Abstract
Zfhx2 (also known as zfh-5) encodes a transcription factor containing three homeobox domains and 18 Zn-finger motifs. We have reported that Zfhx2 mRNA is expressed mainly in differentiating neurons in the mouse brain and its expression level is negatively regulated by the antisense transcripts of Zfhx2. Although the expression profile of Zfhx2 suggests that ZFHX2 might have a role in a particular step of neuronal differentiation, the specific function of the gene has not been determined. We generated a Zfhx2-deficient mouse line and performed a comprehensive battery of behavioral tests to elucidate the function of ZFHX2. Homozygous Zfhx2-deficient mice showed several behavioral abnormalities, namely, hyperactivity, enhanced depression-like behaviors, and an aberrantly altered anxiety-like phenotype. These behavioral phenotypes suggest that ZFHX2 might play roles in controlling emotional aspects through the function of monoaminergic neurons where ZFHX2 is expressed. Moreover, considering their phenotypes, the Zfhx2-deficient mice may provide a novel model of human psychiatric disorders.
Introduction
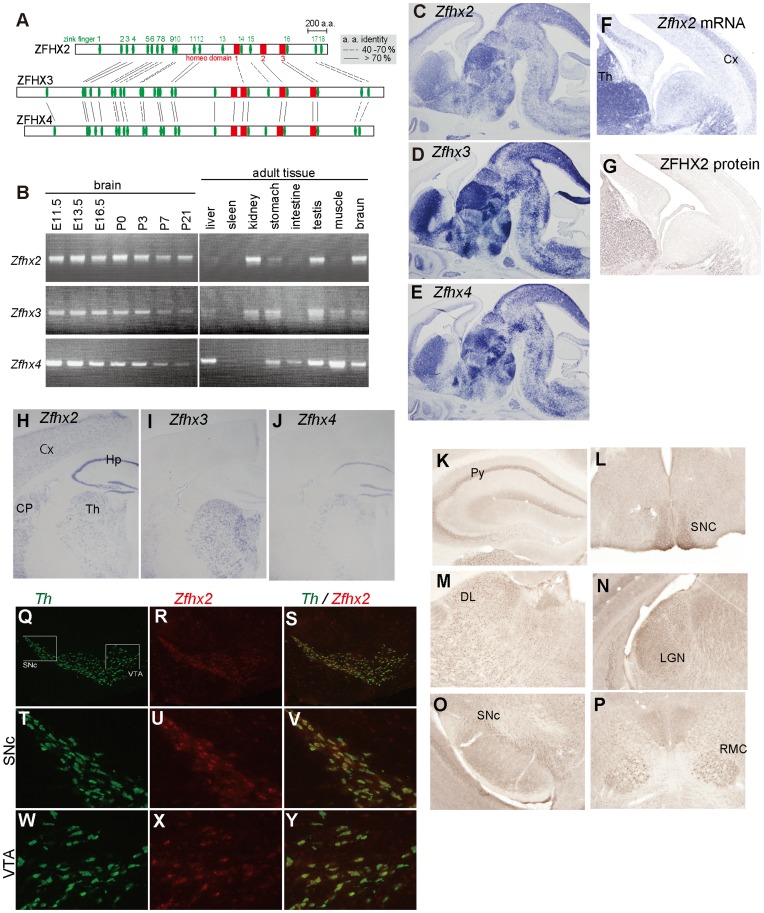
Mammalian Zfhx2 (also known as zfh-5) encodes a transcription factor of unknown function containing three homeobox domains and 18 Zn-finger motifs. Zfhx2 is highly expressed in the developing mouse brain particularly in differentiating neurons and continues to be expressed throughout adulthood at a low level [1]. Two other phylogenically related genes, Zfhx3 (atbf1) and Zfhx4 (zfh-4), have been identified in the Zfhx family [2], the members of which contain three to four homeobox domains and 18 to 23 Zn-finger motifs (Fig. 1A). Zfhx3 is expressed in the developing brain in a manner dependent on neuronal differentiation [3] and was also reported to be relevant in the malignancy of some classes of cancer [4], [5]. Zfhx4 is a candidate gene causing congenital bilateral isolated ptosis [6]. Although these three genes are expressed in substantially similar patterns in the developing brain (Figs. 1C–E), common functional features have not been clarified.
Figure 1. Structure and expression of mouse Zfhx2.
(A) Structure of ZFHX2 and related proteins. ZFHX2 is a protein of 2562 amino acids containing 18 zinc fingers (green ovals) and three homeodomains (red squares). (B) Zfhx2, Zfhx3, and Zfhx4 mRNA detected by semi-quantitative RT-PCR in various RNA sources. Note that cDNAs were amplified for 30 cycles for brains of different developmental stages, whereas for 35 cycles for various adult tissues. (C–E) Expression of Zfhx2 (C), Zfhx3 (D), and Zfhx4 (E) mRNA in the parasagittal sections of an E15.5 mouse brain. These three genes were expressed in substantially similar patterns with the highest expression level of Zfhx3. (F, G) Expression of Zfhx2 mRNA (F) and the ZFHX2 protein (G) was compared on adjacent coronal sections of an E15.5 brain. mRNA expressed in the thalamic region (Th) was translated, whereas mRNA expressed in the cerebral cortex (Cx) was not translated: this situation made the expression patterns of ZFHX2 and ZFHX3 more alike in the protein level than in the mRNA level. (H–J) Expression of Zfhx2 (H), Zfhx3 (I), and Zfhx4 (J) mRNA in the coronal sections of an adult brain. Cerebral cortex (Cx), hippocampus (Hp), thalamus (Th), caudate putamen (CP). Expression levels of all three genes were decreased compared with those in the embryonic brain, but Zfhx2 maintained higher level of expression than the others. (K–P) Expression of ZFHX2 protein in the adult brain. The pyramidal layer of hippocampus (K, Py), the suprachiasmatic nucleus (L, SCN), laterodorsal thalamic nucleus (M, LD), lateral geniculate nucleus (N, LGN), substantia nigra pars compacta (O, SNc), and magnocellular part of the red nucleus suprachiasmic (P, RMC). (Q–Y) Double-color in situ hybridization with Zfhx2 and tyrosine hydroxylase (Th) probes. The Zfhx2 mRNA (red) was highly expressed in the Th mRNA (green)-positive cells in the substantia nigra pars compacta (SNc). Zfhx2 mRNA was coexpressed with Th mRNA also in the ventral tegmental area (VTA) at a slightly lower level.
