directives for the remediation of asbestos-cement roofs to be treated prior to disposal on landfill [146]. Some processes are able to eliminate the hazard of these wastes in order to recycle mineral components in new building materials [147,148]. Asbestos-cement wastes are milled in a cyclic process leading to mineralogical and morphological transformations of asbestos while keeping interesting physical properties for building use. On the other hand, two million metric tons of asbestos were consumed in developing countries in 2007, illustrating the need for regulating the use of this carcinogen in developing nations [149].
Strategies against radon rely on radiation detectors and implementation of radon-resistant features; for example, houses in potentially high exposure zones should be equipped with pipes to vent radon gas generated in the ground, and sealed with plastic sheeting and caulking. Ideally, active mitigation techniques involving physical alterations such as sub-slab depressurization should be instigated, as these methods are more effective [150].
Conclusions
In the next decades, an increasing proportion of lung cancer cases will arise in former or never smokers. While the reduction of environmental carcinogen exposure is certainly a very important cancer prevention issue, understanding the mechanisms of carcinogenesis will facilitate targeted treatment design.
Although tobacco smoke is the major cause of lung cancer, environmental carcinogens, such as arsenic, asbestos and radon play an increasingly important role in this disease, either independently or through additive or multiplicative effects [151,152]. While the number of individuals exposed to these carcinogens is significant, the difficulty to associate tumor cases directly with exposure to these agents (mainly due to the long latency period between exposure and disease onset) may be highly underestimated.
The growing interest in non-tobacco induced causes of lung cancer is reflected in the increasing number of reports describing molecular alterations correlated with exposure to these carcinogens. In this article, we have collected evidence of the involvement of specific molecular mechanisms that can lead to genetic and epigenetic aberrations in lung tumor genomes as a result of exposure to these agents. While sharing a few carcinogenic mechanisms, each agent may induce specific sets of alterations which might affect tumor biology and define tumor behavior, presenting therefore a unique opportunity for developing diagnostic and treatment options. Future research, including the integration of different genetic and epigenetic dimensions, will further the characterization of these etiologically distinct tumors and identify actionable candidates for therapeutic targets.
Abbreviations
ARUBA: Arsenic removal using bottom ash; Bq: Becquerel; CAREX: CARcinogen exposure canadian surveillance project; DMAV: Dimethylarsenate; GSH: Glutathione; H2O2: Hydrogen peroxide; HBEC: Human bronchial epithelial cell line; MMAIII: Monomethylarsonous acid; MMAV: Methylarsonate; mtDNA: Mitochondrial DNA; RNS: Reactive nitrogen species; ROS: Reactive oxygen species; SAM: s-adenosylmethionine; SCC: Lung small cell carcinoma; SqCC: Lung squamous cell carcinoma.
Competing interests
The authors declared they have no competing interests.
Authors’ contributions
RH, DDB, KSSE and VDM designed and collected information for this review. RH and DDB drafted the manuscript. SL and WLL are principal investigators of related projects. All authors have been involved in revision and approved the final manuscript.
Contributor Information
Roland Hubaux, Email: rhubaux@bccrc.ca.
Daiana D Becker-Santos, Email: dbecker@bccrc.ca.
Katey SS Enfield, Email: kenfield@bccrc.ca.
Stephen Lam, Email: slam@bccrc.ca.
Wan L Lam, Email: wanlam@bccrc.ca.
