Abstract
Background
Contradictory results have been reported regarding the association between Pro12Ala polymorphism of PPARγ2 and coronary artery disease (CAD). We sought to estimate the inconsistent results by performing a comprehensive meta-analysis.
Methods
Studies in English or Chinese publications were identified by screening MEDLINE, Embase, CNKI, Wanfang and CBM. 22 studies including 8948 cases and 14427 controls were selected. A random-effects model was applied to combine the divergent outcomes of the individual studies, while addressing between-study heterogeneity and publication bias.
Results
The Pro12Ala polymorphism of control population followed Hardy-Weinberg equilibrium for all studies (P>0.05). Overall, a marginal increased risk of CAD under the recessive genetic model (AlaAla vs ProAla+ProPro: P = 0.04, OR = 1.31, 95%CI 1.01–1.69, Pheterogeneity = 0.67, I2 = 0%) and the homozygote comparison (AlaAla vs ProPro: P = 0.04,OR = 1.30, 95%CI 1.01–1.68, Pheterogeneity = 0.68, I2 = 0%) was observed. In the subgroup analysis by ethnicity, carriers of AlaAla homozygotes had a significant increased risk for CAD among Caucasians (AlaAla vs ProAla+ProPro: P = 0.01, OR = 1.45, 95%CI 1.08–1.96, Pheterogeneity = 0.48, I2 = 0%; AlaAla vs ProPro: P = 0.02,OR = 1.44, 95%CI 1.07–1.93, Pheterogeneity = 0.46, I2 = 0%). After dividing into population source, the CAD risk magnitude of hospital-based studies was distinctly strengthened under the recessive model (P = 0.03,OR = 1.85,95%CI 1.07–3.19, Pheterogeneity = 0.87,I2 = 0%) and the homozygote comparison (P = 0.03,OR = 1.83, 95%CI 1.06–3.16, Pheterogeneity = 0.88, I2 = 0%). There was no observable publication bias as reflected by funnel plot and Egger’s linear regression test (t = -0.12, P = 0.91).
Conclusion:
Our results demonstrated that the PPARγ2 Pro12Ala polymorphism might be risk-conferring locus for the progression of CAD among Caucasians, but not among Asians.
Introduction
Coronary artery disease (CAD) and myocardial infarction (MI), the leading causes of morbidity and death in industrialized countries, represent heavy economic and social burdens on the public health system. CAD and MI are common disorders resulted from the interaction of numerous risk factors [1],including Diabetes,obesity, hypercholesterolemia, hypertension, smoke and so on. In the past few years, large quantities of evidences have documented that the genetic factors may contribute to the majority of variation in susceptible to CAD. Nevertheless, little crucial genetic variants that determined the progression of CAD were found out.
The gene peroxisome proliferator-activated receptor γ (PPARγ), located on chromosome 3p25, is a member of the nuclear receptor superfamily. PPARγ is considered as a “master regulator” in the course of glucose homeostasis, lipoprotein metabolism and vascular homeostasis [2], [3]. PPARγ has two isoforms (γ1 and γ2), which differ at their N terminus [4]. The PPARγ2 isoform is mostly expressed in adipose tissue [5]. The most common gene polymorphism in human PPARγ2 gene is cytosine-guanine exchange in exon B (codon12) which results proline to alanine (Pro12Ala) substitution in the protein [6].The Pro12Ala polymorphism was first identified by Yen et al. [7] in 1997 and regarded to reduce transcriptional activity of PPARγ2 [8], resulting in lower transcription levels of target genes [9],including tumor necrosis factor α (TNF α), leptin, resistin, adiponectin, and plasminogen activator in hibitor-1(PAI-1), which play important roles in the process of inflammation and atherosclerosis. There are evidences that the resulting mutant transcription factor profoundly affects the energy metabolism and energy balance and is associated with the risks of atherogenesis [10]–[12] and diabetes [13], [14]. In addition, the Pro12Ala polymorphism was regarded to change the response of synthetic PPARγ agonists– thiazolidinediones (TZDs) treatment [15], which seemed to improve the insulin resistance [16] and limit atherosclerosis development [17]. Many studies have been performed to explore the association between PPARγ2 Pro12Ala polymorphism and CAD, but data are inconsistent [18]–[23]. As a matter of fact, single studies with restrictive sample sizes have insufficient statistical powers to determine the common variants with moderate effects on CAD and the results are not replicated most of time. Given the limitation of these individual researches, large meta-analysis is a feasible strategy to reliably assess the predetermined candidates in genetic-related researches. To derive a more precise estimation, we performed a meta- analysis of published studies to date in order to evaluate the association of PPARγ2 gene Pro12Ala polymorphism with CAD, while addressing between-study heterogeneity and publication bias.
Materials and Methods
Search Strategy
To identify all studies that examined the relationship between PPARγ2 Pro12Ala polymorphism and CAD, a systematic computerized literature search was conducted. The search was done on August, 2012. All published studies were found with PubMed/MEDLINE, Embase, CNKI (China Nation Knowledge Infrastructure Platform), Wanfang, CBM (China Biological Medicine Database) electronic databases by using the following combinations of text search string: ‘Pro12Ala’, ‘Peroxisome proliferator -activated receptor gamma’ and ‘coronary’ or ‘CAD’ or ‘myocardial infarction’ or ‘MI’. We also retrieved additional studies through the MEDLINE option ‘related articles’ and manual bibliography review was added. References from the retrieved articles, reviews, and previous meta-analysis were also screened to complete the data bank. If the data were incomplete in an appropriate format, we connected to the corresponding author to obtain the data. The following constraints were applied to the search: (1) Articles published in English or Chinese journals or their supplements; (2) Studies in human subjects without country restrictions; (3) When studies from the same research group with overlapped population were found, only the one with largest population was included to avoid data duplication; (4) Have available genotype frequency; (5) If articles containing more than one geographic or ethnic heterogeneous group, each group was treated separately; (6) genotype distribution of control population must be consistent with Hardy-Weinberg equilibrium (HWE).
CAD was defined as documented evidence of a previous MI, coronary bypass operation or coronary catheterization findings of significant stenosis of 50% or more in at least one major coronary artery together with clinical symptoms of angina [24]. Acute coronary syndromes (ACS) included unstable angina pectoris, fatal and non-fatal MI [25] and MI was defined as the presence of typical electrocardiographic changes and elevation in the levels of cardiac enzymes [26].
Data Extraction
With the purpose of extracting the necessary characteristics, all relevant articles were collated independently and entered into separate databases by two investigators (ZW and Y. Lou). They checked for any encountered discrepancies and reached a consensus. The following information was collected on the genotype of Pro12Ala according to different cohort: First author’s name, publication year, geographic location and population ethnicity, study design, population source, diagnostic criteria, baseline characteristics of the study population (such as age, gender, and body mass index [BMI]), the proportion of diabetes and smoking, the Pro12Ala genotype frequency in patients and controls, genotyping methods and consistency of genotype frequencies with Hardy-Weinberg equilibrium (HWE). Quantitative variables expressed as mean ± standard deviation (SD) or median (5th and 95th percentiles).
Quality Score Assessment
The quality of studies was also separately assessed by the same two investigators. Quality scoring criteria were modified from the genetic association study by Thakkinstian et al [27]. Total scores ranged from 0 (worst) to 13 (best). The criteria of quality assessment for the association of the Pro12Ala polymorphism and CAD were described in Table S1.