Previously, we found that the antisense strand of Zfhx2 is also expressed in the mouse brain in a manner complementary to the expression of Zfhx2 mRNA. Although most neurons express Zfhx2 mRNA immediately after their final mitosis, several types of neuron (e.g., granule cells in the olfactory bulb and pyramidal and granule cells in the hippocampus) express antisense RNA prior to Zfhx2 mRNA during the early phase of their differentiation. By generating a gene-targeting mouse line in which Zfhx2 sense RNA is expressed but not antisense RNA, we showed that this antisense RNA has a negative regulatory role in the expression of Zfhx2 mRNA. These observations suggest that the ZFHX2 protein might have a role in a particular step of neuronal differentiation, and in some types of neuron, this step might be delayed by the expression of antisense RNA [1]. The specific function of ZFHX2, however, remains to be revealed.
To elucidate the function of ZFHX2, we have generated a Zfhx2-deficient mouse line. Although the production of the ZFHX2 protein is completely abolished in the homozygous mutant mice, the mice appear grossly normal and healthy. No anatomical abnormality has been observed in the mutant mouse brain so far examined. We hence subjected the Zfhx2-deficient mice to a comprehensive battery of behavioral tests [7], [8] to explore the physiological function of ZFHX2 in the nervous system. The homozygous Zfhx2 deficient mice showed several behavioral abnormalities particularly in emotional aspects, such as a depression-like phenotype. In this paper, we report the behavioral phenotypes of the Zfhx2-deficient mice and discuss their possible relevance to human psychiatric disorders.
Results
Expression Profile of Zfhx2
The expression profile of Zfhx2 mRNA and the ZFHX2 protein analyzed by reverse-transcriptase (RT)-PCR, in situ hybridization, and immunohistochemistry was summarized in Figure 1, together with those of the related genes, Zfhx3 and Zfhx4. All three genes were expressed in various structures of the developing brain, and continued to be expressed throughout adulthood at lower expression levels. The expression profiles of these genes will be discussed again in the later section.
General Characteristics of Zfhx2-deficient Mice
The Zfhx2-deficient mice appear grossly normal and healthy. No anatomical abnormality has been observed in the mutant mouse brain so far examined (data not shown). We subjected the Zfhx2-deficient mice and their wild-type littermates to a comprehensive battery of behavior tests to determine whether Zfhx2 deficiency causes behavioral alternations (summarized in Table 1). The physical and neurological characteristics (i.e., body weight, body temperature, state of whiskers and fur, neurological reflexes, and muscle strength) were normal in the Zfhx2-deficient mice as compared with the control mice (Figs. S1A–D).
Table 1. List of behavioral tests.
| Group | Test | Age * | Graphs | Descriptions ** | Phenotypes of mutant mice suggested |
| 1st | General health/neurological screen | 11 | Figs. S1A–D | Results-2 | |
| Light/dark transition | 11 | Figs. 3C–F | Results-3 | hypoactivity in novel environments | |
| Open field | 12 | Figs. 2A–E | Results-3 | hyperactivity, hypoactivity in novel environments, unusual anxiety | |
| Elevated plus maze | 12 | Figs. S2A–D | Results-6 | ||
| Hot plate | 12 | Fig. S1F | Results-6 | ||
| Social interaction (novel environment) | 13 | Figs. S3A–D | Results-6 | ||
| Rotarod | 13 | Fig. S1E | Results-6 | ||
| Social interaction (Crawly version) | 14 | Figs. S3E–H | Results-6 | ||
| Prepulse inhibition/startle response | 14 | Figs. 5A, B | Results-6 | ||
| Porsolt forced swim | 15 | Figs. 4A, B | Results-4 | depression-like behavior | |
| Gait analysis | 15 | Figs. S4A, B | Results-6 | ||
| Barnes maze | 24 | Figs. 5A–C | Results-5 | ||
| Cued and contextual fear conditioning | 27 | Figs. S2E–G | Results-6 | ||
| Tail suspension | 29 | Fig. 4C | Results-4 | depression-like behavior | |
| 24-h homecage monitoring | 48 | Figs. 3A, B | Results-3, 6 | hyperactivity | |
| 2nd | General health/neurological screen | 12 | |||
| Light/dark transition | 13 | Fig. S6D | Results-3 | hypoactivity in novel environments | |
| Open field | 14 | Figs. S6A, B | Results-3 | hyperactivity, hypoactivity in novel environments, unusual anxiety | |
| 24-h homecage monitoring, Circadian rhythm | 24 | Figs. S5A, B, S6C | Results-3, 6 | hyperactivity |
; Age (weeks old) of the youngest animals of the group at the start of the test. The oldest animals are 3 weeks (1st group) or 4 weeks (2nd group) older than the age indicated.
; Sections in the text where the results are described. Subsections of Results are; 2, General characteristics of Zfhx2-deficient mice; 3, Locomotor activity and anxiety-like behavior; 4, Depression-like behavior; 5, Sensorimotor gating; 6, Spatial reference memory; 7, Other behavioral tests.