Victor D Martinez, Email: vmartinez@bccrc.ca.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research (CIHR) (MOP-86731, MOP-77903, MOP-110949, MOP-230517), Canadian Cancer Society (CCS20485), NIH/NCI 1R01CA164783-01 and Department of Defence (CDMRP W81XWH-10-1-0634). D.D.B.S. is supported by a University of British Columbia 4YF scholarship, and K.S.S.E. by a Frederick Banting and Charles Best Canada Graduate Scholarship from CIHR.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers–a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Badar F, Meerza F, Khokhar RA, Ali FA, Irfan N, Kamran S, Shahid N, Mahmood S. Characteristics of lung cancer patients–the shaukat khanum memorial experience. Asian Pac J Cancer Prev. 2006;7(2):245–248. [PubMed] [Google Scholar]
- Subramanian J, Govindan R. Lung cancer in never smokers: a review. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(5):561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- IARC. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: International Agency for Research on Cancer; 1987. [PMC free article] [PubMed] [Google Scholar]
- IARC. Some drinking-water disinfectants and contaminants, including arsenic. Monographs on chloramine, chloral and chloral hydrate, dichloroacetic acid, trichloroacetic acid and 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. IARC Monogr Eval Carcinog Risks Hum. 2004;84:269–477. [PMC free article] [PubMed] [Google Scholar]
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(14):3175–3185. doi: 10.1200/JCO.2005.10.462. [DOI] [PubMed] [Google Scholar]
- Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res. 2006;608(2):157–162. doi: 10.1016/j.mrgentox.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Nyberg F. Contribution of environmental factors to cancer risk. Br Med Bull. 2003;68:71–94. doi: 10.1093/bmp/ldg023. [DOI] [PubMed] [Google Scholar]
- De Matteis S, Consonni D, Lubin JH, Tucker M, Peters S, Vermeulen RC, Kromhout H, Bertazzi PA, Caporaso NE, Pesatori AC. et al. Impact of occupational carcinogens on lung cancer risk in a general population. Int J Epidemiol. 2012;41(3):711–721. doi: 10.1093/ije/dys042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley NH, Harley JH. Potential lung cancer risk from indoor radon exposure. CA Cancer J Clin. 1990;40(5):265–275. doi: 10.3322/canjclin.40.5.265. [DOI] [PubMed] [Google Scholar]
- Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-Up of the harvard Six cities study from 1974 to 2009. Environ Health Perspect. 2012;120(7):965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VD, Becker-Santos DD, Vucic EA, Lam S, Lam WL. Induction of human squamous cell-type carcinomas by arsenic. J Skin Cancer. 2011;2011:454157. doi: 10.1155/2011/454157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol. 2012;10(3):157–164. [PubMed] [Google Scholar]
- Turner MC, Krewski D, Pope Iii CA, Chen Y, Gapstur SM, Thun MJ. Long-term ambient fine particulate matter Air pollution and lung cancer in a large cohort of never smokers. Am J Respir Crit Care Med. 2011;84(12):1374–1381. doi: 10.1164/rccm.201106-1011OC. [DOI] [PubMed] [Google Scholar]
- Veloso B, Nogueira JR, Cardoso MF. Lung cancer and indoor radon exposure in the north of Portugal–an ecological study. Cancer Epidemiol. 2012;36(1):e26–e32. doi: 10.1016/j.canep.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Yang M. A current global view of environmental and occupational cancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29(3):223–249. doi: 10.1080/10590501.2011.601848. [DOI] [PubMed] [Google Scholar]
- Virta RL. Worldwide asbestos supply and consumption trends from 1900 through 2003. Reston, Virginia: U.S. Geological Survey; 2006. p. 80. (Circular 1298). http://pubs.usgs.gov/circ/2006/1298/c1298.pdf. [Google Scholar]
- U.S. Geological Survey. Mineral commodity summaries 2011. Reston, Virginia: U.S. Geological Survey; 2011. p. 198. http://minerals.usgs.gov/minerals/pubs/mcs/2011/mcs2011.pdf. [Google Scholar]
- Brunt RVL, Vasak L, Griffioen J. Arsenic in groundwater worldwide: probability of occurrence of excesive concentration on global scale. Utrecht; 2004. p. 15. (International groundwater resources assessment centre). http://www.un-igrac.org/dynamics/modules/SFIL0100/view.php?fil_Id=124. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR 2000 Report. UNSCEAR; 2000. p. 649. (Sources and effects of ionizing radiation). http://www.unscear.org/unscear/publications/2000_1.html. [Google Scholar]
- Mclaughlin Centre. World Map of national residential radon levels. http://www.mclaughlincentre.ca/research/map.shtml.
