Abstract
Purpose
This study was performed to evaluate the trabecular pattern on panoramic radiographs to predict age-related osteoporosis in postmenopausal women.
Materials and Methods
Thirty-one postmenopausal osteoporotic women and 25 postmenopausal healthy women between the ages of 50 and 88 were enrolled in this study. The bone mineral density (BMD) of the lumbar vertebrae and femur were calculated using dual-energy X-ray absorptiometry (DXA), and panoramic radiographs were obtained. Fractal dimension (FD) was measured using the box counting method from 560 regions of interest (51×51 pixels) in 6 sites on the panoramic radiographs. The relationships between age and BMD and between FD and BMD were assessed, and the intraobserver agreement was determined.
Results
There was a significant difference in the FD values between the osteoporotic and normal groups (p<0.05). There was a significant difference in the FD values at three sites in the jaws (p<0.05). Age was significantly correlated with the BMD measurements, with an odds ratio of 1.25. However, the FD values were not significantly correlated with the BMD measurements, with an odds ratio of 0.000. The intraobserver agreement showed relatively higher correlation coefficients at the upper premolar, lower premolar, and lower anterior regions than the other sites.
Conclusion
Age was an important risk factor for predicting the presence of osteoporosis in postmenopausal women. The lower premolar region was the most appropriate site for evaluating the FD value on panoramic radiographs. However, further investigation might be needed to predict osteoporosis using an FD value on panoramic radiographs.
Keywords: Osteoporosis, Age-Related; Fractals; Radiography, Panoramic
Introduction
Osteoporosis is a systemic disease characterized by low bone density, deterioration of the bone structure, and increased bone fragility.1 Therefore, bone mineral density (BMD) and trabecular bone structure are important for diagnosing of osteoporosis.
Age is the most important predictor for osteoporosis,2,3 and women tend to lose BMD more rapidly than men, especially in the 5-10 year period after menopause. Geraets and van der Stelt2 reported that the characteristics of the trabecular pattern demonstrated a reasonably good correlation with BMD. Yaşar and Akgünlü3 showed that age was an important risk factor for osteoporosis by using binary logistic regression analysis.
Dual-energy x-ray absorptiometry (DXA)4-6 is known as the most accurate clinical method for identifying those with low BMD. By using a DXA scanner, the BMD of the lumbar spine and femoral neck are calculated. The BMD of the mandible is positively correlated with that in the lumbar spine and femoral neck in osteoporosis.6
Dental radiographs are frequently used imaging modalities for the elderly population. Therefore, dental radiographs may offer an opportunity for a screening tool to predict the presence of osteoporosis.1,7-9 Taguchi et al7 observed a significant linear correlation between lumbar BMD and morphology of the mandibular inferior cortex on panoramic radiographs. Verheij et al1 reported that taking into account both age and the trabecular pattern from dental radiographs increased the sensitivity of predicting the presence of osteoporosis.
The osseous fractal analysis is a useful method to identify the bone trabecular pattern.10,11 Fractal dimension (FD) is a method of quantitatively measuring complex geometric structures that exhibit patterns throughout the image. The FD represents the complexity of the structure with an increasing number, indicating an increasing complexity.10 Fractal analysis has also been used to identify and understand most structures on dental radiographs. Jolley et al11 showed that periapical radiographs could provide a reliable method for determining the FD by analyzing the changes in the alveolar bone density. However, there have been few studies on fractal analysis of panoramic radiographs for predicting osteoporosis.
The aim of the present study was to evaluate the trabecular pattern on panoramic radiographs to predict the presence of age-related osteoporosis.
Materials and Methods
Thirty-one postmenopausal osteoporotic and 25 postmenopausal healthy Korean women between the ages of 50 and 88 years (mean 64.3 years) were enrolled as the study subjects. The exclusion criteria were metabolic bone diseases such as hyperparathyroidism, hypoparathyroidism, diabetes, osteomalacia, thyrotoxicosis, and renal disease. Informed consent was obtained from the patients who participated in this study.
