Abstract
Purpose
Precocious puberty is defined as breast development before the age of 8 years in girls. The present study aimed to reveal the diagnosis of Korean girls referred for precocious puberty and to compare the constitutional and endocrinological features among diagnosis groups.
Methods
The present study used a retrospective chart review of 988 Korean girls who had visited a pediatric endocrinology clinic from 2006 to 2010 for the evaluation of precocious puberty. Study groups comprised fast puberty, true precocious puberty (PP), pseudo PP, premature thelarche, and control. We determined the height standard deviation score (HSDS), weight standard deviation score (WSDS), and body mass index standard deviation score (BMISDS) of each group using the published 2007 Korean growth charts. Hormone tests were performed at our outpatient clinic.
Results
The PP groups comprised fast puberty (67%), premature thelarche (17%), true PP (15%), and pseudo PP (1%). Advanced bone age and levels of estradiol, basal luteinizing hormone (LH), and peak LH after gonadotropin-releasing hormone stimulation testing were significantly high in the fast puberty and true PP groups compared with the control group. HSDS, WSDS, and BMISDS were significantly higher in the true PP group than in the control group (P<0.05).
Conclusion
The frequent causes of PP were found to be fast puberty, true PP, and premature thelarche. Furthermore, BMISDS were significantly elevated in the true PP group. Therefore, we emphasize the need for regular follow-up of girls who are heavier or taller than others in the same age group.
Keywords: Precocious puberty, Puberty, Premature thelarche, Body mass index, Sexual maturation
Introduction
Precocious puberty (PP) is the precocious onset of pubertal changes in girls and boys. It is mainly due to the precocious activation of a pulsatile activity of the gonadotropic axis with pulsatile secretion of hypothalamic gonadotropin-releasing hormone (GnRH), leading to an elevation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion1). PP is clinically defined by the onset of breast development before the age of 8 years in girls and by the onset of testicular development (testicular volume≥4 mL) before the age of 9 years in boys2). The diagnostic cut-off age in girls is determined from studies of normal pubertal development, which showed that Tanner stage 2 breast development was presented at 10.9±1.2 (±1 standard deviation [SD]) years of age in Swiss girls3) and at 11.2±1.1 years in British girls4). However, another prospective study about pubertal development in American boys and girls5) has led to a newly revised criteria proposed by the American Academy of Pediatrics. The proposed cut-off age used to determine whether girls with pubertal change should be evaluated for PP is 7 years in girls, although the traditional limit of 8 years is still considered in specific circumstances (rapidly progressing puberty, neurogenic PP, or bad compliance to treatment)6). However, in a study about sexual maturation in Korean girls and boys, Tanner stage 2 breast development was presented at 9.11±1.86 years of age, which is similar to that of European studies7). Therefore, this issue is still in debate.
Many studies about the possible causes of the earlier puberty have been performed. There are several possible causes, such as improvement in socio-economic conditions, general health8,9), and exposure to endocrine-disrupting chemicals10,11). Some studies have found associations between obesity and sexual maturation in girls7,12), or both genders13). A study about Korean girls and boys showed obesity as a significant influencing factor in advanced puberty by using an obesity scale7). However, there have been few constitutional studies about PP using 2007 Korean growth charts which were most recently published Korean national constitutional data. Therefore we analyzed constitutional features of Korean girls referred for suspected PP using 2007 Korean growth charts. Furthermore, we analyzed endocrinologic hormonal features and determined causes of PP in Korean girls.
Materials and methods
1. Subjects
Nine hundred and eighty eight Korean girls (ages 6 years to 9 years 11 months) were enrolled in this study, performed at the Samsung Medical Center at Seoul, South Korea, from 2006 to 2010. These girls visited the outpatient clinic because of their parents' concerns over suspicious pubertal changes, or were referred by general physicians for the evaluation of suspected pubertal changes.
2. Methods
We did a detailed history taking and physical examination (including pubertal stage using Tanner stage for breast) of each patient. Age, height, weight, and body mass index (BMI) of the each visit were obtained in each group. Palpation and observation of breast were done by a single pediatric endocrinologist at each visit. Bone age was determined by both of our pediatric endocrinologists and pediatric radiologists with the mean bone age of the Atlas of Greulich and Pyle, Tanner-Whitehouse 3 system, and computer aided skeletal maturity assessment system. Serum estradiol (E2) level was obtained at outpatient clinic blood test. Serum LH and FSH was determined at baseline and every 30 minutes up to 2 hours after the intravenous bolus administration of GnRH (100 mcg Relefact; Sanofi-Aventis, Frankfurt am Main, Germany).