- Loeb LA, Harris CC. Advances in chemical carcinogenesis: a historical review and prospective. Cancer Res. 2008;68(17):6863–6872. doi: 10.1158/0008-5472.CAN-08-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Evaluation of certain contaminants in food. 959. India; 2011. pp. 1–105. (World health organ tech Rep Ser). 115p. [ http://whqlibdoc.who.int/trs/who_trs_959_eng.pdf] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Mahfuzar R. Contamination of drinking-water by arsenic in bangladesh: a public health emergency. Bull World Health Organ. 2000;78(9):1093–1103. [PMC free article] [PubMed] [Google Scholar]
- Andrew AS, Bernardo V, Warnke LA, Davey JC, Hampton T, Mason RA, Thorpe JE, Ihnat MA, Hamilton JW. Exposure to arsenic at levels found inU.S. Drinking water modifies expression in the mouse lung. Toxicological sciences: an official journal of the Society of Toxicology. 2007;100(1):75–87. doi: 10.1093/toxsci/kfm200. [DOI] [PubMed] [Google Scholar]
- Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, Duell EJ. Lung cancer in a U.S. Population with low to moderate arsenic exposure. Environ Health Perspect. 2009;117(11):1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat Res. 2006;612(3):215–246. doi: 10.1016/j.mrrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) Arsenic in drinking water: 2001 update. Washington DC: The National Academy Press; 2001. p. 244. [Google Scholar]
- Putila JJ, Guo NL. Association of arsenic exposure with lung cancer incidence rates in the united states. PLoS One. 2011;6(10):e25886. doi: 10.1371/journal.pone.0025886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Hsu LI, Chiou HY, Hsueh YM, Chen SY, Wu MM, Chen CJ. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in taiwan. JAMA. 2004;292(24):2984–2990. doi: 10.1001/jama.292.24.2984. [DOI] [PubMed] [Google Scholar]
- Toh CK, Gao F, Lim WT, Leong SS, Fong KW, Yap SP, Hsu AA, Eng P, Koong HN, Thirugnanam A. et al. Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(15):2245–2251. doi: 10.1200/JCO.2005.04.8033. [DOI] [PubMed] [Google Scholar]
- Toh CK, Lim WT. Lung cancer in never-smokers. J Clin Pathol. 2007;60(4):337–340. doi: 10.1136/jcp.2006.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Chiou HY, Hsu LI, Hsueh YM, Wu MM, Chen CJ. Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern taiwan. Environ Res. 2010;110(5):455–462. doi: 10.1016/j.envres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Martinez VD, Buys TP, Adonis M, Benitez H, Gallegos I, Lam S, Lam WL, Gil L. Arsenic-related DNA copy-number alterations in lung squamous cell carcinomas. Br J Cancer. 2010;103(8):1277–1283. doi: 10.1038/sj.bjc.6605879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol. 2002;29(1):2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- McCormack V, Peto J, Byrnes G, Straif K, Boffetta P. Estimating the asbestos-related lung cancer burden from mesothelioma mortality. Br J Cancer. 2012;106(3):575–584. doi: 10.1038/bjc.2011.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos MD, Bizekis C, Pass HI. Malignant mesothelioma 2008. Curr Opin Pulm Med. 2008;14(4):303–309. doi: 10.1097/MCP.0b013e328302851d. [DOI] [PubMed] [Google Scholar]
- van Loon AJ, Kant IJ, Swaen GM, Goldbohm RA, Kremer AM, van den Brandt PA. Occupational exposure to carcinogens and risk of lung cancer: results from the netherlands cohort study. Occup Environ Med. 1997;54(11):817–824. doi: 10.1136/oem.54.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42(2):133–139. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira RM, Ghio AJ, Effros RM, Morrisey J, Dawson CA, Hacker AD. Hydroxyl radicals are formed in the rat lung after asbestos instillation in vivo. Am J Respir Cell Mol Biol. 1994;10(5):573–579. doi: 10.1165/ajrcmb.10.5.8179922. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Brown RC, Turim J. Biopersistence, fiber length, and cancer risk assessment for inhaled fibers. Inhal Toxicol. 2001;13(9):755–772. doi: 10.1080/089583701316941294. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Bignon J, Corn M, Seaton A, Gee JB. Asbestos: scientific developments and implications for public policy. Science. 1990;247(4940):294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- Lin RT, Takahashi K, Karjalainen A, Hoshuyama T, Wilson D, Kameda T, Chan CC, Wen CP, Furuya S, Higashi T. et al. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. Lancet. 2007;369(9564):844–849. doi: 10.1016/S0140-6736(07)60412-7. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Radon health risks. Washington D.C.: U.S. Environmental Protection Agency; 2012. [Google Scholar]