Locomotor Activity and Anxiety-like Behavior
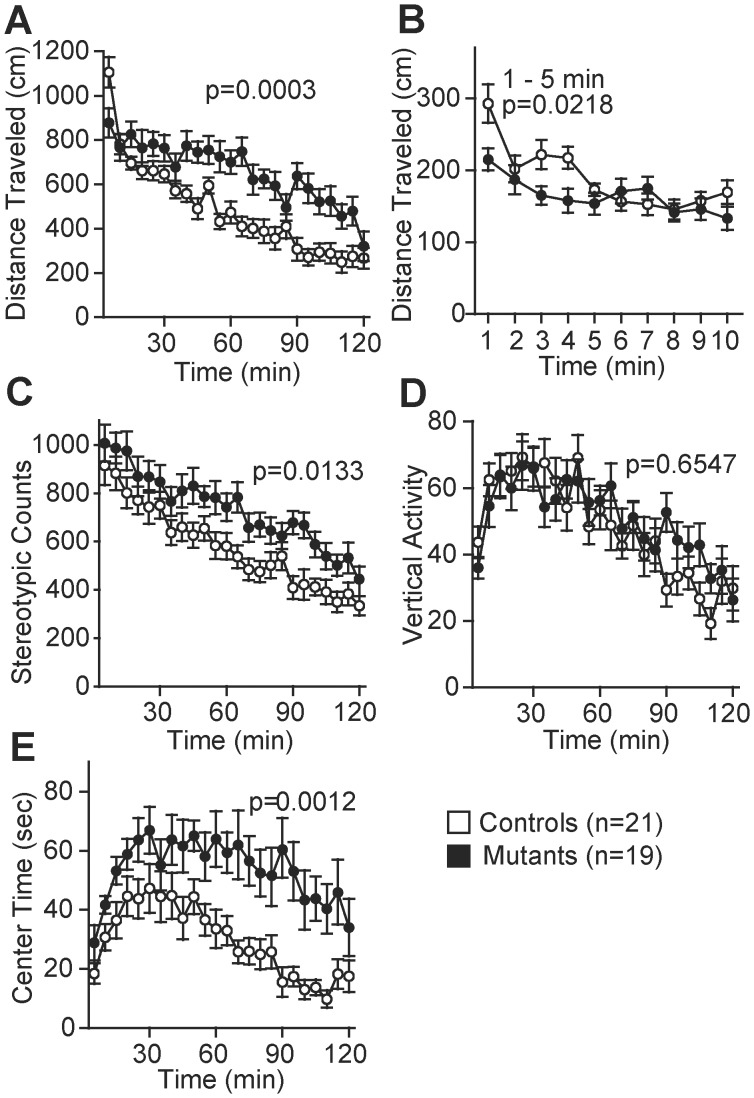
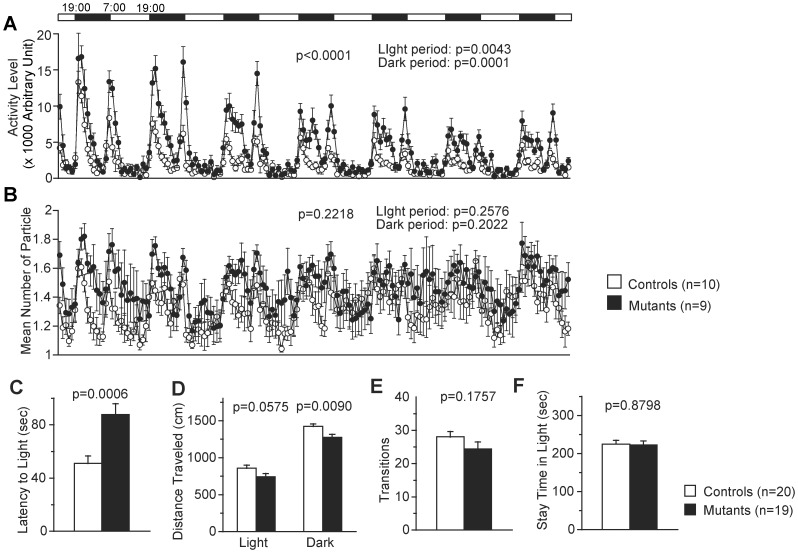
In the open field test, the distance traveled (F 1,38 = 15.999, p = 0.0003, Fig. 2A) and stereotypic counts (F 1,38 = 6.737, p = 0.0133, Fig. 2C) of the mutant mice during the entire 120-min period were significantly higher than those of the control mice suggesting a hyperactive phenotype of the Zfhx2-deficient mice. This phenotype was confirmed by the activity level measured in the 24-h homecage monitoring test (light period, F 1,17 = 10.828, p = 0.0043; dark period, F 1,17 = 25.235, p = 0.0001; total, F 1,17 = 25.645, p<0.0001, Fig. 3A). In the open field, however, the pattern of temporal change in activity of the mutant mice was different from that of the control mice (genotype × time interaction, F 23,874 = 3.877, p<0.0001). In the first 5 min, the mutant mice were less active than the control mice (F 1,38 = 5.724, p = 0.0218, Fig. 2B). This observation was consistent with the following two results of the light/dark transition test: the mutant mice demonstrated a longer latency to enter into the light chamber for the first time (F 1,37 = 14.015, p = 0.0006, Fig. 3C) and traveled a shorter distance in 10 min than the control mice, although the difference in the distance traveled in the light chamber did not reach a statistically significant level (light, F 1,37 = 3.842, p = 0.0575; dark, F 1,37 = 7.599, p = 0.0090, Fig. 3D) nor was observed a significant difference in the number of transitions between the light and dark chambers (Fig. 3E). Taken together, the Zfhx2-deficient mice were generally hyperactive, although they were specifically less active immediately after being transferred to novel environments.
Figure 2. Locomotor activity measured in open field test.
Distance traveled (A, B), stereotypic behavior (C), vertical activity (D), and time spent in the center area (E). Measurements are blocked in 5 min (A, C, D, and E) or in 1 min (B, shown only for the first 10 min). The Zfhx2-deficient mice were generally hyperactive but were hypoactive in the initial period in the open field.
Figure 3. 24-h homecage monitoring test and light/dark transition test.
(A, B) 24-h homecage monitoring test: two mice of the same genotype were housed in a single cage and continuously monitored by a video camera. The activity level of the mutant mice was significantly higher (A), but the mean number of particles, which represented the social interaction level between cagemates, was not significantly different (B). Top bar indicates light/dark cycle. (C–F) Light/dark transition test: latency to enter the light chamber for the first time was significantly longer in the mutant mice (C), the mutant mice traveled shorter distances particularly in the dark chamber (D), no significant difference was observed in the number of transitions between the light and dark chambers (E) or in the time spent in the light chamber (F).
In the open field test, the time spent in the center area by the mutant mice was significantly longer than that by the control mice (F 1,38 = 12.275, p = 0.0012, Fig. 2E), which may be related to an abnormal anxiety-like behavior. On the other hand, no significant difference was observed in the time spent in the light compartment in the light/dark transition test, which is another anxiety-related measurement, between the mutant and control mice (Fig. 3F) (see Discussion).
Finally, using another group of animals, we repeated the light/dark transition test, open field test, and 24-h homecage monitoring test (Fig. S6) and confirmed these phenotypes of the Zfhx2-deficient mice, namely, the hypoactivity during the initial period after being transferred into novel environments and the hyperactivity in familiar environments, together with the phenotype of spending longer time in the center area of the open field.