The BMDs of the lumbar spine and femoral neck classified by the World Health Organization (WHO) classification were calculated using DXA with a DXA scanner (Hologic Discovery®, Hologic Inc., Bedford, MA, USA). Osteoporosis was determined according to the criteria from the WHO, in that a subject was classified as having osteoporosis if the T-score of the lumbar spine and femoral neck was -2.5 or less. Panoramic radiographs of the subjects were taken using a Planmeca Proline XC (Planmeca, Helsinki, Finland).
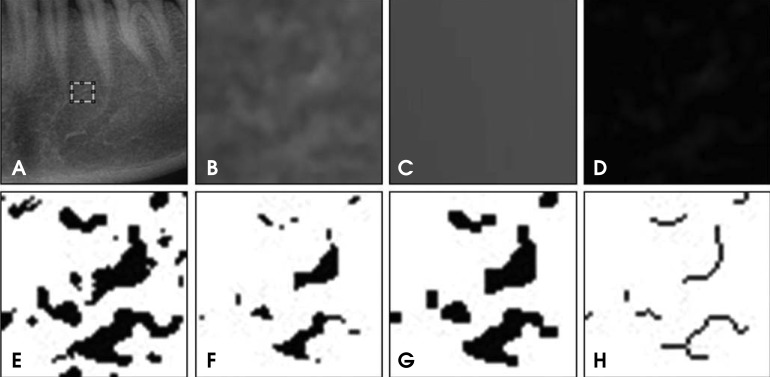
The FDs were measured using the box counting method from 560 regions of interest (ROIs: 51×51 pixels) in 6 sites on the panoramic radiographs. Square ROIs were selected at the interdental 1/3 region including 6 sites (anterior, premolar, and molar regions of the jaws). The ImageJ (ver. 1.45s National Institute of Health, Bethesda, MD, USA) program was used to calculate the FDs. Figure 1 shows the image procedure to make the skeletonized image from a panoramic radiograph. The resultant image was used for the FD.
Fig. 1.
Image processing procedures. A. The ROI (51×51 pixels) on a panoramic radiograph. B. The raw image before processing. C. A gaussian blurred image, D. A subtraction image. E. A binary image. F. An eroded image. G. A dilated image. H. A skeletonized image.
The selected area was processed using the method designed by White and Rudolph.12 Briefly, the transferred ROI was filtered using Gaussian blur with 35 pixels to remove the fine and medium scale variations in the image brightness, and then saved again. Using Scion image, the blurred image was then subtracted from the original image, and gray value of 128 was added. The image was then made binary by threshold at a gray value of 128. The resultant image was converted to a binary image. The binary image was eroded and dilated to reduce the noise. After dilation, the binary image was outlined and skeletonized. Finally, the skeletonized image was used for fractal analysis. All digital manipulations and measurements were made within ROIs. The FD values of the skeletonized images were calculated using the box counting method using the ImageJ program. Initially, the image was converted using a square grid of equally sized tiles; the widths of the square boxes were 2, 3, 4, 6, 8, 12, 16, 32, and 64 pixels. Subsequently, the numbers of the counted tiles were plotted against the total number of tiles in a double logarithmic scale. Finally, the FD values were calculated from the slope of the line filtered on the data points. The FD values were calculated three times with a two week interval by one oral and maxillofacial radiologist.
Statistical analysis of the FD values was performed using 2-way ANOVA to evaluate the difference between the groups and sites. The correlation coefficient was analyzed using the Pearson correlation coefficient. Statistical analysis was performed using statistical software (SPSS, version 12.0 for Windows, Chicago, IL, USA). The relationships between age and BMD and between FD and BMD were assessed, and the intraobserver agreement was determined.