Statistical Analysis
We examined the extent of the association between the PPARγ2 pro12ala polymorphism and CAD risk by calculating odd ratio (OR) with 95% confidence interval (CI). The result of allele comparison (Ala vs Pro), the dominant genetic model (ProAla+AlaAla vs ProPro), the recessive genetic model (AlaAla vs ProAla+ProPro), and homozygote comparison (AlaAla vs ProPro) were obtained through assessing the pooled studies’ ORs. The random-effects model using the DerSimonian & Laired method was applied to calculate individual effect size together and the Mantel-Haenszel model [28] was used to evaluate the heterogeneity of the studies. The random-effects method adjusted the study weights according to the in-study variance. We assessed the between-study heterogeneity in approach to a Chi-square-based Q statistic test [29]. P<0.10 was considered significantly heterogenetic among the studies. The inconsistency index I2 statistic (ranging from 0 to 100%) was also documented to estimate the degree of heterogenetic variation [30], with higher values suggesting the variability of between-study was caused by heterogeneity rather than chance. The significance of the pooled OR was determined by the Z test and P<0.05 was considered to be significant. Initially studies were categorized into subgroups based on ethnicity. Three subgroups (Asian, Caucasian and others) according to different descent were analyzed for ethnic-specific genetic comparison. The population of Indian was grouped as “others” since its lineage was complicated and cannot simply be grouped as Asian or Caucasian [31]–[34]. The Costa Rica population was of Mestizo background and also grouped as “others” [35]. Pischon et al. [22] and Dallongeville et al. [36] had provided data from two different studies respectively (Nurses’ Health Study [NHS] and Health Professionals Follow-up Study [HPFS] by Pischon et al.; Prospective Study of Myocardial Infarction [PRIME] and Atherosclerotic Disease, Vascular Function, and Genetic Epidemiology [ADVANCE] by Dallongeville et al.). Although the predominant ethnicity of these studies was Caucasian, these four studies were performed in different geographical population (The PRIME study was based in France and in Northern Ireland; The patients of the ADVANCE study came from different countries globally; The NHS study and the HPFS study recruited US participates) and in distinct periods (PRIME in 1991, ADVANCE in 2001, NHS in 1976 and HPFS in 1986). Considering the heterogeneity of environment exposures of the different region and era, we regarded these studies as four independent studies in our meta-analysis. Then we estimated the other study characteristics that could classify the studies into subgroups with homogeneous effects, such as study design, population source and endpoints.
Cumulative-analysis was performed to determine the impact of the first published research on the subsequent publication and the evolution of the pooled estimates over time in accordance with the ascending date of published articles.
Sensitivity analysis was conducted by sequential deleting a single study each time in an attempt to identify the potential influence of the individual data set to the pooled ORs. Furthermore, meta-regression was used as an extension to random-effects meta-analysis. In addition, we used the funnel plot to estimate potential publication bias. The standard error of log (OR) of each study was plotted against its OR. An asymmetric plot suggested publication bias probably. Egger’s linear regression test [37] was examined to verify Funnel-plot asymmetry. We also performed a T-test to determine the significance of intercept and P<0.05 of I2 statistic and Egger’s test was considered to be statistically significant. HWE was tested by the chi-square test or Fisher's exact test for goodness of fit based on a Web program (http://ihg2.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl). Review Manager software release 5.0 (Oxford, England) and Stata 11.0 (Stata Corporation, College Station, Texas, USA) were used for combined data in all studies, and all P values were 2-sided.
Result
Description of Studies Search Result
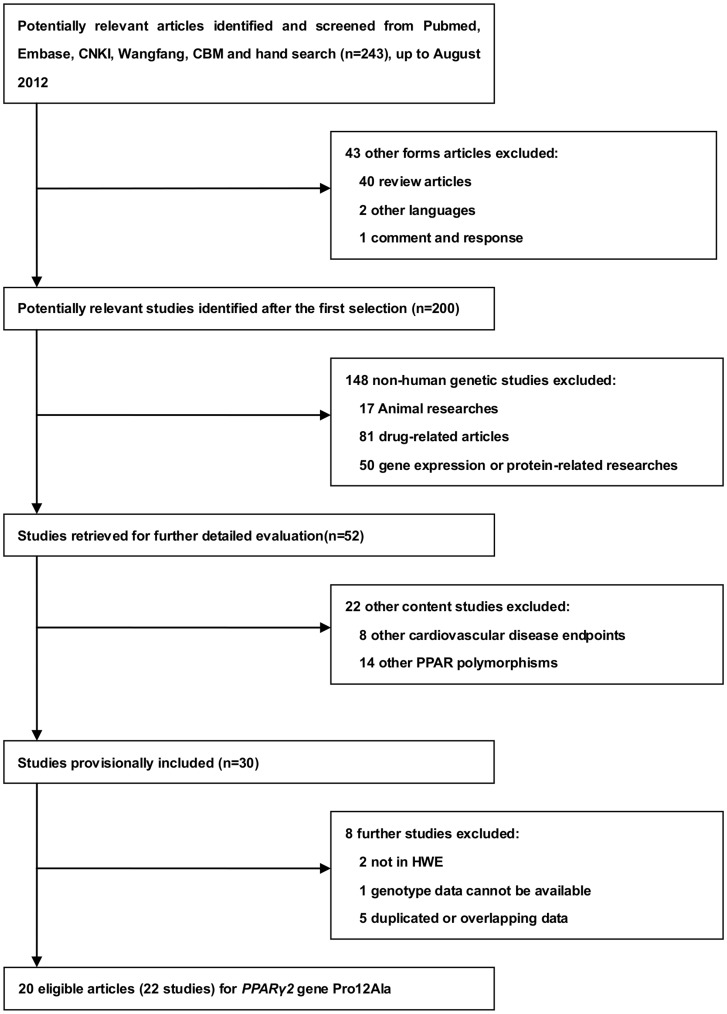
The flowchart summarizing the process of study search and selection was presented in Figure 1. After initial literature search in PubMed, EMBASE, CNKI, Wangfang and CBM with our search strategy and manual bibliography review, a total of 243 relevant articles were yielded. After the subsequent selection, 30 studies focusing on the relationship between PPARγ2 Pro12Ala polymorphism and CAD was provisionally included. Among these studies, 8 articles were further excluded: 2 study [19], [38] and 3 abstracts [39]–[41] were overlapped by other 3 studies [42]–[44] with larger population. The genotyping data in the controls of Wang et al. [45] and Galgani et al. [46] was deviated from HWE in control population (PHWE = 8.865×10−7 & 0.044). These two articles were excluded. Two studies had insufficient data, so we tried to connect with the corresponding or original authors for detailed data by E mail. Until recently, the raw data by Ho JS et al. [43] were provided by the original author and Doney et al. [20] did not reply. The result of our quality score assessment varied between 8 and 12, suggesting that all the studies contained in our meta-analysis were of medium or high quality.
Figure 1. Flow diagram of search strategy and study selection for the meta-analysis.
20 articles included 22 studies with sufficient information were identified in the light of the inclusion criteria [18], [21]–[23], [36], [42]–[44], [47]–[54]. All the eligible studies were published between 2000 and 2012, with 7 in Asian, 13 in Caucasian and 2 in others (Costa Rican & Indian). 8 of the 22 qualified studies were prospective [22], [36], [43], [44], [53], [54] and the others were retrospective. 11 studies were population-based (P–B) [22], [36], [44], [47], [49], [50], [52]–[54] and the rest half were hospital-based (H–B). 15 studies were analyzed for CAD [22], [36], [42], [43], [47], [48], [50], [51], [54] as the primary outcome, 2 studies for ACS [49], [53] and 5 studies for MI [18], [21], [23], [44], [52] as an end point.
Overall Analysis
22 studies comprising 8948 cases and 14427 controls were selected for the meta-analysis. The baseline characteristics of the qualified studies were summarized in Table 1. The distribution of PPARγ2 Pro12Ala genotypes and alleles in the individual studies was listed in Table 2. Genotype distribution of the Pro12Ala polymorphism of control population were in line with HWE for all eligible studies (P>0.05). The pooled overall frequency of the Ala allele was 10.6% in cases and 10.1% in controls. The highest frequency of Ala allele was observed in Caucasian population (11.6% cases vs 12.1% controls). The frequency among Asians (5.2% cases vs 3.6% controls) was much lower than that among Caucasians and others of mixed origin (10.4% cases vs 9.5% controls).