Parameters include age, difference between bone age and chronological age (BA-CA), weight standard deviation score (WSDS), height standard deviation score (HSDS), BMI standard deviation score (BMISDS), estradiol, basal LH, basal FSH, peak LH after GnRH stimulation test, peak FSH after GnRH stimulation test, peak LH to basal LH ratio, peak FSH to basal FSH ratio, and peak LH to FSH ratio. Constitutional parameters such as WSDS, HSDS, and BMISDS were calculated by the modified LMS (lambda, mu, sigma) statistical procedure used in the 2007 Korean growth charts in Korean children and adolescents published by the Korea Center for Disease Control and Prevention and the Korea National Institute of Health14). The standard deviation score (SDS) formula is as follows15):
L, box-cox power; M, median; S, coefficient of variation
All girls were classified as true PP, pseudo PP, fast puberty, premature thelarche, and control group. We diagnosed PP by the following criteria: patients developed Tanner stage 2 or above by palpation of breast before 8 years old, and advanced bone age compared to chronological age by 1 year or more. True PP was diagnosed by the peak LH level more than 5 IU/L after GnRH stimulation test in PP patients16); pseudo PP was determined by the peak LH level less than 5 IU/L after GnRH stimulation test. Neurogenic true PP was determined by the abnormalities on brain magnetic resonance imaging (MRI); idiopathic true PP was diagnosed when there were no abnormal findings on brain MRI. Abdominal and pelvic ultrasonograms were performed in pseudo PP patients to identify the status of adrenal glands, ovaries, and uterus. Premature thelarche was classified in case of the girls show accelerated breast development more than Tanner stage 2, but have normal range in bone age and estradiol level. The fast puberty group includes girls with onset of breast development after the age of 8 years but before the age of 10 years and with positive diagnostic criteria of true PP17). Girls who have been diagnosed to normal pubertal onset with normal bone age were served as control group.
3. Statistical methods
All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). We used Kruskal-Wallis test and Mann-Whitney test to compare multiple parameters among the true PP, pseudo PP, fast puberty, premature thelarche, and control group. The results were marked as mean±SD. Findings were considered statistically significant at P value less than 0.05.
Results
1. Diagnoses
The diagnostic distribution shows as this; fast puberty (67%), premature thelarche (17%), true PP (15%), and pseudo PP (1%). Abnormalities on central nervous system were found in 4 girls of true PP patients group; Rathke's cleft cysts were diagnosed in 2 cases, one hamartoma, and one pituitary gland hyperplasia was diagnosed. Total number of pseudo PP case was 4; McCune-Albright syndrome case number was 2, and ovarian cysts case number was one.
2. Age
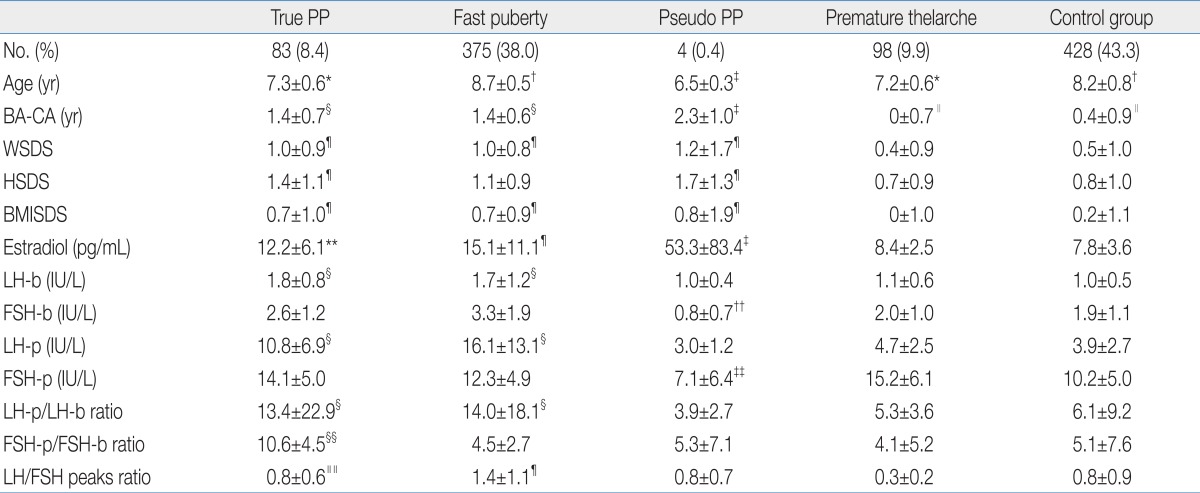
Ages of fast puberty and control group were significantly higher than those of true PP, pseudo PP, and premature thelarche (P<0.05). Age of pseudo PP was significantly lower than those of true PP and premature thelarche (P<0.05). There was no significant difference between true PP and PT group (Table 1).