Results
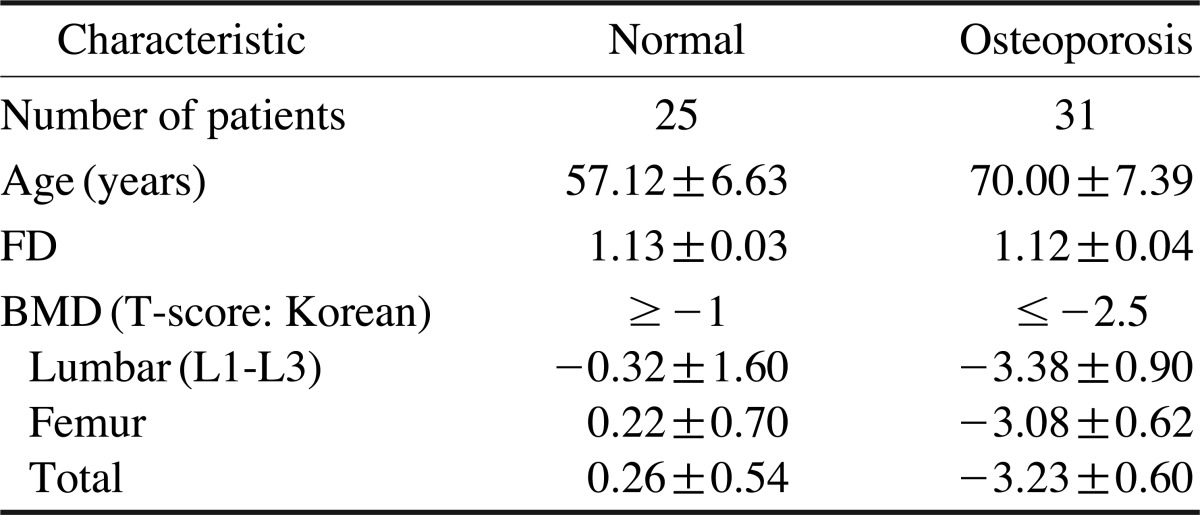
Table 1 shows the characteristics of the study subjects. The mean age of the control group was 57 years and that of the osteoporosis group was 70 years. The mean FD value was 1.13 in the normal group and 1.12 in the osteoporosis group. The mean BMD T-score was -0.26 in the normal group and -3.23 in the osteoporosis group. There was a significant difference in the FD values between the osteoporotic and normal groups (p<0.01).
Table 1.
Age and fractal dimension (FD) of the study subjects
FD: fractal dimension; BMD: bone mineral density
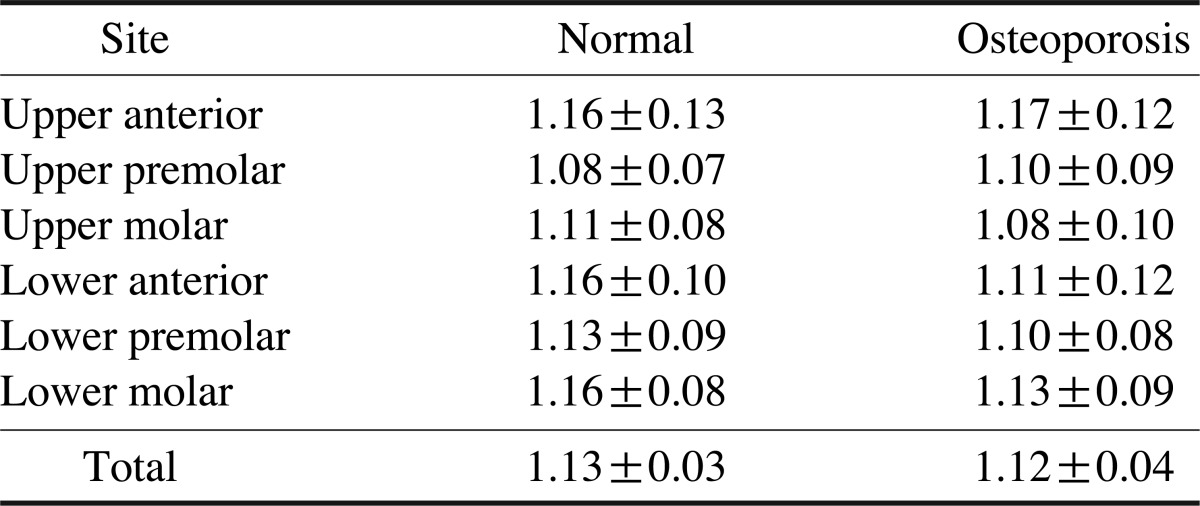
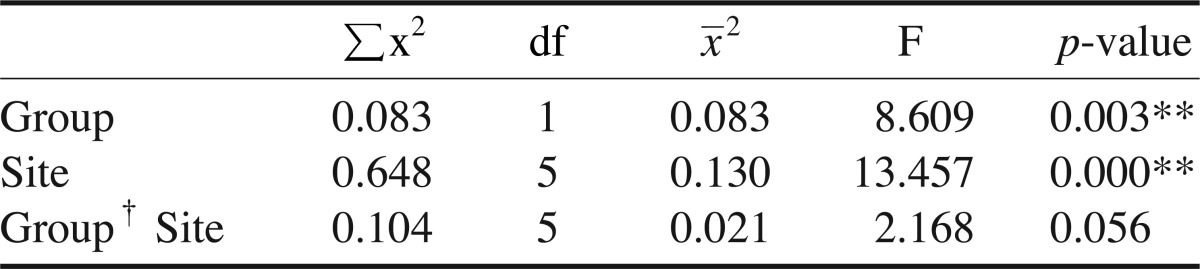