Depression-like Behavior
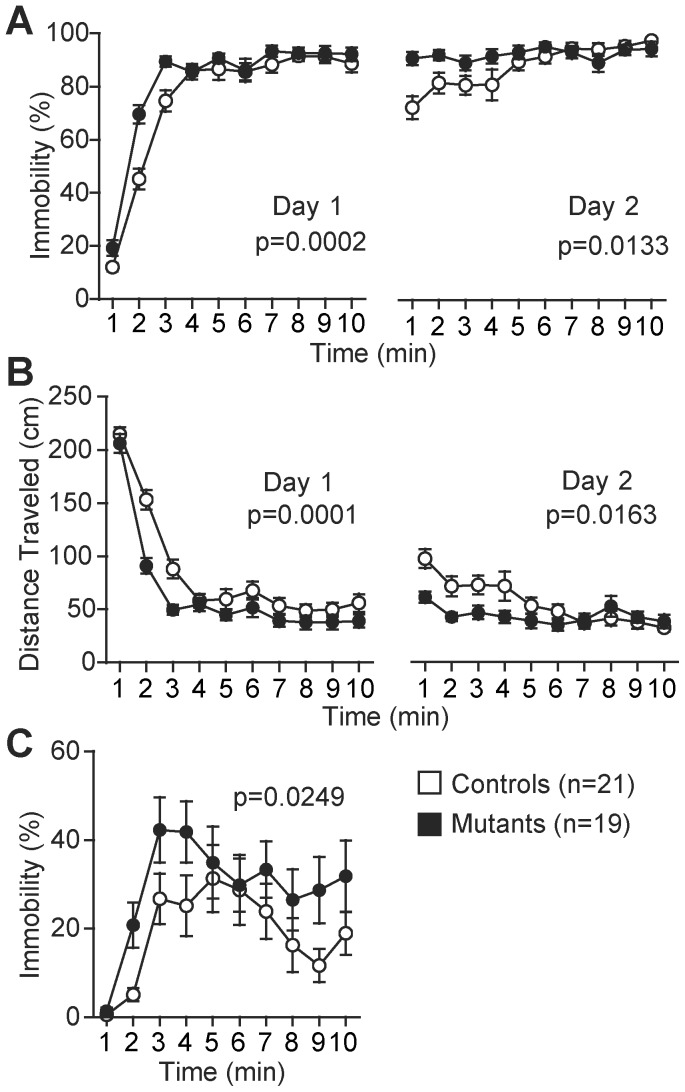
In the Porsolt forced swim test, the Zfhx2-deficient mice showed an immobile posture, which is considered as a reflection of a state of behavioral despair and commonly used as an index for evaluating depression-like behavior, for a significantly longer time than the control mice did (day 1, F 1,38 = 17.343, p = 0.0002; day 2, F 1,38 = 6.739, p = 0.0133, Fig. 4A). The distance traveled by the mutant mice was also significantly shorter than that by the control mice (day 1, F 1,38 = 18.797, p = 0.0001; day 2, F 1,38 = 6.322, p = 0.0163, Fig. 4B). In addition, the mutant mice showed a significantly increased immobility in the tail suspension test (F 1,38 = 5.453, P = 0.0249, Fig. 4C). These results suggest that the Zfhx2-deficient mice showed an enhanced depression-like phenotype.
Figure 4. Enhanced depression-like behavior of Zfhx2-deficient mice.
(A, B) Porsalt forced swim test: the percentage of time in immobile posture of the mutant mice was significantly higher (A) and the distance traveled by the mutant mice was significantly shorter (B) than those of the controls. (C) Tail suspension test: the percentage of time in immobile posture of the mutant mice was significantly higher than that of the control mice.
Spatial Reference Memory
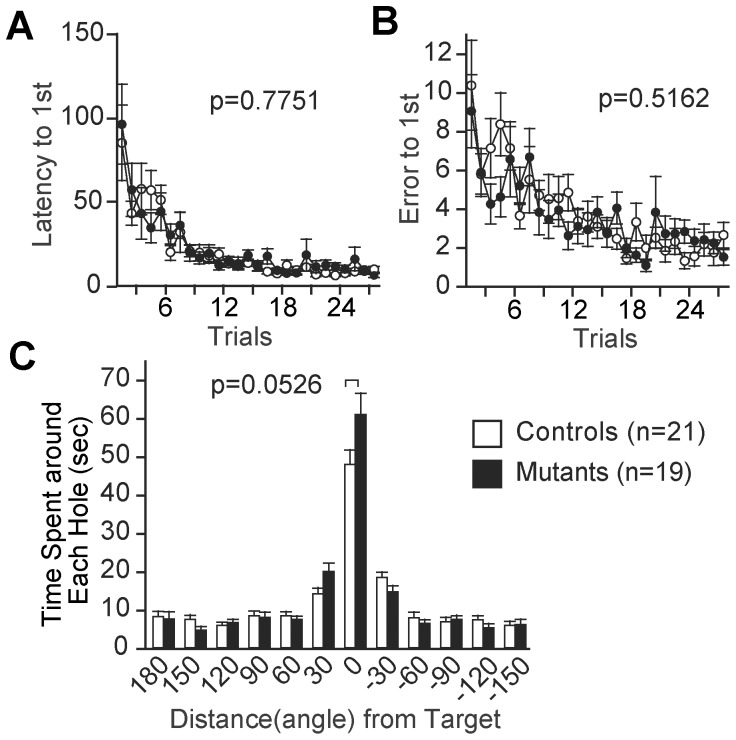
To assess spatial reference memory, we performed the Barnes circular maze test, which resembles the Morris water maze test, using a white circular board instead of a milk pool. The Zfhx2-deficient mice had no defect in the memory acquisition process, judging from the latency and number of errors before the first time they reached to the target where an escape box was attached (Figs. 5A and B). In the probe test (exploration on the same circular board without an escape box attached for 3 min) conducted on the next day of the last training session (Fig. 5C), both mutant mice and control mice spent significantly longer time around the target hole than around the others (the effect of hole position ( = distance from the target in Fig. 5C), F 11,240 = 56.229, p<0.0001 for controls, F 11,216 = 59.340, p<0.0001 for mutants), suggesting that mice of both genotypes remembered the position of the target. However, there was a significant difference in the effect of (genotype) × (hole position) interaction (F 11,418 = 2.989, p = 0.0008), i.e., the mutant mice and control mice preferred the target hole at different degrees. This result may suggest an increased perseveration tendency of the Zfhx2-deficient mice, although the difference of the time spent around the target did not reach a statistically significant level (genotype effect, F 1,38 = 4.003, p = 0.0526).