Table 1. The baseline charcteristics of all eligible studies in the meta-analysis.
| First Author | Year | Ethnicity | geographiclocation | Design | Source | Endpoint | Status | Age,year | Gender,M(%) | T2DM,% | BMI,kg/m2 | QualityScore |
| Pischon T | 2005 | Caucasian | US | prospective | P-B | CAD | cases | 65.2±0.5 | 100 | 9.2 | 26.2±0.2 | 10 |
| (HPFS study) | controls | 65.1±0.4 | 100 | 4.4 | 25.7±0.2 | |||||||
| Pischon T | 2005 | Caucasian | US | prospective | P-B | CAD | cases | 60.4±0.4 | 0 | 19.6 | 26.8±0.4 | 10 |
| (NHS study) | controls | 60.3±0.3 | 0 | 6.6 | 25.4±0.2 | |||||||
| Nassar BA | 2006 | Caucasian | Canada | retrospective | P-B | CAD | cases | 45.5±4.0 (<50y) | 74.5 | 0 | a– | 10 |
| 73.7±5.4 (>65y) | ||||||||||||
| controls | 67±6.4 | 46 | 0 | – | ||||||||
| Zee RY | 2006 | Caucasian | US | prospective | P-B | MI | cases | 58.3±0.4 | 100 | 5.6 | 25.5±0.1 | 9 |
| (PHS study) | controls | 58.4±0.2 | 100 | 2.7 | 25±0.1 | |||||||
| Zafarmand MH | 2008 | Caucasian | Netherlands | Prospective | P-B | CAD | cases | 60.5±5.9 | 0 | 5.7 | 26.8±3.9 | 10 |
| (prospect-EPICstudy) | controls | 57.1±6.1 | 0 | 2.2 | 25.8±4.0 | |||||||
| Dallongeville J | 2009 | Caucasian | Global | prospective | P-B | CAD | cases | 61.5±7.9(M) | 65.6 | 22.5 | 29.1±4.8(M) | 9 |
| (ADVANCEstudy) | 60.0±8.8(F) | 29.6±7.2(F) | ||||||||||
| controls | 65.8±3.3(M) | 53.8 | 10.7 | 28.3±4.4(M) | ||||||||
| 61.5±6.9(F) | 27.4±6.3(F) | |||||||||||
| Dallongeville J | 2009 | Caucasian | France& | prospective | P-B | CAD | cases | 55.3±3.0 | 100 | 8.8 | 27.1±3.4 | 10 |
| (PRIME study) | Northern Ireland | controls | 55.1±2.8 | 100 | 4.9 | 26.7±3.5 | ||||||
| Evangelisti L | 2009 | Caucasian | Italy | retrospective | P-B | ACS | cases | 66(25–89) | 70.8 | 26.7 | 27(17–39.6) | 11 |
| controls | 64(20–89) | 69.6 | 4.7 | 26(15.7–40.9) | ||||||||
| Vogel U | 2009 | Caucasian | Denmark | prospective | P-B | ACS | cases | 58(51–65)(M) | 76.2 | 5.4 | 26.9(22.4–34.1)(M) | 11 |
| (DHC study) | 60(52–65)(F) | 26.4(20.1–35.2)(F) | ||||||||||
| controls | 56(51–64)(M) | 53.2 | 1.7 | 26.3(21.6–32.4)(M) | ||||||||
| 56(51–64)(F) | 24.6(19.7–33.7)(F) | |||||||||||
| vos HL | 2000 | Caucasian | Netherlands | retrospective | H-B | MI | cases | – | 100 | – | – | 9 |
| controls | – | 100 | – | – | ||||||||
| Bluher M | 2002 | Caucasian | Germany | retrospective | H-B | CAD | cases | 67.1(43–91) | 67.2 | 100 | 28.68(17.9–44.1) | 12 |
| controls | 63.3(33–87) | 43.9 | 100 | 28.77(17.6–44.4) | ||||||||
| Tobin MD | 2004 | Caucasian | UK | retrospective | H-B | MI | cases | 61.9±9.2 | 68 | 8.7 | 25.9±3.9 | 9 |
| controls | 58.6±10.7 | 62 | 2 | 25.7±3.6 | ||||||||
| Yilmaz-Aydogan H | 2011 | Caucasian | Turkey | retrospective | H-B | CAD | cases | 59.22±11.96b | 50.5 | 50.5 | 26.32±4.01b | 10 |
| 57.10±10.93c | 26.55±3.15c | |||||||||||
| controls | 57.03±12.65 | 55.2 | – | 25.33±3.54 | ||||||||
| Shen D | 2005 | Asian | China | retrospective | H-B | CAD | cases | 58.6±11.7 | 60.4 | 0 | 25.5±2.9 | 9 |
| controls | 52.9±13.3 | 60.8 | 0 | 24.3±2.5 | ||||||||
| Li L | 2006 | Asian | China | retrospective | H-B | MI | cases | 64.95±10.79 | 73.4 | – | 24.21±3.54 | 8 |
| controls | 62.1±8.23 | 55.3 | – | 24.57±3.32 | ||||||||
| Rhee EJ | 2007 | Asian | Korea | retrospective | H-B | CAD | cases | 58.8±9.8d | – | – | 25.8±2.7d | 10 |
| 62.1±9.1e | 25.8±2.9e | |||||||||||
| 65.4±8.0f | 24.7±2.9f | |||||||||||
| controls | 55±11.5 | – | – | 25.3±2.9 | ||||||||
| Wu SR | 2007 | Asian | China | retrospective | H-B | CAD | cases | 69.55±10.58 | 65.1 | – | – | 10 |
| controls | 64.76±11.93 | 57.1 | – | – | ||||||||
| wang JJ | 2008 | Asian | China | retrospective | H-B | CAD | cases | – | – | 59.2 | – | 8 |
| controls | – | – | 45.2 | – | ||||||||
| Wang YX | 2009 | Asian | China | retrospective | H-B | CAD | cases | 63.8±7.4 | 58.1 | 100 | 25.1±3.2 | 8 |
| controls | 53.7±10.4 | 54.2 | 100 | 24.9±3.8 | ||||||||
| Ho JS | 2012 | Asian | China | prospective | H-B | CAD | cases | 58(48.5–68) | 43.5 | 100 | 24.9(23.2–26.9) | 9 |
| controls | 46(38–60) | 40.3 | 100 | 24.7(22.3–27.3) | ||||||||
| Ruiz-Narvaez EA | 2007 | Others | Costa Rica | retrospective | P-B | MI | cases | 58±11 | 74 | 25.5 | 26±4.1 | 8 |
| controls | 58±11 | 74 | 14.5 | 26.5±4.2 | ||||||||
| AshokKumar M | 2010 | Others | India | retrospective | P-B | CAD | cases | 53.2±7.8 | 77.8 | 43.7 | 25.8±3.9 | 10 |
| controls | 53.5±8.2 | 74.8 | 12.7 | 24.8±2.8 | ||||||||
P-B, population-based study; H-B, hospital-based study; M(%): male(percent); F: female; T2DM: type 2 diabetes mellitus; BMI: body mass index; a: Data not available; b: diabetes; c: non-diabetes; d: 1-stenotic vessel; e: 2-stenotic vessels; f: 3-stenotic vessels; Age and BMI are expressed as mean ± SD (standard deviation ) or median (5th and 95th percentiles ).
Table 2. The distribution of Pro12Ala genotypes and alleles among cases and controls, and P-values of HWE in controls.
| First Author | sample size | Ala allele, % | Pro allele, % | AlaAla genotype | ProAla genotype | ProPro genotype | HWE, | ||||||
| cases | controls | cases | controls | cases | controls | cases | controls | cases | controls | cases | controls | P value | |
| AshokKumar M | 414 | 424 | 8.7 | 7.3 | 91.3 | 92.7 | 5 | 4 | 62 | 54 | 347 | 366 | 0.21 |
| Bluher M | 201 | 164 | 7.7 | 7.9 | 92.3 | 92.1 | 4 | 2 | 23 | 22 | 174 | 140 | 0.30 |
| Dallongeville J(ADVANCE study) | 1076 | 805 | 12.0 | 12.2 | 88.0 | 87.8 | 12 | 9 | 231 | 174 | 816 | 605 | 0.37 |
| Dallongeville J(PRIME study) | 249 | 494 | 11.0 | 11.5 | 89.0 | 88.5 | 7 | 4 | 40 | 104 | 198 | 378 | 0.28 |
| Evangelisti L | 202 | 295 | 9.0 | 6.0 | 91.0 | 94.0 | 3 | 0 | 30 | 38 | 169 | 258 | 0.24 |
| Ho JS | 108 | 1309 | 0.9 | 2.7 | 99.1 | 97.3 | 0 | 0 | 2 | 71 | 105 | 1229 | 0.31 |
| Li L | 218 | 626 | 5.3 | 3.2 | 94.7 | 96.8 | 0 | 2 | 23 | 36 | 195 | 588 | 0.08 |
| Nassar BA | 300 | 150 | 10.3 | 12.0 | 89.7 | 88.0 | 0 | 0 | 62 | 36 | 238 | 114 | 0.09 |
| Pischon T(NHS study) | 245 | 485 | 12.7 | 10.8 | 87.3 | 89.2 | 4 | 6 | 54 | 93 | 187 | 386 | 0.88 |
| Pischon T(HPFS study) | 250 | 502 | 13.4 | 9.9 | 86.6 | 90.1 | 4 | 4 | 59 | 91 | 187 | 407 | 0.66 |
| Rhee EJ | 150 | 117 | 9.3 | 8.5 | 90.7 | 91.5 | 0 | 0 | 14 | 10 | 136 | 107 | 0.63 |
| Ruiz-Narvaez EA | 1805 | 1805 | 11.0 | 10.0 | 89.0 | 90.0 | 24 | 25 | 341 | 310 | 1440 | 1470 | 0.06 |
| Shen D | 96 | 125 | 6.2 | 3.6 | 93.8 | 96.4 | 1 | 1 | 10 | 7 | 85 | 117 | 0.14 |
| Tobin MD | 547 | 505 | 11.2 | 12.7 | 88.8 | 87.3 | 10 | 4 | 103 | 120 | 434 | 381 | 0.10 |
| Vogel U | 1031 | 1703 | 13.8 | 13.5 | 86.2 | 86.5 | 23 | 27 | 238 | 397 | 770 | 1245 | 0.47 |
| vos HL | 563 | 646 | 13.1 | 11.3 | 86.9 | 88.7 | 21 | 12 | 105 | 122 | 437 | 512 | 0.14 |
| wang JJ | 147 | 219 | 10.5 | 10.0 | 89.5 | 90.0 | 0 | 0 | 31 | 44 | 116 | 175 | 0.10 |
| Wang YX | 258 | 288 | 1.9 | 3.5 | 98.1 | 96.5 | 0 | 1 | 10 | 18 | 248 | 269 | 0.25 |
| Wu SR | 152 | 49 | 5.3 | 3.1 | 94.7 | 96.9 | 0 | 0 | 16 | 3 | 136 | 46 | 0.83 |
| Yilmaz-Aydogan H | 202 | 105 | 6.4 | 8.1 | 93.6 | 91.9 | 0 | 0 | 26 | 17 | 176 | 88 | 0.37 |
| Zafarmand MH | 211 | 1519 | 11.1 | 13.4 | 88.9 | 86.6 | 3 | 30 | 41 | 346 | 167 | 1143 | 0.52 |
| Zee RY | 523 | 2092 | 9.9 | 12.3 | 90.1 | 87.8 | 6 | 31 | 92 | 452 | 425 | 1611 | 0.91 |
| Total | 8948 | 14427 | 10.6 | 10.1 | 89.5 | 89.9 | 127 | 162 | 1613 | 2565 | 7187 | 11644 | |
HWE: Hardy–Weinberg equilibrium. The P-value of HWE determined by the χ2 test or Fisher's exact test in control groups.