Table 1.
Constitutional Parameters and Hormonal Features in 988 Korean Girls
Values are presented as mean±SD.
PP, precocious puberty; BA, bone age; CA, chronological age; WSDS, weight standard deviation score; HSDS, height standard deviation score; BMISDS, body mass index standard deviation score; LH-b, basal luteinizing hormone; FSH-b, basal follicle-stimulating hormone; LH-p, peak LH after gonadotropin-releasing hormone (GnRH) stimulation test; FSH-p, peak FSH after GnRH stimulation test.
*P<0.05 vs. fast puberty, pseudo PP and control group. †P<0.05 vs. true PP, pseudo PP and premature thelarche. ‡P<0.05 vs. fast puberty, true PP, premature thelarche and control group. §P<0.05 vs. pseudo PP, premature thelarche and control group. ∥P<0.05 vs. fast puberty, true PP and pseudo PP. ¶P<0.05 vs. premature thelarche and control group. **P<0.05 vs. control group. ††P<0.05 vs. fast puberty and true PP. ‡‡P<0.05 vs. fast puberty, true PP and premature thelarche. §§P<0.05 vs. fast puberty, pseudo PP, premature thelarche and control group. ∥∥P<0.05 vs. premature thelarche.
3. Constitutional parameters
1) Difference between BA-CA
BA-CA of pseudo PP was significantly higher than those of other four groups (P<0.05). BA-CA of fast puberty and true PP were significantly higher than those of premature thelarche and control group (P<0.05). There was no significant statistical difference between premature thelarche and control group (Table 1).
2) WSDS
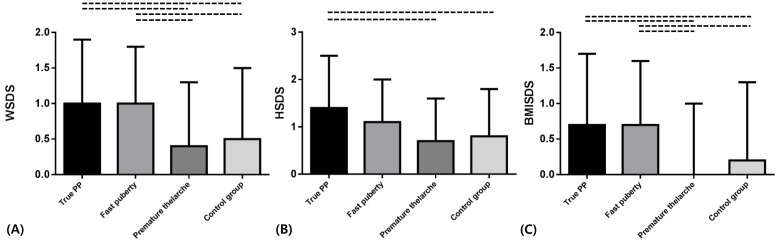
WSDS were significantly higher than those of premature thelarche and control group in fast puberty, true PP, and pseudo PP (P<0.05). There were no significant difference between premature thelarche and control group (Fig. 1A).
Fig. 1.
Constitutional parameters obtained from major precocious puberty (PP) groups in Korean girls. (A) Weight standard deviation score (WSDS). Mean data showing WSDS in the 3 major precocious puberty groups (true precocious puberty, fast puberty, and premature thelarche) compared with the control group. WSDS were significantly higher in the true puberty and fast puberty groups than in the premature thelarche and control groups. There was no significant difference between the premature thelarche and control groups. (B) Height standard deviation score (HSDS). True precocious puberty group had higher HSDS than the premature thelarche and control groups. (C) Body mass index standard deviation score (BMISDS) showed the same result as those obtained for WSDS. Error bars represent mean±SD. --, P<0.05 between group pairs linked with this symbol.
3) HSDS
HSDS of true PP and pseudo PP were significantly higher than those of fast puberty, premature thelarche, and control group (P<0.05). HSDS of true PP was not significantly different with that of pseudo PP (Fig. 1B).
4) BMISDS
BMISDS of fast puberty, true PP, pseudo PP were significantly higher than those of premature thelarche and control group (P<0.05). It showed no significant difference between premature thelarche and control group (Fig. 1C). Also it showed no significant differences among fast puberty, true PP, and pseudo PP group.
4. Endocrinologic hormonal features
1) Estradiol (E2)
Estradiol level of pseudo PP was significantly high compared with other four groups (P<0.05). Estradiol levels of fast puberty and true PP were significantly higher than those of premature thelarche and control group (P<0.05). There was no significant difference between premature thelarche and control group (Table 1).