Figure 5. Spatial reference memory.
Barnes maze test was performed to assess spatial reference memory. There was no significant difference in latency (A) and number of errors (B) to reach the target hole during the training period. (C) Probe trial conducted 1 day after the last training session. The results were analyzed using two-way ANOVA followed by Fisher’s PLSD post hoc test. A significant difference was found in the effect of (genotype) × (hole position ( = distance from target)) interaction (F 11,418 = 2.989, p = 0.0008), although the difference of the time spent around the target did not reach a statistically significant level (genotype effect, F 1,38 = 4.003, p = 0.0526).
Other Behavioral Tests
Besides the tests described above, we also conducted the following behavioral tests: the elevated plus maze test, social interaction test in a novel environment, Crawley version social interaction test, rotarod test, hot plate test, startle response/prepulse inhibition test, cued and contextual fear conditioning test, gait analysis, and circadian rhythm analysis. Results of these behavioral tests are presented as supporting information (Figs. S1, S2, S3, S4, and S5).
In the startle response/prepulse inhibition test, the response of the mutant mice to sound stimuli was greater than that of the control mice (F 1,38 = 6.095, p = 0.0182, Fig. S2H) and the percentage of inhibition of the response to a 120-dB stimulus by a low intensity ‘prepulse’ of the mutant mice was lower than that of the controls (F 1,38 = 4.917, p = 0.0326, Fig. S2I). Although the differences are not significant enough considering multiplicity of measurements done on the first group of mice, these results suggest that the sensorimotor gating might be impaired in the Zfhx2-deficient mice. In the gate analysis, the mutant mice showed slightly longer durations of the ‘swing’ phase, indicating possibility of subtle defects in the limb muscles and/or joints of the mutant mice (Fig. S4). In the other tests, significant differences in results were not observed between the Zfhx2-deficient mice and control mice. In addition, no significant difference was observed in the mean number of particles in the 24-h homecage monitoring test, which was a measurement of the social interaction behavior, between the mutant and control mice (Fig. 3B).
Discussion
Mammalian ZFHX2 is a large transcription factor (2562 amino acids, 273 kD) expressed mainly in the brain. To elucidate its function, we conducted a comprehensive battery of behavioral tests on the Zfhx2-deficient mice. Here, we report that Zfhx2 deficiency caused several behavioral abnormalities in mice, particularly in the emotional aspects.
The open field test (Fig. 2A) and 24-h monitoring homecage test (Fig. 3A) clearly showed that the Zfhx2-deficient mice were generally hyperactive. They were, however, hypoactive during the initial period in the open field (Fig. 2B). Similar tendency was also observed in other genetically modified mice, i.e., hyperactivity of the mutant mice was less prominent during the initial period in the open field than during the later period (DAT knockdown [9], CN knockout [10], GSK3β transgenic [11], Disc1 dominant-negative [12], Dtnbp1 deletion [13]). In some reports, these observations were explained as a habituation defect [9], [11], [13]. In the Zfhx2-deficient mice, however, we assume that the exploratory motivation of a novel environment might be reduced because they were less active than the control mice immediately after being transferred to a novel environment. A longer latency to enter the light compartment in the light/dark transition test (Fig. 3C) supported this idea although further analysis remains to be carried out.
The Porsolt forced swim test and tail suspension test are representative behavioral tests commonly used for assessing depression-like behavior in rodents [14], [15]. In both tests, the Zfhx2-deficient mice showed the immobilized posture, which is considered as a reflection of their state of behavioral despair or ‘helplessness’, for longer time than the control mice (Fig. 4). These observations indicated that Zfhx2 deficiency caused the enhanced depression-like phenotype. The Zfhx2-deficient strain may serve as a novel model of depression, although several questions remain to be resolved including relationships among various phenotypes observed in the mutant mice.
The anxiety-related phenotype of the Zfhx2 mutant mice was complicated. In general, the center area of the open field apparatus, the light chamber of the light/dark transition box, and the open arms of the elevated plus maze are more aversive areas for the mice than the peripheral area, the dark chamber, and the closed arms, respectively. Thus, the measurements of the time spent in these aversive areas are considered to reflect the anxiety level of the mice. The Zfhx2 mutant mice, however, showed inconsistent results among these measurements. The time spent by the mutant mice in the center area of the open field was significantly longer than that spent by the control mice throughout the test period (Fig. 2E), which indicated a reduced anxiety-like phenotype of the Zfhx2-deficient mice. In contrast, the time spent in the light chamber of the light/dark transition box (Fig. 3F), the number of entries into and the time spent in the open arms of the elevated plus maze (Figs. S2B, D) were not significantly different between the mutant and control mice. As discussed previously, ‘anxiety’ is not a single phenomenon, rather, it has a multidimensional nature [16], [17]. We presume that each test that we conducted may have assessed separate aspects of the anxiety-related phenotype of the Zfhx2 mutant mice.
In the wild-type mouse, Zfhx2 was highly expressed in the developing brain and continued to be expressed throughout adulthood at a lower expression level (Figs. 1B, C, and H). It is not clear, however, whether abnormalities observed in the Zfhx2-deficient mice were caused by developmental defects or malfunction of the mature brain (or both). It is possible that Zfhx3, a gene phylogenically related to Zfhx2, compensated for the deficiency of Zfhx2 in the developing mutant brain because Zfhx3 was expressed in a similar pattern to and at a higher level than Zfhx2 in the wild-type brain (Figs.1C–G). As the brain matures, however, the expression level of Zfhx3 quickly decreased and finally became much lower than that of Zfhx2 in the adult brain (Figs. 1B, H–J). Hence, the effect of Zfhx2 deficiency may appear more clearly in the adult nervous system.
In the adult brain, the highest expression level of the ZFHX2 protein was observed in the pyramidal cell layer of the hippocampus, the suprachiasmatic nucleus, laterodorsal thalamic nucleus, lateral geniculate nucleus, substantia nigra pars compacta, and magnocellular part of the red nucleus (Figs. 1K–P). Dopaminergic neurons in the substantia nigra pars compacta, one of the major dopaminergic cell sources, expressed Zfhx2 mRNA at a high level, and dopaminergic neurons in other regions such as the ventral tegmental area also expressed the Zfhx2 mRNA at a slightly lower level (Figs. 1Q–Y). It is possible that the loss of ZFHX2 may cause some impairment in the dopaminergic neurons, which may result in disturbance of the dopaminergic inputs into various parts of the brain. Further investigation may clarify the relationship of Zfhx2 with the dopaminergic system and also with other monoaminergic systems, which may underlie the behavioral phenotypes of the Zfhx2-deficient mice.