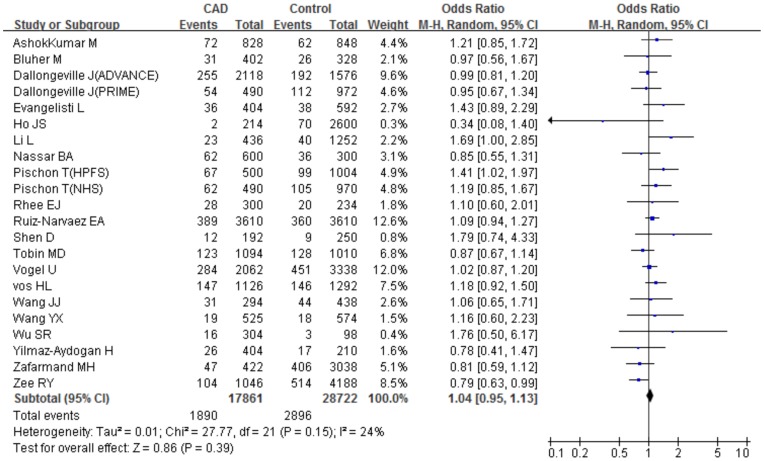
The main results of the meta-analysis and the heterogeneity test were presented in Table 3. For each study, we investigated the association between the PPARγ2 Pro12Ala polymorphism and CAD risk under different genetic models. Overall, We did not detect any significant association under the allele comparison (Ala vs Pro: P = 0.39, OR = 1.04, 95%CI 0.95–1.13, Pheterogeneity = 0.15, I2 = 24%) and under the dominant genetic model with heterogeneity (ProAla+AlaAla vs ProPro: P = 0.88, OR = 1.01, 95%CI 0.91–1.11, Pheterogeneity = 0.06, I2 = 33%). However, a marginal increased risk of CAD under the recessive genetic model (AlaAla vs ProAla+ProPro: P = 0.04, OR = 1.31, 95%CI 1.01–1.69, Pheterogeneity = 0.67, I2 = 0%) and the homozygote comparison (AlaAla vs ProPro: P = 0.04, OR = 1.30, 95%CI 1.01–1.68, Pheterogeneity = 0.68, I2 = 0%) was observed for all the subjects (Figure 2).
Table 3. Summary estimates for ORs and 95% CI in different subgroups under various genetic contrasts.
| Genotype contrasts | Study population | study number, (case/control), n(n/n) | Pheterogeneity | I2,% | P valuea | OR | 95% CI |
| Total studies | |||||||
| Allele comparison | 22(8948/14427) | 0.15 | 24 | 0.39 | 1.04 | 0.95–1.13 | |
| (Ala vs Pro) | |||||||
| Dominant model | 22(8948/14427) | 0.06 | 33 | 0.88 | 1.01 | 0.91–1.11 | |
| (ProAla+AlaAla vs ProPro) | |||||||
| Recessive model | 16(7889/12478) | 0.67 | 0 | 0.04 | 1.31 | 1.01–1.69 | |
| (AlaAla vs ProAla+ProPro) | |||||||
| Homozygote comparison | 16(7889/12478) | 0.68 | 0 | 0.04 | 1.30 | 1.01–1.68 | |
| (ALaAla vs ProPro) | |||||||
| Ethnicity | |||||||
| Allele comparison | Asian | 7(1129/2733) | 0.41 | 3 | 0.11 | 1.24 | 0.96–1.61 |
| Caucasian | 13(5600/9465) | 0.13 | 31 | 0.94 | 1.00 | 0.90–1.10 | |
| Others | 2(2219/2229) | 0.60 | 0 | 0.15 | 1.11 | 0.96–1.27 | |
| Dominant model | Asian | 7(1129/2733) | 0.10 | 43 | 0.56 | 1.13 | 0.75–1.69 |
| Caucasian | 13(5600/9465) | 0.18 | 26 | 0.44 | 0.96 | 0.86–1.07 | |
| Others | 2(2219/2229) | 0.67 | 0 | 0.12 | 1.13 | 0.97–1.31 | |
| Recessive model | Asian | 3(572/1039) | 0.83 | 0 | 0.68 | 0.69 | 0.12–3.9 |
| Caucasian | 11(5098/9210) | 0.48 | 0 | 0.01 | 1.45 | 1.08–1.96 | |
| Others | 2(2219/2229) | 0.69 | 0 | 0.99 | 1.00 | 0.60–1.69 | |
| Homozygote comparison | Asian | 3(572/1039) | 0.82 | 0 | 0.7 | 0.71 | 0.13–4.02 |
| Caucasian | 11(5098/9210) | 0.46 | 0 | 0.02 | 1.44 | 1.07–1.93 | |
| Others | 2(2219/2229) | 0.69 | 0 | 0.92 | 1.03 | 0.61–1.72 | |
| Study design | |||||||
| Allele comparison | prospective | 8(3693/8909) | 0.06 | 48 | 0.79 | 0.98 | 0.85–1.13 |
| retrospective | 14(5255/5518) | 0.55 | 0 | 0.07 | 1.09 | 0.99–1.20 | |
| Dominant model | prospective | 8(3693/8909) | 0.06 | 48 | 0.58 | 0.96 | 0.82–1.12 |
| retrospective | 14(5255/5518) | 0.25 | 18 | 0.39 | 1.06 | 0.93–1.20 | |
| Recessive model | prospective | 7(3585/7600) | 0.47 | 0 | 0.21 | 1.25 | 0.88–1.77 |
| retrospective | 9(4304/4878) | 0.61 | 0 | 0.09 | 1.39 | 0.95–2.01 | |
| Homozygote comparison | prospective | 7(3585/7600) | 0.44 | 0 | 0.24 | 1.23 | 0.87–1.74 |
| retrospective | 9(4304/4878) | 0.65 | 0 | 0.08 | 1.39 | 0.96–2.03 | |
| Population source | |||||||
| Allele comparison | P-B | 11(6306/10274) | 0.10 | 38 | 0.64 | 1.03 | 0.93–1.14 |
| H-B | 11(2642/4153) | 0.33 | 12 | 0.39 | 1.07 | 0.92–1.26 | |
| Dominant model | P-B | 11(6306/10274) | 0.09 | 39 | 0.86 | 1.01 | 0.9–1.13 |
| H-B | 11(2642/4153) | 0.13 | 34 | 0.96 | 1.01 | 0.81–1.25 | |
| Recessive model | P-B | 10(6006/10124) | 0.51 | 0 | 0.24 | 1.19 | 0.89–1.59 |
| H-B | 6(1883/2354) | 0.87 | 0 | 0.03 | 1.85 | 1.07–3.19 | |
| Homozygote comparison | P-B | 10(6006/10124) | 0.49 | 0 | 0.24 | 1.19 | 0.89–1.58 |
| H-B | 6(1883/2354) | 0.88 | 0 | 0.03 | 1.83 | 1.06–3.16 | |
| Endpoint | |||||||
| Allele comparison | CAD | 15(4059/6755) | 0.46 | 0 | 0.5 | 1.04 | 0.93–1.15 |
| ACS | 2(1233/1998) | 0.19 | 41 | 0.45 | 1.12 | 0.84–1.49 | |
| MI | 5(3656/5674) | 0.02 | 67 | 0.78 | 1.03 | 0.85–1.25 | |
| Dominant model | CAD | 15(4059/6755) | 0.27 | 17 | 0.97 | 1.00 | 0.88–1.15 |
| ACS | 2(1233/1998) | 0.29 | 9 | 0.71 | 1.04 | 0.85–1.27 | |
| MI | 5(3656/5674) | 0.01 | 70 | 0.93 | 1.01 | 0.81–1.26 | |
| Recessive model | CAD | 9(3000/4806) | 0.77 | 0 | 0.23 | 1.32 | 0.84–2.07 |
| ACS | 2(1233/1998) | 0.19 | 43 | 0.35 | 2.22 | 0.41–11.91 | |
| MI | 5(3656/5674) | 0.27 | 22 | 0.33 | 1.25 | 0.80–1.97 | |
| Homozygote comparison | CAD | 9(3000/4806) | 0.77 | 0 | 0.23 | 1.32 | 0.84–2.07 |
| ACS | 2(1233/1998) | 0.18 | 44 | 0.36 | 2.26 | 0.40–12.74 | |
| MI | 5(3656/5674) | 0.28 | 21 | 0.35 | 1.24 | 0.79–1.94 |
a:Test for overall effect;P-B: population-based, H-B: hospital-based.