2) Basal LH and FSH levels
Basal LH levels of fast puberty and true PP were significantly higher than those of pseudo PP, premature thelarche and control group (P<0.05). It showed no significant difference between fast puberty and true PP. Also there were no significant differences among pseudo PP, premature thelarche, and control group. Basal FSH levels of fast puberty and true PP were significantly higher than that of pseudo PP (P<0.05). But it showed no significant differences among pseudo PP, premature thelarche, and control group (Table 1).
3) Peak LH and FSH levels and ratios after GnRH stimulation test
Peak LH levels after GnRH stimulation test of fast puberty and true PP were significantly higher than those of pseudo PP, premature thelarche, and control group (P<0.05). There were no significant differences among pseudo PP, premature thelarche and control group. Peak FSH levels after GnRH stimulation test of fast puberty, true PP, and premature thelarche were significantly higher than that of pseudo PP (P<0.05). It showed no significant difference between pseudo PP and control group.
Peak LH levels after GnRH stimulation test to basal LH level ratios were significantly high in fast puberty and true PP compared with pseudo PP, premature thelarche and control group (P<0.05). There were no significant differences among pseudo PP, premature thelarche and control group. Peak FSH level after GnRH stimulation test to basal FSH level was significantly high in true PP compared with other four groups (P<0.05). Peak LH level to peak FSH level after GnRH stimulation test ratio was significantly high in fast puberty compared with premature thelarche and control group (P<0.05). Also it showed significantly high ratio in true PP compared with premature thelarche (P<0.05).
Discussion
Increasing prevalence of PP has elevated the needs for identifying the distribution of diagnosis to determine initiation of GnRH agonist therapy in girls with early sexual maturation. According to an American study about PP which was performed in 2004, the distribution of diagnosis is as these: true PP (9%), premature adrenarche (46%), premature thelarche (18%), and premature menarche (5%)18). According to a Korean study about 948 PP patients, which were published in 2007, fast puberty was 39%, premature thelarche was 31%, true PP was 27% and pseudo PP was 1%19). Another Korean study reported that fast puberty was 36.3% of 375 PP patients, true PP was 30.4%, premature thelarche was 29.1%, pseudo PP was 3.7%, and that premature adrenarche was 0.5% of those20). In the present study, 67% of 560 Korean girls with early sexual maturation were diagnosed as fast puberty. Premature thelarche were diagnosed at 17% of total cases. True PP was 15%, and pseudo PP was 1%. Therefore, we can suggest that the causes of early sexual maturation are varied by races, environmental circumstances as well as diagnostic criteria for PP.
There have been several studies on association of BMI with sexual maturation in girls. Increasing BMI is associated with earlier menstruation in Black American girls rather than White American girls21), suggesting an interaction of environmental factors with racial and genetic factors22). A cross-sectional study including 819 Portuguese girls and boys showed association between early sexual maturation and the prevalence of being overweight in both genders23). Also a study including 170 Korean girls and boys suggested earlier sexual maturation in a higher obesity scale group7). However, others suggested that contemporary changes in BMI and timing of menarche are coincident but independent24).
According to contemporary studies, factors influencing timing of puberty include genetic and environmental factors and changes in socio-economic status. However, difference of leptin levels between races is the main influencing factor25). Leptin serves as a metabolic signal for progression of puberty, and appears to be a permissive factor rather than a pubertal onset trigger26).
Several previous studies have concluded that true PP patients are more obese than control patients are. In the present study, the fast puberty group, true PP, and pseudo PP group had significantly higher BMISDS than those of premature thelarche and control group in the present study. This result means that 6- to 9-year-old girls meeting the diagnostic criteria of fast puberty and true PP showed higher obesity compared with premature thelarche and control group.
In the present study, true PP group also showed significantly high WSDS, HSDS compared with premature thelarche and control group. These results suggest us that the constitutional parameters are apparently advanced in true PP group. And, these are similar to other previous studies18-20). In the early pubertal period, growth development is accelerated by growth hormone and insulin-like growth factor 1 which were stimulated by sexual hormone. However, accelerated bone maturation causes early epiphyseal fusion which decreases final adult height in the end. Therefore, regular follow-up is required to girls with high percentiles of height or body weight in order to differentiate true PP which needs GnRH agonist therapy.