In this study, we found that Zfhx2 deficiency caused multiple behavioral abnormalities in mice, namely, hyperactivity, enhanced depression-like behavior, and anxiety-related abnormalities. In addition to these major phenotypes, the Zfhx2-deficient mice may have a reduced motivation to explore a novel environment. It is noteworthy that the Zfhx2-deficient mice appeared quite normal with regard to general cognitive abilities, such as motor learning (rotarod test, Fig. S1E), reference memory (acquisition process of the Barnes maze test, Figs. 5A and B) and context and episodic-like memory (fear conditioning test, Figs. S2E–G), in contrast to the wide range of impairments observed in emotional aspects. Despite these behavioral phenotypes of the Zfhx2-deficient mice, functional relationships have not been known between Zfhx2 and other genes related to neuropsychiatric disorders, such as serotonin-related genes, dopamine-related genes, calcineurin and bdnf. Thus, further analysis of the function of Zfhx2 may provide a key to the clarification of novel aspects of the pathophysiology of these neuropsychiatric disorders.
Materials and Methods
Ethics Statement
All animal procedures were approved by the Institutional Animal Care and Use Committee of National Institutes of Natural Sciences (approval numbers: 10A173 and 11A102) or the Animal Care and Use Committee of Kyoto University Graduate School of Medicine (approval number: MedKyo09539) and performed in accordance with their guidelines.
Animals
Generation of the Zfhx2-deficient mouse line was described by Komine et al. [1], where Zfhx2 and the Zfhx2-deficient mouse line were referred to as zfh-5 and the ORF replacement line, respectively. Heterozygous mutant mice, which had been backcrossed on a C57B6/J background for at least 12 generations, were intercrossed and the resulting homozygous and wild-type males were used for behavioral tests. Behavioral test battery was started after animals reached 11 weeks of age. Four mice were housed in one cage in a room with a 12-hr light/dark cycle with access to food and water ad libitum. Behavioral tests were performed between 9:00 and 18:00.
RT-PCR
Reverse transcription (RT) was performed with 1 µg total RNA from mouse brains of various developmental stages and various adult tissues using SuperScript II (Invitrogen), and 1/100 of the resulting cDNA was amplified using ExTaq (Takara) for 30 cycles (E11.5 - P21 brains) or 35 cycles (adult tissues). Sequences of the primers used are available on request.
In situ Hybridization
Embryonic brains fixed with 4% paraformamide or fresh adult brains were cryosectioned and in situ hybridization was performed as previously described [1], [18]. Briefly, after postfixation in 4% paraformaldehyde, acetylation and prehybridization, sections were hybridized with a digoxigenin (DIG)-labeled RNA probe. Following posthybridization washing, DIG-probes were detected using an alkaline-phosphatase (AP)-conjugated anti-DIG antibody and BCIP/NBT as an AP substrate.
For the double-color detection of Zfhx2 and tyrosine hydroxylase (Th), a mixture of four fluorescein (FITC)-labeled Zfhx2 antisense probes (in order to improve detection sensitivity) and a DIG-labeled Th probe was used. DIG-probes were detected using an AP-conjugated anti-DIG antibody and the HNPP Fluorescein Detection Set (Roche), and FITC-probes were detected using a horseradish-peroxidase (HRP)-conjugated anti-FITC antibody, amplified with the TSA-Plus DNP system (PerkinElmer), and finally visualized using an Alexa Fluor 488-conjugated anti-DNP antibody (Molecular Probes). Information about regions of the genes (Zfhx2, Zfhx3, Zfhx4, and Th) used for probes is available on request.
Immunohistochemistry
A rabbit antibody raised against an N-terminal portion (amino acid 11-360) of ZFHX2 protein was used to examine localization of ZFHX2 protein. Staining was performed on paraformamide-fixed cryosections by a standard method using vecstain elite ABC kit (Vector laboratories) and DAB as a HRP substrate.
Behavioral Tests
We performed a battery of behavioral tests consisting of the following experiments (listed in the order conducted): the general health and neurological screening test, light/dark transition test, open field test, elevated plus maze test, hot plate test, social interaction in novel environment test, rotarod test, startle response/prepulse inhibition test, Porsolt forced swim test, Crawley version social interaction test, gait analysis, Barnes maze test, cued and conditional fear conditioning test, tail suspension test and 24-h homecage monitoring test. Results of all tests of the battery are summarized in the supporting table (Table S1). Details of the experiments not mentioned below are described elsewhere [8], [19], [20]. Raw data from the behavioral tests are available in the mouse phenotype database (http://www.mouse-phenotype.org/).
Open Field Test
Each mouse was placed in the corner of the open field apparatus (40×40×30 cm; Accuscan Instruments, Columbus, OH). The chamber of the test was illuminated at 100 lux. Distance traveled, vertical activity (rearing measured by counting the number of the times the high-position photobeam was interrupted), time spent in the center area (20×20 cm), and counts of stereotypical behaviors (counted when the same beam was repeatedly interrupted) were recorded. Data were collected for 120 min.
Light/dark Transition Test
The apparatus consisted of a cage (21 cm×42 cm×25 cm) divided into two chambers of equal sizes by a partition with a door (O’HARA & Co., Tokyo, Japan). One chamber was brightly illuminated (390 lux), whereas the other chamber was dark (2 lux). Mice were placed into the dark chamber and allowed to move freely between the two chambers with the door open for 10 min. The latency to the first entry to the light chamber, distance traveled, total number of transitions between chambers, and time spent in each chamber were recorded and analyzed using Image LD software. Details of the experiment are described in Takao and Miyakawa [21].
Porsolt Forced Swim Test
The apparatus consisted of a Plexiglas cylinder (20 cm height×10 cm diameter) placed in the center of an opaque plastic chamber (30 cm×30 cm×30 cm). The cylinder was filled with water (23°C) up to a height of 7.5 cm. Mice were placed into the cylinder, and their behavior was recorded for 10 min and analyzed using Image PS software. Retention test trials were administered 24 hours after the first trials.