- Lubin JH, Boice JD Jr, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA, Morrison HI, Radford EP, Samet JM. et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst. 1995;87(11):817–827. doi: 10.1093/jnci/87.11.817. [DOI] [PubMed] [Google Scholar]
- Simon S. Lung cancer also affects nonsmokers. The American Cancer Society; 2011. http://www.cancer.org/cancer/news/lung-cancer-also-affects-nonsmokers. [Google Scholar]
- Al-Zoughool M, Krewski D. Health effects of radon: a review of the literature. Int J Radiat Biol. 2009;85(1):57–69. doi: 10.1080/09553000802635054. [DOI] [PubMed] [Google Scholar]
- Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626–5645. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on the Biological Effects of Ionizing Radiations, National Research Council. Health risks of radon and other internally deposited alpha-emitters:BEIR IV. Washington D.C.: The National Academies Press; 1988. [PubMed] [Google Scholar]
- Samet JM, Kutvirt DM, Waxweiler RJ, Key CR. Uranium mining and lung cancer in navajo men. N Engl J Med. 1984;310(23):1481–1484. doi: 10.1056/NEJM198406073102301. [DOI] [PubMed] [Google Scholar]
- Archer VE, Wagoner JK, Lundin FE. Lung cancer among uranium miners in the united states. Health Phys. 1973;25(4):351–371. doi: 10.1097/00004032-197310000-00001. [DOI] [PubMed] [Google Scholar]
- Roscoe RJ, Steenland K, Halperin WE, Beaumont JJ, Waxweiler RJ. Lung cancer mortality among nonsmoking uranium miners exposed to radon daughters. JAMA. 1989;262(5):629–633. doi: 10.1001/jama.1989.03430050045024. [DOI] [PubMed] [Google Scholar]
- Health Canada Dp. Presented to the Federal Provincial Territorial Radiation Protection Committee. Ottawa: Health Canada D; 2006. Report of the radon working group on a new radon guideline for Canada (Rev. 03-10-2006) [Google Scholar]
- Barros-Dios JM, Ruano-Ravina A, Perez-Rios M, Castro-Bernardez M, Abal-Arca J, Tojo-Castro M. Residential radon exposure, histologic types, and lung cancer risk. A case–control study in galicia, spain. Canc Epidemiol Biomarkers Prev. 2012;21(6):951–958. doi: 10.1158/1055-9965.EPI-12-0146-T. [DOI] [PubMed] [Google Scholar]
- Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89:713. doi: 10.1021/cr00094a002. [DOI] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethyl-ation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Persp. 2002;110:767. doi: 10.1289/ehp.02110s5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176(2):127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74(6):289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163(2):203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Ebert F, Weiss A, Bultemeyer M, Hamann I, Hartwig A, Schwerdtle T. Arsenicals affect base excision repair by several mechanisms. Mutat Res. 2011;715(1–2):32–41. doi: 10.1016/j.mrfmmm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Nemeti B, Regonesi ME, Tortora P, Gregus Z. Polynucleotide phosphorylase and mitochondrial ATP synthase mediate reduction of arsenate to the more toxic arsenite by forming arsenylated analogues of ADP and ATP. Toxicological sciences: an official journal of the Society of Toxicology. 2010;117(2):270–281. doi: 10.1093/toxsci/kfq141. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Styblo M, Thomas DJ. Purification of arsenic (+3 oxidation state) methyltransferase from rat liver cytosol. Curr Protoc Toxicol. 2009;42(4.34):1–13. doi: 10.1002/0471140856.tx0434s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533(1–2):37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Klein CB. Genetic and epigenetic effects of environmental arsenicals. Metallomics. 2011;3(11):1135–1141. doi: 10.1039/c1mt00074h. [DOI] [PubMed] [Google Scholar]
- Sung TI, Wang YJ, Chen CY, Hung TL, Guo HR. Increased serum level of epidermal growth factor receptor in liver cancer patients and its association with exposure to arsenic. Sci Total Environ. 2012;424:74–78. doi: 10.1016/j.scitotenv.2012.02.079. [DOI] [PubMed] [Google Scholar]
- Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255(1–2):67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Ahmad S. Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett. 2003;137(1–2):3–13. doi: 10.1016/s0378-4274(02)00376-4. [DOI] [PubMed] [Google Scholar]