Another diagnostic criteria not used in the present study is peak LH level to peak FSH level after GnRH stimulation test. In girls with early sexual maturation, however, the response to GnRH may be quite low at breast stage 2 to early 3, and the peak LH to peak FSH ratio can remain low until mid-puberty2). In the present study, estradiol, basal LH, peak LH level after GnRH stimulation test, and peak LH to basal LH ratio were significantly higher in fast puberty and true PP compared with premature thelarche and control group. These results are similar to other previous studies. However, advanced bone age, elevated estradiol or LH or FSH levels cannot be evident in the early stage of true PP, therefore it is recommended to follow up those girls without definite evidence of true PP regularly.
The present study applied detailed diagnostic criteria of PP, which resulted in well-defined grouping (true PP versus pseudo PP versus premature thelarche versus fast puberty versus control group). Previous studies about association between obesity and early sexual maturation had relatively simple criteria for early sexual development, such as menarche12,21,27), Tanner stage13,23,28,29), or both Tanner stage and menarche30), compare to the detailed criteria for PP used in the present study. Our criteria include Tanner stage, carpal bone age, age (girls younger than 8 years old), and serum LH peak level obtained by GnRH stimulation test. We determined true PP, pseudo PP, fast puberty, premature thelarche, and control groups by these detailed diagnostic criteria; consequently, significant differences in BMI of fast puberty, true PP, pseudo PP group compared with premature thelarche and control group. This conclusion well matched with many those of previous studies about sexual maturation, adding positive evidence about the association of increased BMI with early sexual maturation.
Nevertheless, we have to consider possible selection bias of our patients group. Our patients were referred for evaluation of PP by general physicians or visited our clinic for examination by their parents' concern. However, the selection bias is compensated for by our detailed diagnostic criteria for confirming diagnosis of PP. We should consider another selection bias from the location of our hospital (Seoul, the largest city of Korea). According to the population data from the Korean National Indicators website, the population of Seoul metropolitan area was 49.4% of the Korean population in 201031). Although our patient group represents the urban population in Korean, our hospital is a tertiary center with referred patients from nation-wide hospitals. Given these facts, we can expect the compensation of selection bias in our patient group.
We used BMI as an indicator of obesity. However, there are several other parameters to evaluate obesity, such as waist-hip ratio or triceps skinfold thickness. Thus, the present study is limited given that only one parameter is used for evaluation of obesity, although BMI is the most useful parameter for obesity scale.
In conclusion, the main causes of early sexual maturation were fast puberty, true PP, and premature thelarche in the present study about 988 Korean girls. Furthermore, constitutional parameters such as WSDS, HSDS, and BMISDS were significantly elevated in true PP group compared with premature thelarche or control group. Therefore, the need for regular follow up of girls who are more obese or taller than the same age group is emphasized.
References
- 1.Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update. 2004;10:135–147. doi: 10.1093/humupd/dmh012. [DOI] [PubMed] [Google Scholar]
- 2.Kliegman RM, Stanton B, St. Geme J, Schor N, Behrman RE. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. pp. 1887–1889. [Google Scholar]
- 3.Largo RH, Prader A. Pubertal development in Swiss girls. Helv Paediatr Acta. 1983;38:229–243. [PubMed] [Google Scholar]
- 4.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 6.Kaplowitz PB, Oberfield SE Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Pediatrics. 1999;104(4 Pt 1):936–941. doi: 10.1542/peds.104.4.936. [DOI] [PubMed] [Google Scholar]
- 7.Park YJ, Moon CM, Yoo HJ. A study of factors influencing advanced puberty. Korean J Pediatr. 2010;53:146–151. [Google Scholar]
- 8.Hauspie RC, Vercauteren M, Susanne C. Secular changes in growth and maturation: an update. Acta Paediatr Suppl. 1997;423:20–27. doi: 10.1111/j.1651-2227.1997.tb18364.x. [DOI] [PubMed] [Google Scholar]
- 9.Herman-Giddens ME, Kaplowitz PB, Wasserman R. Navigating the recent articles on girls' puberty in pediatrics: what do we know and where do we go from here? Pediatrics. 2004;113:911–917. doi: 10.1542/peds.113.4.911. [DOI] [PubMed] [Google Scholar]