Table 2 shows the FD values of the study subjects according to the sites, and Table 3 shows the differences in the FD values between the groups and sites. A significant difference in the FD values was found between the groups, and at three sites (p<0.01). However, there was no reciprocal action between the groups and sites.
Table 2.
Fractal dimension according to the site
Table 3.
Differences in fractal dimension between the groups and sites
Group† Site: reciprocal action, **: p<0.01
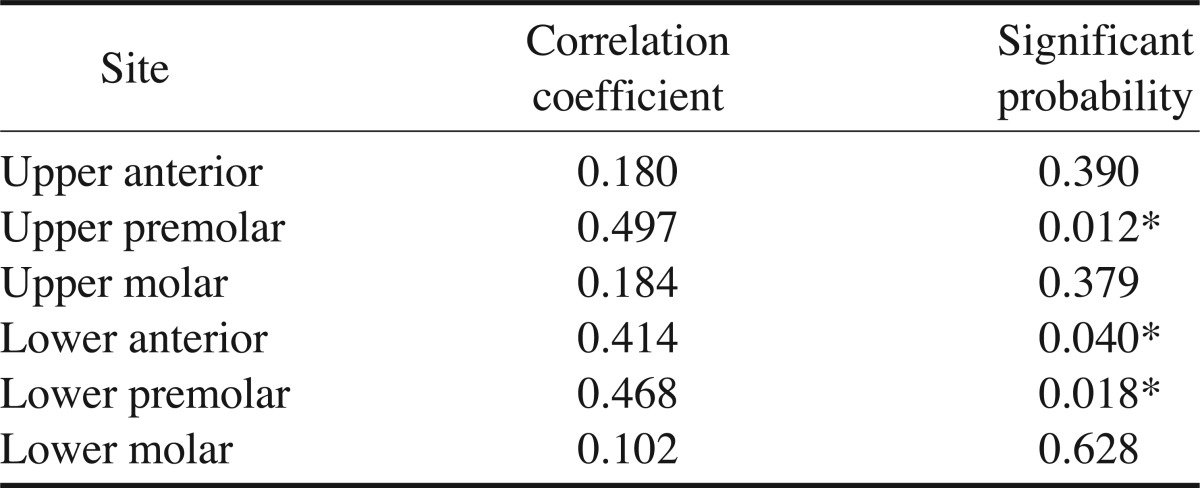
Table 4 shows the intraobserver agreement. The intraobserver agreement showed relatively higher correlation coefficients of over 0.4 at the upper premolar, lower premolar, and lower anterior regions compared to the other regions. However, the correlation coefficient was very low at the upper anterior, upper molar, and lower molar regions.