Figure 2. Meta-analysis for the overall association between the PPARγ2 Pro12Ala polymorphism and CAD under the allele comparison (Ala vs Pro).
’Events’ indicates the total number of Ala allele. ‘Total’ indicates the total number of Pro allele plus Ala allele.
Subgroup Analysis
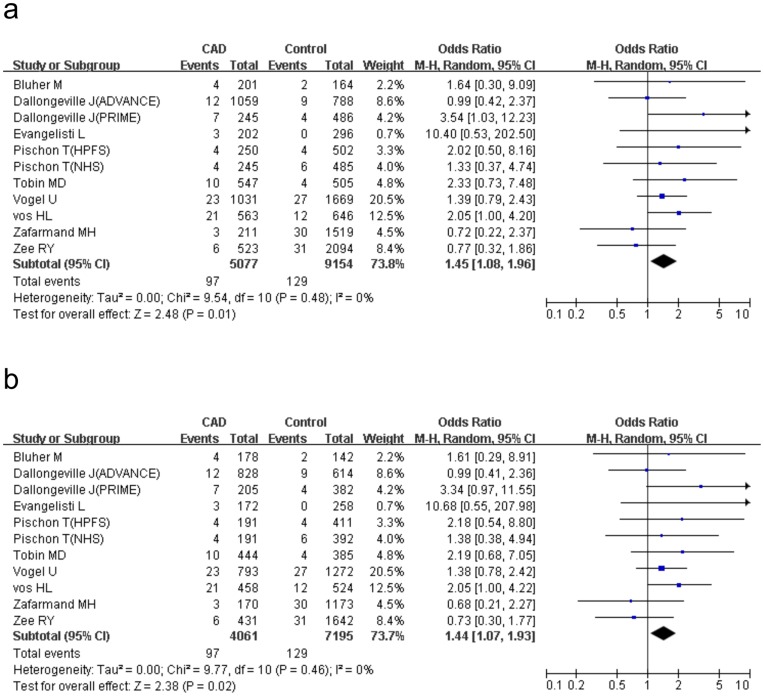
We conducted a series of subgroup analysis on ethnicity, study design, population source and endpoints to explore the potential causes of the heterogeneity (Table 3). Data of all the 22 studies were stratified according to the 3 different ethnic groups: Asian (7 studies involved 1129 cases and 2733 controls), Caucasian (13 studies involved 5600 cases and 9465 controls) and others (1 study recruited Costa Rican and the other recruited Indian). The “others” group contained 2219 cases and 2229 controls. Non-significant association was observed in all ethnic subgroups under the allele comparison and the dominant genetic model. Nevertheless, the significance of the increased CAD risk was augmented among Caucasians under the recessive model (P = 0.01, OR = 1.45, 95%CI 1.08–1.96, Pheterogeneity = 0.48, I2 = 0%) and the homozygote comparison (P = 0.02, OR = 1.44, 95%CI 1.07–1.93, Pheterogeneity = 0.46, I2 = 0%) compared with the overall estimation (Figure 3). In contrast, there was non-significant changes in CAD risk among Asians (recessive model:P = 0.68, OR = 0.69, 95%CI 0.12–3.90, Pheterogeneity = 0.83, I2 = 0% ; homozygote comparison:P = 0.70,OR = 0.71, 95%CI 0.13–4.02, Pheterogeneity = 0.82, I2 = 0%) and mixed-blood population (recessive model:P = 0.99, OR = 1.00, 95%CI 0.60–1.69, Pheterogeneity = 0.69, I2 = 0%;homozygote comparison:P = 0.92,OR = 1.03, 95%CI 0.61–1.72, Pheterogeneity = 0.69, I2 = 0%).
Figure 3. Meta-analysis for the association between PPARγ2 Pro12Ala polymorphism and CAD among Caucasians.
The AlaAla homozygote shows a significant increased risk of CAD under the recessive model (AlaAla vs ProAla+ ProPro, Figure 3a) and under the homozygote comparison (AlaAla vs ProPro, Figure 3b). ‘Events’ indicates the total number of AlaAla genotype. ‘Total’ indicates the total number of AlaAla genotype plus ProAla+ ProPro genotype (Figure 3a) and the total number of AlaAla genotype plus ProPro genotype (Figure 3b) respectively.
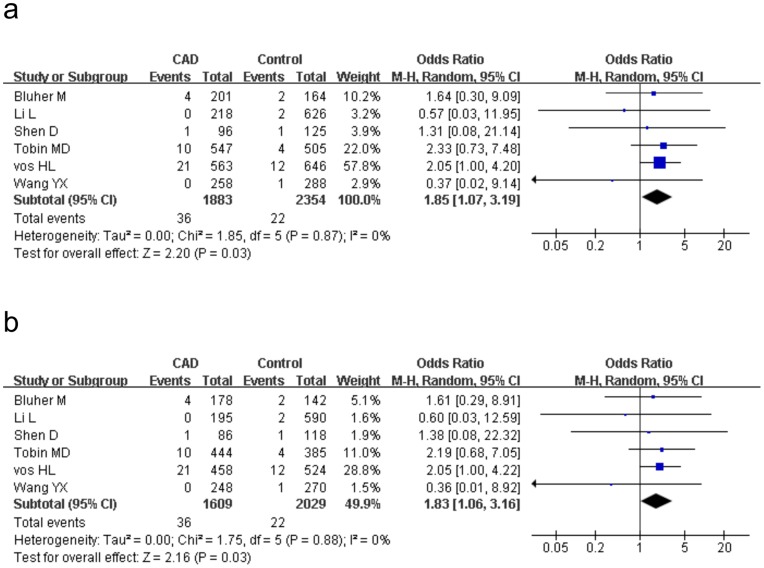
A further subgroup analysis was performed in light of study design. 11 studies included 2642 cases and 4113 controls were H–B and the other half involved 6306 cases and 10274 controls were P-B. After dividing into population source, the CAD risk magnitude of H-B studies was distinctly strengthened under the recessive model (P = 0.03, OR = 1.85, 95%CI 1.07–3.19, Pheterogeneity = 0.87, I2 = 0%) and the homozygote comparison (P = 0.03,OR = 1.83, 95%CI 1.06–3.16, Pheterogeneity = 0.88, I2 = 0%) (Figure 4), whereas in P-B subjects, the lack of remarkable association was found between the PPARγ2 Pro12Ala polymorphism and CAD under all genetic models (allele comparison: P = 0.64, OR = 1.03, 95%CI 0.93–1.14, Pheterogeneity = 0.10, I2 = 38%). Further analysis stratifying on study design (prospective versus retrospective) or endpoints (CAD versus ACS versus MI) yielded no significant association under the four genetic models in all the subgroups.