Tail Suspension Test
Mice were suspended from the ceiling of an opaque plastic chamber (30 cm×30 cm×30 cm) by an adhesive tape placed <1 cm from the tip of the tail. Their behavior was recorded for 10 min, and image data were analyzed automatically using Image TS software.
Barnes Circular Maze Test
The Barnes circular maze test was conducted on a white circular board, 100 cm in diameter, with 12 holes equally spaced around the perimeter (O’HARA & Co., Tokyo, Japan). The circular board was elevated 75 cm from the floor. A black Plexiglas escape box (17 cm×13 cm×7 cm), which had paper bedding on its bottom, was located under one of the holes. The hole above the escape box represented the target, analogous to the hidden platform in the Morris water maze task. The location of the target was consistent for a given mouse, but was randomized across mice. The maze was rotated daily, with the spatial location of the target unchanged with respect to the visual room cues, to prevent a bias based on olfactory or proximal cues within the maze. Mice were placed at the center of the circular board, and the latency, number of errors and distance traveled to reach the target hole were recorded. Three trials per day were conducted for nine days. One day after the last training, a probe trial, in which mice navigated on the same circular board without the escape box for 3 min, was conducted and the time spent around each hole was recorded.
24-hour Monitoring in Home Cage
A system that automatically analyzes the locomotor activity of mice in the home cage was used. The system contains a home cage (29 cm×18 cm×12 cm) and a filter cage top, separated by a 13-cm-high metal stand containing an infrared video camera, which is attached to the top of the stand. Two mice of the same genotype that had been housed separately were placed together in a home cage. Their locomotor activity and social behavior were monitored for 1 week. Outputs from the video cameras were fed into a computer and images from each cage were captured at a rate of one frame per second. Distance traveled was measured automatically using Image HA software. Social interaction was measured by counting the number of particles detected in each frame: two particles indicted that the mice were not in contact with each other, and one particle (i.e., the tracking software could not distinguish two separate bodies) indicated contact between the two mice.
Using the same system, circadian rhythms of locomotor activity were also analyzed. Each mouse was individually housed for 2 weeks under a 12-hour light-dark cycle condition (LD) and then for 2 weeks in constant darkness (DD). Distance traveled was automatically measured and analyzed.
Data Analysis
Behavioral data were obtained automatically using applications based on the public domain NIH Image program and Image J program, with modifications for each test by the authors (available through O’HARA & Co., Tokyo, Japan). Statistical analysis was conducted using StatView (SAS Institute, Cary, NC). Data were analyzed by one-way or two-way ANOVA, or two-way repeated measures ANOVA. F and p values indicated in the text and graphs represent the effects of genotype unless otherwise noted. Values in graphs are expressed as mean ± SEM.
Supporting Information
No abnormality was observed in the physical characteristics of the Zfhx2 -deficient mice. Body weight (A), body temperature (B), grip strength (C), wire hang test (D), latency to fall in the rotarod test (E), and latency to the first response in the hot plate test (F).
(TIF)
Elevated plus maze test, cued and context fear conditioning test, and startle response/prepulse inhibition test. (A–D) Elevated plus maze test: no significant difference was observed in the number of entries (A), percent entries into open arms (B), distance traveled (C), and time spent on open arms (D). (E–G) Fear conditioning test: percent freezing in conditioning (E), context testing (F), and cued testing (G). Horizontal bars and arrows indicate tone and foot shock presentation, respectively. (H, I) Startle response/prepulse inhibition test: the amplitude of the startle response to the acoustic stimuli (H), and the percentage of prepulse inhibition of the startle response (I). p-values represent the effects of genotype using the sound level (H) or prepulse level (I) as repeated measures.
(TIF)
Zfhx2 -deficient mice showed normal performances in social interaction tests. (A–D) Social interaction test in novel environment: total duration of contact (A), number of contacts (B), mean duration of contacts (C), and distance traveled (D). (E–H) Crawley version social interaction test: (E, F) Number of entries (G) and time spent (H) around a cage with a stranger mouse and an empty cage: no significant effect of (genotype) × (stranger/empty) interaction was observed. (I, J) Number of entries (I) and time spent (J) around a cage with a stranger mouse and a cage with a familiar mouse: no significant effect of (genotype) × (stranger/familiar) interaction was observed.
(TIF)
Results of gait analysis. The Zfhx2-deficient mice showed slightly longer durations of the ‘swing’ phase, indicating possibility of subtle defects in the limb muscles and/or joints.
(TIF)
Circadian rhythm of locomotor activity. (A) Representative double-plotted activity records from two control mice and two mutant mice. Data from the last 7 days under 12-h light-dark cycle condition (LD) and 16 days under constant dark condition (DD) are shown. (B) Circadian period length estimated from activity records under DD condition. The Zfhx2-deficient mice showed a normal period length.
(TIF)
Additional behavioral tests. The second group of animals (homozygous mutant males and their wild-type littermates) was subjected to the light/dark transition test, open field test, and 24-h homecage monitoring test to confirm the phenotypes observed in the first group of animals. (A, B) Open field test. Total distance traveled (A) and time spent in the center area (B) blocked in 5 minutes. In (A), genotype effect for the entire period, F 1,38 = 0.751, p = 0.3916; for initial 30 min, F 1,38 = 4.342, p = 0.044; for the last 30 min, F 1,38 = 6.670, p = 0.0138; the effect of (genotype) × (time) interaction for the entire period, F 23,874 = 6.388, p<0.0001. (C) Total distance traveled in the 24-h monitoring cage. Note that each mouse was separately housed in this experiment, whereas two mice were housed together in the experiment of Fig. 2. (D) Latency to enter the light chamber for the first time.
(TIF)
Results of comprehensive battery of behavioral tests. Values are shown as means ± SEM. F and p values are effects of genotype, except *. *; effects of stranger/empty or stranger/familiar.
(XLS)
Acknowledgments
We thank T. Ishikawa, M. Furuyama, and Y. Kon for technical assistances, and the members of Yamamori’s lab and Miyakawa’s lab for their help.