Table 4.
Intraobserver agreement
*: p<0.05
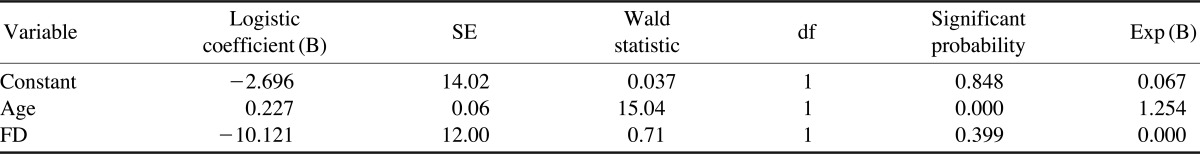
Table 5 shows the logistic coefficient and odds radio. To analyze the effects of age and FD values on osteoporosis, the logistic coefficient and odds ratio were evaluated. Age was significantly correlated with the BMD measurements, with an odds ratio of 1.25; however, the FD values were not significantly correlated with the BMD measurements, with an odds ratio of 0.000.
Table 5.
Logistic regression analysis and odds ratio
P̂=e0.227X1/1+e0.227X1, P̂=Incidence rate of osteoporosis, X1=Age
Discussion
Some studies2,3 have demonstrated that age was the most important predictor for osteoporosis. Amer et al13 showed that there was a significant difference in the FD values among the different age groups. In 1996, the WHO reported that postmenopausal women constituted more than 15% of the population in the developed countries whereas this rate was 5-8% in the less developed regions of the world.14 The subjects of the present study consisted of postmenopausal women over the age of 50. According to the result, age was an important risk factor, with an odds ratio of 1.25, in predicting the presence of osteoporosis.
Fractal analysis appears to be a useful method for estimating the bone density on dental radiographs2,11,13,15,16 and calcaneus radiographic images.17 Amer et al13 demonstrated that there was a significant difference in the FD values among different sites of the jaws on intraoral radiographs. Heo et al15 showed that the FD might be closely related to the radiographic characteristics observed during the healing process. The FD values decreased immediately after the operation and increased gradually according to the bony healing process. Otis et al16 showed that the FD values of the dentoalveolar complex remained relatively unchanged during tooth movement.
Prouteau et al17 showed that the stress fracture group demonstrated a significantly lower FD, reflecting a more complex structure of the trabecular microarchitectural organization with a lower bone mass. In fractal analysis, a tile counting15 or box counting18,19 algorithm has been mainly used to quantify the trabecular pattern by counting the trabecular bone and bone marrow interface. A higher box counting volume indicates a more complex structure. Southard et al20 found that the average FD decreased from 1.26 to 1.1 on the radiographs of decalcified human alveolar bone. However, Ruttimann and Ship10 reported that the FD increased with decalcification.
Southard et al,20 in an in vivo study, confirmed the clinical relevance of this, finding that the FD and density of the alveolar process bone were highly correlated, meaning the FD could represent an objective way to quantify bone quality, irrespective of variations in the exposure settings. Shrout et al21 reported that absolute ROI placement might not be necessary since the FD values as determined from the ROIs of the digital radiographic images of the alveolar bone were insensitive to small variations in X-ray exposure, beam alignment, and ROI position. Jolley et al11 demonstrated that the non-standardized periapical radiographs might provide a reliable method for determining the FD, which could be useful in analyzing changes in alveolar bone density. In our preliminary study, the size and position of the ROI on panoramic radiographs did not affect the FD values.