Figure 4. Meta-analysis for the association between PPARγ2 Pro12Ala polymorphism and CAD in hospital-based studies.
The Pro12Ala polymorphism shows a signification increased risk of CAD under the recessive model (AlaAla vs ProAla+ ProPro, Figure 4a) and under the homozygote comparison (AlaAla vs ProPro, Figure 4b). ‘Events’ indicates the total number of AlaAla genotype. ‘Total’ indicates the total number of AlaAla genotype plus ProAla+ ProPro genotype (Figure 4a) and the total number of AlaAla genotype plus ProPro genotype (Figure 4b) respectively.
Sensitivity Analysis
Sensitivity analysis was performed to estimate the heterogeneity among all the studies in our meta-analysis. We sequentially removed the single study every time to ascertain the cause of heterogeneity. As a result, 2 independent studies (Zee et al. [44] and Pischon [HPFS] et al. [22]) accounted for the major sources of heterogeneity. The overall heterogeneity of the Pro12Ala polymorphism no longer existed when these 2 studies were ruled out respectively in the total analysis under the four genetic models (Pheterogeneity>0.10) and the total effect estimation remained negative. Meanwhile, similar results was also observed in the subsequent subgroup analysis (Pheterogeneity>0.10). Nevertheless, there was not any single study influencing the pooled ORs significantly in any subgroups.
Cumulative Analysis
There was no remarkable evidence suggesting that the first published study had significant impact on the subsequent publication by the cumulative meta-analysis (data not shown).
Meta-regression Analysis
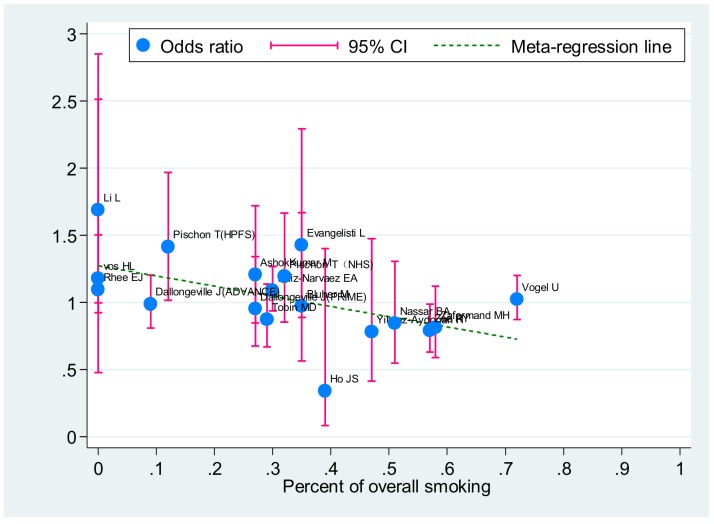
The meta-regression was a feasible scenario to identify the further source of heterogeneity. The mean or median value of age and BMI and the proportion of male, smoking and T2DM were involved in the meta-regression. Among these variable, the association of Pro12Ala polymorphism with CAD risk was shown with a low smoking rate under the allele comparison (correlation coefficient:−0.53, P = 0.02) (Figure 5).
Figure 5. Meta-regression of overall smoking percent on in-allele risk estimates of PPARγ2 Pro12Ala polymorphism.
For each study, OR is shown by the middle of the blue solid circle whose upper and lower extremes represent the corresponding 95%CI. OR values were calculated for the smokers against non-smokers when available. The green dotted line is plotted by fitting OR and overall smoking percent for the included studies.
Publication Bias
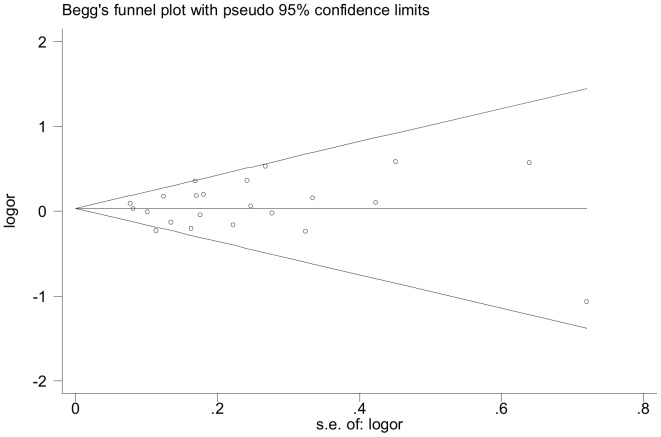
The funnel plot was applied for allele comparison in the OR analysis of the PPARγ2 Pro12Ala polymorphism to evaluate the publication bias of the literatures. There was no evidence for remarkable publication bias of the Pro12Ala polymorphism (t = −0.12, P = 0.91 for Ala vs Pro) confirmed by the Egger’s test and Begg’s funnel plot (Figure 6).
Figure 6. Begg’s funnel plot analysis to detect publication bias for allele comparison (Ala vs Pro) of the Pro12Ala polymorphism.
Discussion
The definite association of PPARγ2 Pro12Ala polymorphism with CAD risk is not precisely clarified yet. Although some studies have been performed to connect PPARγ2 Pro12Ala polymorphism with CAD, the results remained conflicting. The varying results may be explained by a relatively small size of samples and the limited statistical power of single studies but may also be explained by different distributions of potential effect modifiers in the study populations. To our knowledge, this is one of the largest systematic reviews of the literatures via a meta-analysis to investigate the relationship between the PPARγ2 common polymorphism and the potential risk of CAD. Overall results verified that the AlaAla homozygote of PPARγ2 Pro12Ala polymorphism might have marginal significant increased risk of CAD in a recessive inherited pattern. Our result is subtly different from a previous meta-analysis containing 6898 CAD cases and 11287 controls by Dallongeville et al. [36], which revealed that the PPAPγ2 Pro12Ala polymorphism had a borderline non-significant increased risk of CAD (P = 0.06, OR = 1.29, 95%CI 0.99–1.67) under the recessive genetic model. The nuance is probably due to the restricted sample size.
Genetic heterogeneity is inevitable in disease identification strategy [55] and subgroup analysis determined ethnicity as a potential cause of between-study difference. We found that the association of the PPARγ2 Pro12Ala polymorphism with CAD risk was different between Caucasians and other ethnic groups. The AlaAla genotype carriers showed a significant 45% risk increase among Caucasians, whereas the significance was lack among Asians and other mixed-blood population. Our result indicated the Pro12Ala polymorphism might be increased risk conferring locus for CAD in Caucasian population, but not in Asian and other population. The consensus has not been reached, suggesting the racial genetic diversity of the PPARγ2 Pro12Ala polymorphism plays an important role in the etiology of atherosclerosis across various ethnic populations. It also should be noticed that the Pro12Ala polymorphism was hypervariable between different ancestries [56] and might have subtle influences on the result of case-control studies.
Apart from the dramatic impact of ethnicity on total evaluation, another estimate should be treated with caution when studies were stratified by population source. Risk increase given by the AlaAla homozygote in the H-B studies seemed to differ from that in P-B studies, being 85% in H-B studies and 19% in P-B studies under the recessive model. Risk increase was significant in H-B studies but not in P-B studies. Besides the relatively small sample size, population classification was still problematic [57]. Despite high participation and less information bias may favor H-B studies, the weakness of H-B studies is ineluctable. Hospital controls are derived from different source population and partly represent the general population in the study region. In addition, the possibility of biased case-control comparisons should be taken into account when controls were selected from a ill-related study base [43] and could not accurately reflect the exposure experience of the real source population. By contrast, the controls sampled from community or general population are largely regarded as being more advisable than those from hospital for reasons of representativeness. Considering a wide range of confidence intervals of in the H-B subgroup analysis, further studies are called for to ascertain the reliability of effect size.
Furthermore, our meta-regression analysis found out a link of the PPARγ2 Pro12Ala polymorphism with CAD risk in population with lower smoking proportion. Considering smoking is a major risk factor of CAD [58], our result implied potential interaction of the Pro12Ala genotype with environment factors.