- Kessel M, Liu SX, Xu A, Santella R, Hei TK. Arsenic induces oxidative DNA damage in mammalian cells. Mol Cell Biochem. 2002;234-235(1–2):301–308. [PubMed] [Google Scholar]
- Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62(14):3893–3903. [PubMed] [Google Scholar]
- Partridge MA, Huang SX, Hernandez-Rosa E, Davidson MM, Hei TK. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67(11):5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez AD, De Vizcaya-Ruiz A, Cebrian ME. Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat Res. 2009;674(1–2):109–115. doi: 10.1016/j.mrgentox.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Matsui M, Nishigori C, Toyokuni S, Takada J, Akaboshi M, Ishikawa M, Imamura S, Miyachi Y. The role of oxidative DNA damage in human arsenic carcinogenesis: detection of 8-hydroxy-2’-deoxyguanosine in arsenic-related Bowen’s disease. J Invest Dermatol. 1999;113(1):26–31. doi: 10.1046/j.1523-1747.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Arnold LL, Eldan M, Lewis AS, Beck BD. Methylated arsenicals: the implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit Rev Toxicol. 2006;36(2):99–133. doi: 10.1080/10408440500534230. [DOI] [PubMed] [Google Scholar]
- Xu A, Huang X, Lien YC, Bao L, Yu Z, Hei TK. Genotoxic mechanisms of asbestos fibers: role of extranuclear targets. Chem Res Toxicol. 2007;20(5):724–733. doi: 10.1021/tx600364d. [DOI] [PubMed] [Google Scholar]
- Choe N, Tanaka S, Kagan E. Asbestos fibers and interleukin-1 upregulate the formation of reactive nitrogen species in rat pleural mesothelial cells. Am J Respir Cell Mol Biol. 1998;19(2):226–236. doi: 10.1165/ajrcmb.19.2.3111. [DOI] [PubMed] [Google Scholar]
- Ault JG, Cole RW, Jensen CG, Jensen LC, Bachert LA, Rieder CL. Behavior of crocidolite asbestos during mitosis in living vertebrate lung epithelial cells. Cancer Res. 1995;55(4):792–798. [PubMed] [Google Scholar]
- Sanchez VC, Pietruska JR, Miselis NR, Hurt RH, Kane AB. Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):511–529. doi: 10.1002/wnan.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, Ishihara T, Yamashita K, Yoshikawa Y, Yasui H. et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108(49):E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei TK, Xu A, Huang SX, Zhao Y. Mechanism of fiber carcinogenesis: from reactive radical species to silencing of the beta igH3 gene. Inhal Toxicol. 2006;18(12):985–990. doi: 10.1080/08958370600835310. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Lohani M, Pant AB, Rahman Q. Cyto-genotoxicity of amphibole asbestos fibers in cultured human lung epithelial cell line: role of surface iron. Toxicol Ind Health. 2010;26(9):575–582. doi: 10.1177/0748233710374464. [DOI] [PubMed] [Google Scholar]
- Panduri V, Weitzman SA, Chandel NS, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1220–L1227. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- Aljandali A, Pollack H, Yeldandi A, Li Y, Weitzman SA, Kamp DW. Asbestos causes apoptosis in alveolar epithelial cells: role of iron-induced free radicals. J Lab Clin Med. 2001;137(5):330–339. doi: 10.1067/mlc.2001.114826. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Gwinn MR, DeVoney D, Jarabek AM, Sonawane B, Wheeler J, Weissman DN, Masten S, Thompson C. Meeting report: mode(s) of action of asbestos and related mineral fibers. Environ Health Perspect. 2011;119(12):1806–1810. doi: 10.1289/ehp.1003240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SX, Jaurand MC, Kamp DW, Whysner J, Hei TK. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J Toxicol Environ Health B Crit Rev. 2011;14(1–4):179–245. doi: 10.1080/10937404.2011.556051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for radon. Atlanta; U.S: Department of Health and Human Services, Public Health Service; 2012. [PubMed] [Google Scholar]
- Adams GED. Introduction to the cellular and molecular biology of cancer. Oxford: Oxford University Press; 1986. [Google Scholar]
- Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA. 2000;97(5):2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavanja MC. Biologic damage resulting from exposure to tobacco smoke and from radon: implication for preventive interventions. Oncogene. 2002;21(48):7365–7375. doi: 10.1038/sj.onc.1205798. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Thompson RE, Beecher GC. Human lung cancer risks from radon - part I - influence from bystander effects - a microdose analysis. Dose–response: a publication of International Hormesis Society. 2011;9(2):243–292. doi: 10.2203/dose-response.09-057.Leonard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V, Howland M, Kutzner B, McNamee JP, Bellier PV, Wilkins RC. Biological effects of alpha particle radiation exposure on human monocytic cells. Int J Hyg Environ Health. 2012;215(3):339–344. doi: 10.1016/j.ijheh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew AS, Mason RA, Memoli V, Duell EJ. Arsenic activates EGFR pathway signaling in the lung. Toxicological sciences: an official journal of the Society of Toxicology. 2009;109(2):350–357. doi: 10.1093/toxsci/kfp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LZ, Jiang Y, Carpenter RL, Jing Y, Peiper SC, Jiang BH. Role and mechanism of arsenic in regulating angiogenesis. PLoS One. 2011;6(6):e20858. doi: 10.1371/journal.pone.0020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Xia L, Gabrilove J, Waxman S, Jing Y. Down-regulation of Mcl-1 through GSK-3beta activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia. 2012. p. 10. advance online publication. [DOI] [PMC free article] [PubMed]
- Zhang Z, Wang X, Cheng S, Sun L, Son YO, Yao H, Li W, Budhraja A, Li L, Shelton BJ. et al. Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/beta-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol Appl Pharmacol. 2011;256(2):114–121. doi: 10.1016/j.taap.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Osmond MJ, Kunz BA, Snow ET. Age and exposure to arsenic alter base excision repair transcript levels in mice. Mutagenesis. 2010;25(5):517–522. doi: 10.1093/mutage/geq037. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Okada S. Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ Health Perspect. 1994;102(Suppl 3):37–40. doi: 10.1289/ehp.94102s337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G, Calaf GM, Partridge MA, Echiburu-Chau C, Zhao Y, Huang S, Chai Y, Li B, Hu B, Hei TK. Neoplastic transformation of human small airway epithelial cells induced by arsenic. Mol Med. 2008;14(1–2):2–10. doi: 10.2119/2007-00090.Wen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Flanders T, Lounsbury KM, Mossman BT. The gamma-glutamylcysteine synthetase and glutathione regulate asbestos-induced expression of activator protein-1 family members and activity. Cancer Res. 2004;64(21):7780–7786. doi: 10.1158/0008-5472.CAN-04-1365. [DOI] [PubMed] [Google Scholar]
- Nymark P, Lindholm PM, Korpela MV, Lahti L, Ruosaari S, Kaski S, Hollmen J, Anttila S, Kinnula VL, Knuutila S. Gene expression profiles in asbestos-exposed epithelial and mesothelial lung cell lines. BMC Genomics. 2007;8:62. doi: 10.1186/1471-2164-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Barrett TF, Nakayama KI, Nakayama K, Mossman BT, Lounsbury KM. Transcriptional up-regulation of MMP12 and MMP13 by asbestos occurs via a PKCdelta-dependent pathway in murine lung. FASEB J. 2006;20(7):997–999. doi: 10.1096/fj.05-4554fje. [DOI] [PubMed] [Google Scholar]
- Husgafvel-Pursiainen K, Karjalainen A, Kannio A, Anttila S, Partanen T, Ojajarvi A, Vainio H. Lung cancer and past occupational exposure to asbestos. Role of p53 and K-ras mutations. Am J Respir Cell Mol Biol. 1999;20(4):667–674. doi: 10.1165/ajrcmb.20.4.3404. [DOI] [PubMed] [Google Scholar]
- Wang X, Christiani DC, Wiencke JK, Fischbein M, Xu X, Cheng TJ, Mark E, Wain JC, Kelsey KT. Mutations in the p53 gene in lung cancer are associated with cigarette smoking and asbestos exposure. Canc Epidemiol Biomarkers Prev. 1995;4(5):543–548. [PubMed] [Google Scholar]
- Andujar P, Wang J, Descatha A, Galateau-Salle F, Abd-Alsamad I, Billon-Galland MA, Blons H, Clin B, Danel C, Housset B. et al. p16INK4A Inactivation mechanisms in non-small-cell lung cancer patients occupationally exposed to asbestos. Lung Cancer. 2010;67(1):23–30. doi: 10.1016/j.lungcan.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Piao CQ, Zhao YL, Hei TK. Karyotype analysis of tumorigenic human bronchial epithelial cells transformed by chrysolite asbestos using chemically induced premature chromosome condensation technique. Int J Mol Med. 2001;8(1):43–47. doi: 10.3892/ijmm.8.1.43. [DOI] [PubMed] [Google Scholar]