However, many authors2,22,23 have disagreed with these results. An et al22 showed that an adequate exposure time and image resolution was essential for acquiring the FD. Chappard et al23 insisted that the disagreement of the results in their studies could be due to anatomical variations, discrepancies in the methods, techniques for measuring the FD, and/or the differences in selecting the regions to be measured. Geraets and van der Stelt2 and Veenland et al24 showed that the FD could be severely affected by many factors such as the noise produced during the imaging process. Therefore, a study on FD values should be carefully designed to obtain a more conclusive result.15
In fractal analysis, there are different methods for calculating the FD, which can be used to compare normal bone with osteoporotic bone.2 Yaşar and Akgünlü3 demonstrated that the FD values showed no statistically significant difference between the osteoporotic and non-osteoporotic patients.
The present study demonstrated that the FD on the panoramic radiographs decreased in the osteoporosis group compared with the normal group. It seemed that the FD values decreased as bone density decreased.
The present study showed that the intraobserver agreement demonstrated a relatively high correlation coefficient of over 0.4 at the upper and lower premolar, and lower anterior regions than other sites of the jaws.
In conclusion, age was an important risk factor for predicting the presence of osteoporosis in postmenopausal women. The FD values of the jaws on the panoramic radiographs decreased as the bone density decreased. The lower premolar region was the most appropriate site of the jaws for evaluating the FD value on panoramic radiographs. However, further evaluation would be needed when using the FD value to predict osteoporosis from panoramic radiographs.
References
- 1.Verheij JG, Geraets WG, van der Stelt PF, Horner K, Lindh C, Nicopoulou-Karayianni K, et al. Prediction of osteoporosis with dental radiographs and age. Dentomaxillofac Radiol. 2009;38:431–437. doi: 10.1259/dmfr/55502190. [DOI] [PubMed] [Google Scholar]
- 2.Geraets WG, van der Stelt PF. Fractal properties of bone. Dentomaxillofac Radiol. 2000;29:144–153. doi: 10.1038/sj/dmfr/4600524. [DOI] [PubMed] [Google Scholar]
- 3.Yaşar F, Akgünlü F. The differences in panoramic mandibular indices and fractal dimension between patients with and without spinal osteoporosis. Dentomaxillofac Radiol. 2006;35:1–9. doi: 10.1259/dmfr/97652136. [DOI] [PubMed] [Google Scholar]
- 4.Horner K, Karayianni K, Mitsea A, Berkas L, Mastoris M, Jacobs R, et al. The mandibular cortex on radiographs as a tool for osteoporosis risk assessment: the OSTEODENT Project. J Clin Densitom. 2007;10:138–146. doi: 10.1016/j.jocd.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Karayianni K, Horner K, Mitsea A, Berkas L, Mastoris M, Jacobs R, et al. Accuracy in osteoporosis diagnosis of a combination of mandibular cortical width measurement on dental panoramic radiographs and a clinical risk index (OSIRIS): the OSTEODENT project. Bone. 2007;40:223–229. doi: 10.1016/j.bone.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Horner K, Devlin H, Alsop CW, Hodgkinson IM, Adams JE. Mandibular bone mineral density as a predictor of skeletal osteoporosis. Br J Radiol. 1996;69:1019–1025. doi: 10.1259/0007-1285-69-827-1019. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, et al. Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. AJR Am J Roentgenol. 2004;183:1755–1760. doi: 10.2214/ajr.183.6.01831755. [DOI] [PubMed] [Google Scholar]