Although our meta-analysis included relatively large sample size consistent of HWE, there are some methodological limitations should be noticed [59]. The literature bias is a latent issue. Because small negative studies are less likely to be accepted to publish and the articles in languages other than English and Chinese were excluded, the possibility of language bias cannot be ruled out completely, even though the Egger’s test and funnel plots did not provide any evidence of publication bias in our meta-analysis. Although simulation studies of funnel plots have documented publication bias may be inferred by mistake if heterogeneity of the studies is present [60], there is still no gold standard against the methods to compare the results of funnel plot tests and Egger’s test [61].
A majority of in vivo studies showed PPARγ2 exerts direct and indirect anti-inflammatory effects in the arterial cells of the vascular wall. PPARγ2 activation reduces the production of macrophage and lymphocyte cytokine, inhibits the growth, proliferation [62], [63], and migration of vascular smooth muscle cell as well as restrains the expression of endothelial cell adhesion molecule, chemokine, and matrix metalloproteinase [64]. All of these evidences suggested that PPARγ2 is benefit to prevent the initiation of atherosclerosis [65]. Nevertheless, clinical studies determining the role of the PPARγ2 Pro12Ala polymorphism in CAD are scarce. Our meta-analysis complements the evidences that the Pro12Ala polymorphism, a loss of PPARγ2 function mutation, may exert pleiotropic and deleterious effects in the development of atherosclerosis.
In conclusion,our meta-analysis, comprising 23375 participants,implies that homozygosity of the Ala allele might have a potential increased risk of CAD. The effect is at odds, being stronger in Caucasians and barely significant in Asians. Our meta-analysis also emphasizes the necessity of great caution when trying to interpret and reconcile data observed in different ethnic population. More prospective registered studies are helpful to confirm or refuse the present association.
Supporting Information
Criteria of quality assessment for genetic association of the PPARγ2 gene Pro12Ala polymorphism with CAD.
(DOC)
PRISMA Checklist.
(DOC)
Acknowledgments
We really appreciate and thank Dr Ho JS and Dr Chan JC for providing us the additional information and data of their research.
Funding Statement
This work was supported by the Shanghai Excellent Young Teachers Training Fund of Colleges and Universities (jdy09097) and the Science and Technology Fund of Shanghai Jiao Tong University School of Medicine (11XJ21001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ross R (1999) Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 2. Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B (2002) The role of PPARs in atherosclerosis. Trends Mol Med 8: 422–430. [DOI] [PubMed] [Google Scholar]
- 3. Fisman EZ, Tenenbaum A (2009) A cardiologic approach to non-insulin antidiabetic pharmacotherapy in patients with heart disease. Cardiovasc Diabetol 8: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, et al. (2006) International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58: 726–741. [DOI] [PubMed] [Google Scholar]
- 5. Wahli W, Michalik L (2012) PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab 23: 351–363. [DOI] [PubMed] [Google Scholar]
- 6. Meirhaeghe A, Amouyel P (2004) Impact of genetic variation of PPARgamma in humans. Mol Genet Metab 83: 93–102. [DOI] [PubMed] [Google Scholar]
- 7. Yen CJ, Beamer BA, Negri C, Silver K, Brown KA, et al. (1997) Molecular scanning of the human peroxisome proliferator activated receptor gamma (hPPAR gamma) gene in diabetic Caucasians: identification of a Pro12Ala PPAR gamma 2 missense mutation. Biochem Biophys Res Commun 241: 270–274. [DOI] [PubMed] [Google Scholar]
- 8. Tonjes A, Stumvoll M (2007) The role of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma in diabetes risk. Curr Opin Clin Nutr Metab Care 10: 410–414. [DOI] [PubMed] [Google Scholar]
- 9. Montagner A, Rando G, Degueurce G, Leuenberger N, Michalik L, et al. (2011) New insights into the role of PPARs. Prostaglandins Leukot Essent Fatty Acids 85: 235–243. [DOI] [PubMed] [Google Scholar]
- 10. Iwata E, Yamamoto I, Motomura T, Tsubakimori S, Nohnen S, et al. (2003) The association of Pro12Ala polymorphism in PPARgamma2 with lower carotid artery IMT in Japanese. Diabetes Res Clin Pract 62: 55–59. [DOI] [PubMed] [Google Scholar]
- 11. Temelkova-Kurktschiev T, Hanefeld M, Chinetti G, Zawadzki C, Haulon S, et al. (2004) Ala12Ala genotype of the peroxisome proliferator-activated receptor gamma2 protects against atherosclerosis. J Clin Endocrinol Metab 89: 4238–4242. [DOI] [PubMed] [Google Scholar]
- 12. Al-Shali KZ, House AA, Hanley AJ, Khan HM, Harris SB, et al. (2004) Genetic variation in PPARG encoding peroxisome proliferator-activated receptor gamma associated with carotid atherosclerosis. Stroke 35: 2036–2040. [DOI] [PubMed] [Google Scholar]
- 13. Gouda HN, Sagoo GS, Harding AH, Yates J, Sandhu MS, et al. (2010) The association between the peroxisome proliferator-activated receptor-gamma2 (PPARG2) Pro12Ala gene variant and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol 171: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang H, Zhu S, Chen J, Tang Y, Hu H, et al. (2012) Peroxisome Proliferator-Activated Receptor gamma Polymorphism Pro12Ala Is Associated With Nephropathy in Type 2 Diabetes: Evidence from meta-analysis of 18 studies. Diabetes Care 35: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Namvaran F, Azarpira N, Rahimi-Moghaddam P, Dabbaghmanesh MH (2011) Polymorphism of peroxisome proliferator-activated receptor gamma (PPARgamma) Pro12Ala in the Iranian population: relation with insulin resistance and response to treatment with pioglitazone in type 2 diabetes. Eur J Pharmacol 671: 1–6. [DOI] [PubMed] [Google Scholar]
- 16. Chen Z, Vigueira PA, Chambers KT, Hall AM, Mitra MS, et al. (2012) Insulin Resistance and Metabolic Derangements in Obese Mice Are Ameliorated by a Novel Peroxisome Proliferator-activated Receptor gamma-sparing Thiazolidinedione. J Biol Chem 287: 23537–23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurnell M (2003) PPARgamma and metabolism: insights from the study of human genetic variants. Clin Endocrinol (Oxf) 59: 267–277. [DOI] [PubMed] [Google Scholar]
- 18.Vos HL, Doggen C, Rosendaal FR (2000) Effect of the PPAR gamma2 Pro12Ala polymorphism on the risk of myocardial infarction. Blood 96.