- Olofsson K, Mark J. Specificity of asbestos-induced chromosomal aberrations in short-term cultured human mesothelial cells. Cancer Genet Cytogenet. 1989;41(1):33–39. doi: 10.1016/0165-4608(89)90105-2. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Lamb PW, Wiseman RW. Multiple mechanisms for the carcinogenic effects of asbestos and other mineral fibers. Environ Health Perspect. 1989;81:81–89. doi: 10.1289/ehp.898181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegles M, Janson X, Dong HY, Renier A, Jaurand MC. Role of fibre characteristics on cytotoxicity and induction of anaphase/telophase aberrations in rat pleural mesothelial cells in vitro: correlations with in vivo animal findings. Carcinogenesis. 1995;16(11):2751–2758. doi: 10.1093/carcin/16.11.2751. [DOI] [PubMed] [Google Scholar]
- Hesterberg TW, Barrett JC. Induction by asbestos fibers of anaphase abnormalities: mechanism for aneuploidy induction and possibly carcinogenesis. Carcinogenesis. 1985;6(3):473–475. doi: 10.1093/carcin/6.3.473. [DOI] [PubMed] [Google Scholar]
- Prise KM, Pinto M, Newman HC, Michael BD. A review of studies of ionizing radiation-induced double-strand break clustering. Radiat Res. 2001;156(5 Pt 2):572–576. doi: 10.1667/0033-7587(2001)156[0572:arosoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Taylor JA, Watson MA, Saccomanno G, Devereux TR. p53 And K-ras in radon-associated lung adenocarcinoma. Canc Epidemiol Biomarkers Prev. 1995;4(7):791–793. [PubMed] [Google Scholar]
- Vahakangas KH, Samet JM, Metcalf RA, Welsh JA, Bennett WP, Lane DP, Harris CC. Mutations of p53 and ras genes in radon-associated lung cancer from uranium miners. Lancet. 1992;339(8793):576–580. doi: 10.1016/0140-6736(92)90866-2. [DOI] [PubMed] [Google Scholar]
- Nymark P, Wikman H, Ruosaari S, Hollmen J, Vanhala E, Karjalainen A, Anttila S, Knuutila S. Identification of specific gene copy number changes in asbestos-related lung cancer. Cancer Res. 2006;66(11):5737–5743. doi: 10.1158/0008-5472.CAN-06-0199. [DOI] [PubMed] [Google Scholar]
- Kettunen E, Aavikko M, Nymark P, Ruosaari S, Wikman H, Vanhala E, Salmenkivi K, Pirinen R, Karjalainen A, Kuosma E. et al. DNA copy number loss and allelic imbalance at 2p16 in lung cancer associated with asbestos exposure. Br J Cancer. 2009;100(8):1336–1342. doi: 10.1038/sj.bjc.6605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman H, Ruosaari S, Nymark P, Sarhadi VK, Saharinen J, Vanhala E, Karjalainen A, Hollmen J, Knuutila S, Anttila S. Gene expression and copy number profiling suggests the importance of allelic imbalance in 19p in asbestos-associated lung cancer. Oncogene. 2007;26(32):4730–4737. doi: 10.1038/sj.onc.1210270. [DOI] [PubMed] [Google Scholar]
- Bastide K, Guilly MN, Bernaudin JF, Joubert C, Lectard B, Levalois C, Malfoy B, Chevillard S. Molecular analysis of the Ink4a/Rb1-Arf/Tp53 pathways in radon-induced rat lung tumors. Lung Cancer. 2009;63(3):348–353. doi: 10.1016/j.lungcan.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Nelson HH, Christiani DC, Wiencke JK, Mark EJ, Wain JC, Kelsey KT. k-ras mutation and occupational asbestos exposure in lung adenocarcinoma: asbestos-related cancer without asbestosis. Cancer Res. 1999;59(18):4570–4573. [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Iorio MV. Croce CM: microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104(7):528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997;386(3):263–277. doi: 10.1016/S1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]
- Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in a/J mice. Toxicological sciences: an official journal of the Society of Toxicology. 2006;91(2):372–381. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhao Y, Smith E, Goodall GJ, Drew PA, Brabletz T, Yang C. Reversal and prevention of arsenic-induced human bronchial epithelial cell malignant transformation by microRNA-200b. Toxicological sciences: an official journal of the Society of Toxicology. 2011;121(1):110–122. doi: 10.1093/toxsci/kfr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann R, Strunnikova M, Schagdarsurengin U, Rastetter M, Papritz M, Hattenhorst UE, Hofmann HS, Silber RE, Burdach S, Hansen G. CpG island methylation and expression of tumour-associated genes in lung carcinoma. Eur J Cancer. 2005;41(8):1223–1236. doi: 10.1016/j.ejca.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Ahrendt SA, Piantadosi S, Rosell R, Monzo M, Wu L, Westra WH, Yang SC, Jen J, Sidransky D. Chromosomal alterations in lung adenocarcinoma from smokers and nonsmokers. Cancer Res. 2001;61(4):1309–1313. [PubMed] [Google Scholar]