- 8.Nackaerts O, Jacobs R, Devlin H, Pavitt S, Bleyen E, Yan B, et al. Osteoporosis detection using intraoral densitometry. Dentomaxillofac Radiol. 2008;37:282–287. doi: 10.1259/dmfr/30424604. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Jung YH, Nah KS. Prediction of osteoporosis using fractal analysis on periapical and panoramic radiographs. Korean J Oral Maxillofac Radiol. 2008;38:147–151. [Google Scholar]
- 10.Ruttimann UE, Ship JA. The use of fractal geometry to quantitate bone structure from radiographs. J Dent Res. 1990;69:287. (Abstr 1431) [Google Scholar]
- 11.Jolley L, Majumdar S, Kapila S. Technical factors in fractal analysis of periapical radiographs. Dentomaxillofac Radiol. 2006;35:393–397. doi: 10.1259/dmfr/30969642. [DOI] [PubMed] [Google Scholar]
- 12.White SC, Rudolph DJ. Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:628–635. doi: 10.1016/s1079-2104(99)70097-1. [DOI] [PubMed] [Google Scholar]
- 13.Amer ME, Heo MS, Brooks SL, Benavides E. Anatomical variations of trabecular bone structure in intraoral radiographs using fractal and particles count analyses. Imaging Sci Dent. 2012;42:5–12. doi: 10.5624/isd.2012.42.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Scientific Group on Research on the Menopause in the 1990s. WHO technical report series 866: research on the menopause in the 1990s. Geneva: World Health Organization; 1996. [PubMed] [Google Scholar]
- 15.Heo MS, Park KS, Lee SS, Choi SC, Koak JY, Heo SJ, et al. Fractal analysis of mandibular bony healing after orthognathic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:763–767. doi: 10.1067/moe.2002.128972. [DOI] [PubMed] [Google Scholar]
- 16.Otis LL, Hong JS, Tuncay OC. Bone structure effect on root resorption. Orthod Craniofac Res. 2004;7:165–177. doi: 10.1111/j.1601-6343.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- 17.Prouteau S, Ducher G, Nanyan P, Lemineur G, Benhamou L, Courteix D. Fractal analysis of bone texture: a screening tool for stress fracture risk? Eur J Clin Invest. 2004;34:137–142. doi: 10.1111/j.1365-2362.2004.01300.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen SK, Oviir T, Lin CH, Leu LJ, Cho BH, Hollender L. Digital imaging analysis with mathematical morphology and fractal dimension for evaluation of periapical lesions following endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:467–472. doi: 10.1016/j.tripleo.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 19.Ergün S, Saraçoğlu A, Güneri P, Özpinar B. Application of fractal analysis in hyperthyroidism. Dentomaxillofac Radiol. 2009;38:281–288. doi: 10.1259/dmfr/24986192. [DOI] [PubMed] [Google Scholar]
- 20.Southard TE, Southard KA, Jakobsen JR, Hillis SL, Najim CA. Fractal dimension in radiographic analysis of alveolar process bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:569–576. doi: 10.1016/s1079-2104(96)80205-8. [DOI] [PubMed] [Google Scholar]
- 21.Shrout MK, Potter BJ, Hildebolt CF. The effect of image variations on fractal dimension calculations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:96–100. doi: 10.1016/s1079-2104(97)90303-6. [DOI] [PubMed] [Google Scholar]
- 22.An BM, Heo MS, Lee SP, Lee SS, Choi SC, Park TW, et al. Effect of exposure time and image resolution on fractal dimension. Korean J Oral Maxillofac Radiol. 2002;32:75–79. [Google Scholar]
- 23.Chappard C, Brunet-Imbault B, Lemineur G, Giraudeau B, Basillais A, Harba R, et al. Anisotropy changes in post-menopausal osteoporosis: characterization by a new index applied to trabecular bone radiographic images. Osteoporos Int. 2005;16:1193–1202. doi: 10.1007/s00198-004-1829-5. [DOI] [PubMed] [Google Scholar]
- 24.Veenland JF, Grashuis JL, Gelsema ES. Texture analysis in radiographs: the influence of modulation transfer function and noise on the discriminative ability of texture features. Med Phys. 1998;25:922–936. doi: 10.1118/1.598271. [DOI] [PubMed] [Google Scholar]