- 10.Mouritsen A, Aksglaede L, Sorensen K, Mogensen SS, Leffers H, Main KM, et al. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int J Androl. 2010;33:346–359. doi: 10.1111/j.1365-2605.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang RY, Needham LL, Barr DB. Effects of environmental agents on the attainment of puberty: considerations when assessing exposure to environmental chemicals in the National Children's Study. Environ Health Perspect. 2005;113:1100–1107. doi: 10.1289/ehp.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EK, Lee SH. Comparison of obesity and growth development in menarcheal and nonmenarcheal girls. J Korean Diet Assoc. 2003;9:106–113. [Google Scholar]
- 13.Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 14.Korea Center for Disease Control and Prevention; Korean Pediatric Society, Committee for the Development of Growth Standard for Korean Children and Adolescents. 2007 Korean children and adolescents growth standard: commentary for the development of 2007 growth chart [Internet] Cheongwon: KCDC, Division of Chronic Disease Surveillance; c2012. [cited 2012 Jun 10]. Available from: http://www.cdc.go.kr/CDC/info/CdcKrInfo0201.jsp?menuIds=HOME001-MNU0004-MNU0007-MNU0025&fid=28&q_type=&q_value=&cid=1235&pageNum=74. [Google Scholar]
- 15.Lee SY, Kim YN, Kang YJ, Jang MJ, Kim J, Moon JS, et al. The methodology for developing the 2007 Korean growth charts and blood pressure nomogram in Korean children and adolescents. Korean J Pediatr. 2008;51:26–32. [Google Scholar]
- 16.Mogensen SS, Aksglaede L, Mouritsen A, Sorensen K, Main KM, Gideon P, et al. Diagnostic work-up of 449 consecutive girls who were referred to be evaluated for precocious puberty. J Clin Endocrinol Metab. 2011;96:1393–1401. doi: 10.1210/jc.2010-2745. [DOI] [PubMed] [Google Scholar]
- 17.Lazar L, Kauli R, Pertzelan A, Phillip M. Gonadotropin-suppressive therapy in girls with early and fast puberty affects the pace of puberty but not total pubertal growth or final height. J Clin Endocrinol Metab. 2002;87:2090–2094. doi: 10.1210/jcem.87.5.8481. [DOI] [PubMed] [Google Scholar]
- 18.Kaplowitz P. Clinical characteristics of 104 children referred for evaluation of precocious puberty. J Clin Endocrinol Metab. 2004;89:3644–3650. doi: 10.1210/jc.2003-031532. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Coe HJ, Kim S, Lee SW, Chae HW, Kim YS, et al. Clinical and endocrinologic characteristics of children referred for precocious puberty. J Korean Soc Pediatr Endocrinol. 2007;12:119–126. [Google Scholar]
- 20.Na JM, Lee YJ, Kim MS, Lee DY, Yeo CY, Kim CJ, et al. Causes of precocious puberty: multicenter study in Honam area. J Korean Soc Pediatr Endocrinol. 2009;14:30–37. [Google Scholar]
- 21.Wattigney WA, Srinivasan SR, Chen W, Greenlund KJ, Berenson GS. Secular trend of earlier onset of menarche with increasing obesity in black and white girls: the Bogalusa Heart Study. Ethn Dis. 1999;9:181–189. [PubMed] [Google Scholar]
- 22.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro J, Santos P, Duarte J, Mota J. Association between overweight and early sexual maturation in Portuguese boys and girls. Ann Hum Biol. 2006;33:55–63. doi: 10.1080/00207390500434135. [DOI] [PubMed] [Google Scholar]
- 24.Demerath EW, Towne B, Chumlea WC, Sun SS, Czerwinski SA, Remsberg KE, et al. Recent decline in age at menarche: the Fels Longitudinal Study. Am J Hum Biol. 2004;16:453–457. doi: 10.1002/ajhb.20039. [DOI] [PubMed] [Google Scholar]
- 25.Kaminski BA, Palmert MR. Genetic control of pubertal timing. Curr Opin Pediatr. 2008;20:458–464. doi: 10.1097/MOP.0b013e3283060ed4. [DOI] [PubMed] [Google Scholar]
- 26.Shalitin S, Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth: a review. Int J Obes Relat Metab Disord. 2003;27:869–874. doi: 10.1038/sj.ijo.0802328. [DOI] [PubMed] [Google Scholar]
- 27.Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111(4 Pt 1):844–850. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- 28.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 29.de Muinich Keizer SM, Mul D. Trends in pubertal development in Europe. Hum Reprod Update. 2001;7:287–291. doi: 10.1093/humupd/7.3.287. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123:84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 31.Korean Statistical Information Service. Statistical database of Korean population census in 2010 [Internet] Daejeon: Statistics Korea; c2010. [cited 2012 Jun 10]. Available from: http://kosis.kr/eng/database/database_001000.jsp?listid=A&subtitle=Population/Household. [Google Scholar]