- Nymark P, Guled M, Borze I, Faisal A, Lahti L, Salmenkivi K, Kettunen E, Anttila S, Knuutila S. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosomes Cancer. 2011;50(8):585–597. doi: 10.1002/gcc.20880. [DOI] [PubMed] [Google Scholar]
- Su S, Jin Y, Zhang W, Yang L, Shen Y, Cao Y, Tong J. Aberrant promoter methylation of p16(INK4a) and O(6)-methylguanine-DNA methyltransferase genes in workers at a chinese uranium mine. J Occup Health. 2006;48(4):261–266. doi: 10.1539/joh.48.261. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Klinge DM, Liechty KC, March TH, Kang T, Gilliland FD, Sotnic N, Adamova G, Rusinova G, Telnov V. Plutonium targets the p16 gene for inactivation by promoter hypermethylation in human lung adenocarcinoma. Carcinogenesis. 2004;25(6):1063–1067. doi: 10.1093/carcin/bgh096. [DOI] [PubMed] [Google Scholar]
- Intarasunanont P, Navasumrit P, Woraprasit S, Chaisatra K, Suk WA, Mahidol C, Ruchirawat M. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environmental health: a global access science source. 2012;11(1):31. doi: 10.1186/1476-069X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TJ, Novak P, Eblin KE, Gandolfi AJ, Futscher BW. Epigenetic remodeling during arsenical-induced malignant transformation. Carcinogenesis. 2008;29(8):1500–1508. doi: 10.1093/carcin/bgn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TJ, Wozniak RJ, Eblin KE, Wnek SM, Gandolfi AJ, Futscher BW. Epigenetic mediated transcriptional activation of WNT5A participates in arsenical-associated malignant transformation. Toxicol Appl Pharmacol. 2009;235(1):39–46. doi: 10.1016/j.taap.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29(9):1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo WJ, Ren X, Chu F, Aleshin M, Wintz H, Burlingame A, Smith MT, Vulpe CD, Zhang L. Acetylated H4K16 by MYST1 protects UROtsa cells from arsenic toxicity and is decreased following chronic arsenic exposure. Toxicol Appl Pharmacol. 2009;241(3):294–302. doi: 10.1016/j.taap.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci USA. 1997;94(20):10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan D, Pittman CU Jr. Arsenic removal from water/wastewater using adsorbents–a critical review. J Hazard Mater. 2007;142(1–2):1–53. doi: 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Mathieu JL, Gadgil AJ, Addy SE, Kowolik K. Arsenic remediation of drinking water using iron-oxide coated coal bottom ash. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng. 2010;45(11):1446–1460. doi: 10.1080/10934529.2010.500940. [DOI] [PubMed] [Google Scholar]
- Pokhrel D, Viraraghavan T. Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res. 2006;40(3):549–552. doi: 10.1016/j.watres.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Paglietti F, Malinconico S, Molfetta VD, Giangrasso M. Guidelines for asbestos remediation at italian superfund sites. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30(3):253–286. doi: 10.1080/10590501.2012.705161. [DOI] [PubMed] [Google Scholar]
- Colangelo F, Cioffi R, Lavorgna M, Verdolotti L, De Stefano L. Treatment and recycling of asbestos-cement containing waste. J Hazard Mater. 2011;195:391–397. doi: 10.1016/j.jhazmat.2011.08.057. [DOI] [PubMed] [Google Scholar]
- Gualtieri AF, Giacobbe C, Sardisco L, Saraceno M, Gualtieri ML, Lusvardi G, Cavenati C, Zanatto I. Recycling of the product of thermal inertization of cement-asbestos for various industrial applications. Waste Manag. 2011;31(1):91–100. doi: 10.1016/j.wasman.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Rice J. The global reorganization and revitalization of the asbestos industry, 1970–2007. Int J Health Serv. 2011;41(2):239–254. doi: 10.2190/HS.41.2.d. [DOI] [PubMed] [Google Scholar]
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry. 2009;135(4):243–255. doi: 10.1093/rpd/ncp112. [DOI] [PubMed] [Google Scholar]
- Wild CP. Environmental exposure measurement in cancer epidemiology. Mutagenesis. 2009;24(2):117–125. doi: 10.1093/mutage/gen061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, McLaughlin JK, la Vecchia C, Autier P, Boyle P. ‘Environment’ In cancer causation and etiological fraction: limitations and ambiguities. Carcinogenesis. 2007;28(5):913–915. doi: 10.1093/carcin/bgm034. [DOI] [PubMed] [Google Scholar]