Funding Statement
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (Molecular Brain Science) (170245 to TY and YK), a Grant-in-Aid for Scientific Research on Innovative Areas (Neocortical Organization) (22123009 to TY), a Grant-in-Aid for Exploratory Research and Integrative Brain Research (IBR-shien), and a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Komine Y, Nakamura K, Katsuki M, Yamamori T (2006) Novel transcription factor zfh-5 is negatively regulated by its own antisense RNA in mouse brain. Mol Cell Neurosci 31: 273–283. [DOI] [PubMed] [Google Scholar]
- 2. Holland P, Booth H, Bruford E (2007) Classification and nomenclature of all human homeobox genes. BMC Biol 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung C, Kim H, Kawaguchi M, Khanna K, Hida H, et al. (2005) Homeotic factor ATBF1 induces the cell cycle arrest associated with neuronal differentiation. Development 132: 5137–5145. [DOI] [PubMed] [Google Scholar]
- 4. Sun X, Frierson H, Chen C, Li C, Ran Q, et al. (2005) Frequent somatic mutations of the transcription factor ATBF1 in human prostate cancer. Nat Genet 37: 407–412. [DOI] [PubMed] [Google Scholar]
- 5. Kim C, Song J, Cho Y, Cao Z, Lee Y, et al. (2008) Down-regulation of ATBF1 is a major inactivating mechanism in hepatocellular carcinoma. Histopathology 52: 552–559. [DOI] [PubMed] [Google Scholar]
- 6. McMullan T, Crolla J, Gregory S, Carter N, Cooper R, et al. (2002) A candidate gene for congenital bilateral isolated ptosis identified by molecular analysis of a de novo balanced translocation. Hum Genet 110: 244–250. [DOI] [PubMed] [Google Scholar]
- 7. Takao K, Yamasaki N, Miyakawa T (2007) Impact of brain-behavior phenotypying of genetically-engineered mice on research of neuropsychiatric disorders. Neurosci Res 58: 124–132. [DOI] [PubMed] [Google Scholar]
- 8. Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, et al. (2008) Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhuang X, Oosting R, Jones S, Gainetdinov R, Miller G, et al. (2001) Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A 98: 1982–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyakawa T, Leiter L, Gerber D, Gainetdinov R, Sotnikova T, et al. (2003) Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A 100: 8987–8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, et al. (2006) Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci 26: 9022–9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, et al. (2007) Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A 104: 14501–14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox M, Tucker A, Tang J, Talbot K, Richer D, et al. (2009) Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav 8: 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Porsolt R, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730–732. [DOI] [PubMed] [Google Scholar]
- 15. Crowley J, Jones M, O'Leary O, Lucki I (2004) Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav 78: 269–274. [DOI] [PubMed] [Google Scholar]
- 16. Carola V, D'Olimpio F, Brunamonti E, Mangia F, Renzi P (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134: 49–57. [DOI] [PubMed] [Google Scholar]
- 17. Ramos A (2008) Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 29: 493–498. [DOI] [PubMed] [Google Scholar]
- 18. Schaeren-Wiemers N, Gerfin-Moser A (1993) A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100: 431–440. [DOI] [PubMed] [Google Scholar]
- 19. Takao K, Tanda K, Nakamura K, Kasahara J, Nakao K, et al. (2010) Comprehensive behavioral analysis of calcium/calmodulin-dependent protein kinase IV knockout mice. PLoS One 5: e9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao I, Takao K, Miyakawa T, Ito S, Setou M (2011) Synaptic E3 ligase SCRAPPER in contextual fear conditioning: extensive behavioral phenotyping of Scrapper heterozygote and overexpressing mutant mice. PLoS One 6: e17317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takao K, Miyakawa T (2006) Light/dark transition test for mice. J Vis Exp: 104. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No abnormality was observed in the physical characteristics of the Zfhx2 -deficient mice. Body weight (A), body temperature (B), grip strength (C), wire hang test (D), latency to fall in the rotarod test (E), and latency to the first response in the hot plate test (F).
(TIF)
Elevated plus maze test, cued and context fear conditioning test, and startle response/prepulse inhibition test. (A–D) Elevated plus maze test: no significant difference was observed in the number of entries (A), percent entries into open arms (B), distance traveled (C), and time spent on open arms (D). (E–G) Fear conditioning test: percent freezing in conditioning (E), context testing (F), and cued testing (G). Horizontal bars and arrows indicate tone and foot shock presentation, respectively. (H, I) Startle response/prepulse inhibition test: the amplitude of the startle response to the acoustic stimuli (H), and the percentage of prepulse inhibition of the startle response (I). p-values represent the effects of genotype using the sound level (H) or prepulse level (I) as repeated measures.
(TIF)
Zfhx2 -deficient mice showed normal performances in social interaction tests. (A–D) Social interaction test in novel environment: total duration of contact (A), number of contacts (B), mean duration of contacts (C), and distance traveled (D). (E–H) Crawley version social interaction test: (E, F) Number of entries (G) and time spent (H) around a cage with a stranger mouse and an empty cage: no significant effect of (genotype) × (stranger/empty) interaction was observed. (I, J) Number of entries (I) and time spent (J) around a cage with a stranger mouse and a cage with a familiar mouse: no significant effect of (genotype) × (stranger/familiar) interaction was observed.
(TIF)
Results of gait analysis. The Zfhx2-deficient mice showed slightly longer durations of the ‘swing’ phase, indicating possibility of subtle defects in the limb muscles and/or joints.
(TIF)
Circadian rhythm of locomotor activity. (A) Representative double-plotted activity records from two control mice and two mutant mice. Data from the last 7 days under 12-h light-dark cycle condition (LD) and 16 days under constant dark condition (DD) are shown. (B) Circadian period length estimated from activity records under DD condition. The Zfhx2-deficient mice showed a normal period length.
(TIF)
Additional behavioral tests. The second group of animals (homozygous mutant males and their wild-type littermates) was subjected to the light/dark transition test, open field test, and 24-h homecage monitoring test to confirm the phenotypes observed in the first group of animals. (A, B) Open field test. Total distance traveled (A) and time spent in the center area (B) blocked in 5 minutes. In (A), genotype effect for the entire period, F 1,38 = 0.751, p = 0.3916; for initial 30 min, F 1,38 = 4.342, p = 0.044; for the last 30 min, F 1,38 = 6.670, p = 0.0138; the effect of (genotype) × (time) interaction for the entire period, F 23,874 = 6.388, p<0.0001. (C) Total distance traveled in the 24-h monitoring cage. Note that each mouse was separately housed in this experiment, whereas two mice were housed together in the experiment of Fig. 2. (D) Latency to enter the light chamber for the first time.
(TIF)
Results of comprehensive battery of behavioral tests. Values are shown as means ± SEM. F and p values are effects of genotype, except *. *; effects of stranger/empty or stranger/familiar.
(XLS)