- 19. Ridker PM, Cook NR, Cheng S, Erlich HA, Lindpaintner K, et al. (2003) Alanine for proline substitution in the peroxisome proliferator-activated receptor gamma-2 (PPARG2) gene and the risk of incident myocardial infarction. Arterioscler Thromb Vasc Biol 23: 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doney AS, Fischer B, Leese G, Morris AD, Palmer CN (2004) Cardiovascular risk in type 2 diabetes is associated with variation at the PPARG locus: a Go-DARTS study. Arterioscler Thromb Vasc Biol 24: 2403–2407. [DOI] [PubMed] [Google Scholar]
- 21. Tobin MD, Braund PS, Burton PR, Thompson JR, Steeds R, et al. (2004) Genotypes and haplotypes predisposing to myocardial infarction: a multilocus case-control study. Eur Heart J 25: 459–467. [DOI] [PubMed] [Google Scholar]
- 22. Pischon T, Pai JK, Manson JE, Hu FB, Rexrode KM, et al. (2005) Peroxisome proliferator-activated receptor-gamma2 P12A polymorphism and risk of coronary heart disease in US men and women. Arterioscler Thromb Vasc Biol 25: 1654–1658. [DOI] [PubMed] [Google Scholar]
- 23. Li L, Cheng LX, Nsenga R, He MA, Wu TC (2006) Association between Pro12Ala polymorphism of peroxisome proliferator-activated receptor-gamma 2 and myocardial infarction in the Chinese Han population. Clin Cardiol 29: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, et al. (2011) 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 57: e215–367. [DOI] [PubMed] [Google Scholar]
- 25. Joensen AM, Jensen MK, Overvad K, Dethlefsen C, Schmidt E, et al. (2009) Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol 62: 188–194. [DOI] [PubMed] [Google Scholar]
- 26. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, et al. (2011) 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123: e426–579. [DOI] [PubMed] [Google Scholar]
- 27. Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, et al. (2005) Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 162: 201–211. [DOI] [PubMed] [Google Scholar]
- 28. Cohn LD, Becker BJ (2003) How meta-analysis increases statistical power. Psychol Methods 8: 243–253. [DOI] [PubMed] [Google Scholar]
- 29. Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palanichamy MG, Sun C, Agrawal S, Bandelt HJ, Kong QP, et al. (2004) Phylogeny of mitochondrial DNA macrohaplogroup N in India, based on complete sequencing: implications for the peopling of South Asia. Am J Hum Genet 75: 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quintana-Murci L, Chaix R, Wells RS, Behar DM, Sayar H, et al. (2004) Where west meets east: the complex mtDNA landscape of the southwest and Central Asian corridor. Am J Hum Genet 74: 827–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dragojevič J, Marc J, Mlinar B (2008) Association between Pro12Ala and His477His polymorphisms in PPARG gene and insulin resistance in patients with polycystic ovary syndrome. Biochemia Medica 18: 342–350. [Google Scholar]
- 34. Reich D, Thangaraj K, Patterson N, Price AL, Singh L (2009) Reconstructing Indian population history. Nature 461: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kabagambe EK, Baylin A, Allan DA, Siles X, Spiegelman D, et al. (2001) Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol 154: 1126–1135. [DOI] [PubMed] [Google Scholar]
- 36. Dallongeville J, Iribarren C, Ferrieres J, Lyon L, Evans A, et al. (2009) Peroxisome proliferator-activated receptor gamma polymorphisms and coronary heart disease. PPAR Res 2009: 543746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aydogan HY, Kucukhuseyin O, Tekeli A, Isbir T (2012) Associations of receptor for advanced glycation end products -374 T/A and Gly82 Ser and peroxisome proliferator-activated receptor gamma Pro12Ala polymorphisms in Turkish coronary artery disease patients. Genet Test Mol Biomarkers 16: 134–137. [DOI] [PubMed] [Google Scholar]
- 39. Yilmaz-Aydogan H, Kurnaz O, Kur O, Akadam-Teker B, Kucukhuseyin O, et al. (2011) Effects of the PPAR-gamma and apolipoprotein E gene polymorphisms on clinical and lipid characteristics in patients with diabetic and non-diabetic coronary heart disease. In Vivo 25: 513–514. [Google Scholar]
- 40. Kurnaz O, Yilmaz-Aydogan H, Isbir S, Isbir T (2011) The effects of PPAR-γ PR012ALA and LOX-1 K167N variants on Turkish coronary artery disease patients. In Vivo 25: 539. [Google Scholar]
- 41.Ma RC, Ho JS, Tam CH, Wang Y, Lee HM, et al. (2011) Polymorphism of the PPAR-γ2 gene predicts development of coronary heart disease among chinese subjects with type 2 diabetes. Diabetes 60 (A557).
- 42. Yilmaz-Aydogan H, Kurnaz O, Kurt O, Akadam-Teker B, Kucukhuseyin O, et al. (2011) Effects of the PPARG P12A and C161T gene variants on serum lipids in coronary heart disease patients with and without Type 2 diabetes. Mol Cell Biochem 358: 355–363. [DOI] [PubMed] [Google Scholar]
- 43.Ho JS, Germer S, Tam CH, So WY, Martin M, et al. (2012) Association of the PPARG Pro12Ala polymorphism with type 2 diabetes and incident coronary heart disease in a Hong Kong Chinese population. Diabetes Res Clin Pract. [DOI] [PubMed]
- 44. Zee RY, Cook NR, Cheng S, Erlich HA, Lindpaintner K, et al. (2006) Multi-locus candidate gene polymorphisms and risk of myocardial infarction: a population-based, prospective genetic analysis. J Thromb Haemost 4: 341–348. [DOI] [PubMed] [Google Scholar]
- 45.Wang LP, Zhao LR, Cui HW, Yan MR, Yang L, et al. (2012) Association between PPARgamma2 Pro12Ala polymorphism and myocardial infarction and obesity in Han Chinese in Hohhot, China. Genet Mol Res 11. [DOI] [PubMed]
- 46. Galgani A, Valdes A, Erlich HA, Mano C, Cheng S, et al. (2010) Homozygosity for the Ala allele of the PPARgamma2 Pro12Ala polymorphism is associated with reduced risk of coronary artery disease. Dis Markers 29: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AshokKumar M, Veera Subhashini NG, Kanthimathi S, SaiBabu R, Ramesh A, et al. (2010) Associations for lipoprotein lipase and peroxisome proliferator-activated receptor-gamma gene and coronary artery disease in an Indian population. Arch Med Res 41: 19–25 e11. [DOI] [PubMed]
- 48. Bluher M, Klemm T, Gerike T, Krankenberg H, Schuler G, et al. (2002) Lack of association between peroxisome proliferator-activated receptor-gamma-2 gene variants and the occurrence of coronary heart disease in patients with diabetes mellitus. Eur J Endocrinol 146: 545–551. [DOI] [PubMed] [Google Scholar]
- 49. Evangelisti L, Attanasio M, Lucarini L, Sofi F, Marcucci R, et al. (2009) PPARgamma promoter polymorphisms and acute coronary syndrome. Atherosclerosis 205: 186–191. [DOI] [PubMed] [Google Scholar]
- 50. Nassar BA, Rockwood K, Kirkland SA, Ransom TP, Darvesh S, et al. (2006) Improved prediction of early-onset coronary artery disease using APOE epsilon4, BChE-K, PPARgamma2 Pro12 and ENOS T-786C in a polygenic model. Clin Biochem 39: 109–114. [DOI] [PubMed] [Google Scholar]
- 51. Rhee EJ, Kwon CH, Lee WY, Kim SY, Jung CH, et al. (2007) No association of Pro12Ala polymorphism of PPAR-gamma gene with coronary artery disease in Korean subjects. Circ J 71: 338–342. [DOI] [PubMed] [Google Scholar]
- 52. Ruiz-Narvaez EA, Kraft P, Campos H (2007) Ala12 variant of the peroxisome proliferator-activated receptor-gamma gene (PPARG) is associated with higher polyunsaturated fat in adipose tissue and attenuates the protective effect of polyunsaturated fat intake on the risk of myocardial infarction. Am J Clin Nutr 86: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 53. Vogel U, Segel S, Dethlefsen C, Tjonneland A, Saber AT, et al. (2009) PPARgamma Pro12Ala polymorphism and risk of acute coronary syndrome in a prospective study of Danes. BMC Med Genet 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zafarmand MH, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML (2008) Peroxisome proliferator-activated receptor gamma-2 P12A polymorphism and risk of acute myocardial infarction, coronary heart disease and ischemic stroke: a case-cohort study and meta-analyses. Vasc Health Risk Manag 4: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hemminki K, Lorenzo Bermejo J, Forsti A (2006) The balance between heritable and environmental aetiology of human disease. Nat Rev Genet 7: 958–965. [DOI] [PubMed] [Google Scholar]
- 56. Fullerton SM, Yu JH, Crouch J, Fryer-Edwards K, Burke W (2010) Population description and its role in the interpretation of genetic association. Hum Genet 127: 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salanti G, Sanderson S, Higgins JP (2005) Obstacles and opportunities in meta-analysis of genetic association studies. Genet Med 7: 13–20. [DOI] [PubMed] [Google Scholar]
- 58. Castelli WP, Garrison RJ, Dawber TR, McNamara PM, Feinleib M, et al. (1981) The filter cigarette and coronary heart disease: the Framingham study. Lancet 2: 109–113. [DOI] [PubMed] [Google Scholar]
- 59. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 60. Terrin N, Schmid CH, Lau J, Olkin I (2003) Adjusting for publication bias in the presence of heterogeneity. Stat Med 22: 2113–2126. [DOI] [PubMed] [Google Scholar]
- 61. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stumvoll M, Haring H (2002) The peroxisome proliferator-activated receptor-gamma2 Pro12Ala polymorphism. Diabetes 51: 2341–2347. [DOI] [PubMed] [Google Scholar]
- 63. Wang N, Yin R, Liu Y, Mao G, Xi F (2011) Role of peroxisome proliferator-activated receptor-gamma in atherosclerosis: an update. Circ J 75: 528–535. [DOI] [PubMed] [Google Scholar]
- 64. Huang W, Andras IE, Rha GB, Hennig B, Toborek M (2011) PPARalpha and PPARgamma protect against HIV-1-induced MMP-9 overexpression via caveolae-associated ERK and Akt signaling. FASEB J 25: 3979–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qu A, Shah YM, Manna SK, Gonzalez FJ (2012) Disruption of endothelial peroxisome proliferator-activated receptor gamma accelerates diet-induced atherogenesis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol 32: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Criteria of quality assessment for genetic association of the PPARγ2 gene Pro12Ala polymorphism with CAD.
(DOC)
PRISMA Checklist.
(DOC)