Abstract
Cyclooxygenase 2 (COX2) is overexpressed in 80% of colon adenocarcinomas. However, the mechanism leading to aberrant COX2 expression in tumors is unclear. Intestinal epithelium-specific disruption of the von Hippel–Lindau tumor suppressor protein (VHL) in adenomatous polyposis coli (Apc)min/+ mice (VhlΔIE/Apcmin/+) resulted in constitutive activation of hypoxia-inducible factor (HIF), robustly enhanced colon carcinogenesis and potentiated COX2 expression in normal colon epithelium and tumors. In this study, we hypothesize that HIF regulates COX2 expression in colon tumors, and this regulation is critical for HIF-mediated colon carcinogenesis. COX2 was demonstrated to be a direct target gene of HIF-2α, and genetic disruption of HIF-2α abolished the induction of COX2 in tumors. Furthermore, inhibition of COX2 by nimesulide reduced HIF-2α-induced colon tumor formation. Interestingly, the levels of prostaglandin E2 (PGE2), the downstream effector of COX2, remained elevated in normal and tumor tissues of the nimesulide-treated VhlΔIE/Apcmin/+ mice. Further examination revealed that the terminal PGE2 synthesis enzyme microsomal prostaglandin E synthase 1 (mPGES-1) was overexpressed in the colon of VhlΔIE/Apcmin/+ mice. mPGES-1 was demonstrated to be a direct target gene of HIF-2α, and genetic disruption of HIF-2α abolished the induction of mPGES-1 in colon tumors. Together, our findings demonstrate that HIF-2α is a major regulator of COX2/mPGES-1/PGE2 pathway in colon tumors.
Introduction
Cyclooxgenase-2 (COX2), the inducible isoform of cyclooxygenase, is overexpressed in human colorectal adenomas and adenocarcinomas (1). COX2 is the rate-limiting enzyme in the conversion of arachidonic acid into prostaglandin H2 (PGH2), which is then converted into prostaglandin E2 (PGE2) by prostaglandin E synthases (2). Microsomal prostaglandin E synthase 1 (mPGES-1) is the major isoform involved in promoting PGE2 production in inflammatory sites (3). The COX2/mPGES-1/PGE2 pathway is critical in potentiating colon carcinogenesis, and selective COX2 inhibitors have been approved as adjunctive therapy for patients with familial polyposis (4). Currently, the mechanism by which COX2 is overexpressed in colon tumors is unclear. Hypoxia is a notable feature of the tumor microenvironment and can activate COX2 expression in colon cancer-derived cell lines (5–7). However, it is unclear if hypoxia is essential for COX2 increase in colon tumors in vivo.
Hypoxia signaling is mediated by hypoxia-inducible factor (HIF). HIF is a heterodimer of a constitutively expressed beta subunit (HIF-1β), also known as aryl hydrocarbon receptor nuclear translocator, and a hypoxia-inducible alpha subunit (HIF-1α or HIF-2α (8–11)). Under normoxic conditions, von Hippel–Lindau tumor suppressor protein (VHL) coupled to the E3 ubiquitin ligase complex binds and rapidly degrades HIF-α (12,13). In low oxygen environment, the binding of VHL to HIF-α is decreased, leading to accumulation of HIF-α and transcriptional activation of HIF-α-regulated genes (14). HIF-1α and HIF-2α regulate overlapping and distinct target genes in both physiologic and pathologic conditions (15–17). Previously, we have reported that intestine-specific HIF-2α activation increases COX2 expression and facilitated colon tumorigenesis in the adenomatous polyposis coli (Apc)min/+ intestinal tumor model (18). Here, we hypothesize that HIF-2α regulates COX2 expression in colon tumors, and this regulation is critical for HIF-2α-mediated colon carcinogenesis.
This study demonstrates that COX2, which is overexpressed in HIF-2α-activated Apcmin/+ intestinal tumor model, is a direct target gene for HIF-2α. Moreover, the increase in expression of COX2 was completely ablated in tumors from mice with deletion of HIF-2α. Selective COX2 inhibition by nimesulide reduced HIF-2α-induced colon tumor formation. However, the tumors were still increased in these animals compared with littermate controls treated with nimesulide. This suggests that other mechanisms in addition to COX2 elevation could contribute to the HIF-2α-mediated tumorigenesis. Downstream pathways of COX2 were assessed that could mediate the tumor growth under COX2 inhibition. Further analysis revealed that the expression of mPGES-1 is also dependent on HIF-2α in the Apcmin/+ intestinal tumor model. Luciferase reporter assays and chromatin immunoprecipitation (ChIP) analysis further confirmed that mPGES-1 is directly regulated by HIF-2α. Together, these data uncovered a novel role for HIF-2α in regulating the COX2/mPGES-1/PGE2 signaling pathway to modulate the progression of colon cancer.
Materials and methods
Animals and diets
VhlF/F, VhlΔIE, VhlF/F/Apcmin/+, VhlΔIE/Apcmin/+, VhlF/F/Hif-2αF/F/Apcmin/+ and VhlΔIE/Hif-2αΔIE/Apcmin/+ mice were described previously (18–21). All mice were on a 129S6/SvEv background and maintained in standard cages in a light and temperature-controlled room and were allowed standard chow and water ad libitum. For the COX2 inhibition study, 6-week-old VhlΔIE/Apcmin/+ (n = 27) and VhlF/F/Apcmin/+ mice (n = 19) were given powdered laboratory rodent diet 5001 (PMI Nutrition International LLC, Brentwood, MO) with or without 400mg/kg nimesulide (Sigma, St Louis, MO) for 8 weeks. For indoleamine 2,3-dioxygenase (Ido1) inhibition study, 6-week-old VhlΔIE/Apcmin/+ (n = 9) and VhlF/F/Apcmin/+ mice (n = 10) were given 1-methyl-L-tryptophan (Sigma, St Louis, MO) in the drinking water (5mg/ml, pH 9.9). All animal studies were carried out in accordance with the institute of laboratory animal resources guidelines and approved by the university committee on the use and care of animals at the University of Michigan (UCUCA approval number: 10299).
Histology and immunohistochemistry
Following treatment, all mice were euthanized with carbon dioxide. Colons were excised and cut open longitudinally, and tumors were counted and sized. Following tumor counting, the colons were swiss-rolled from the distal to the proximal end, fixed overnight in 10% formalin and embedded in paraffin. About 5 µm tissue sections were prepared and stained with hematoxylin and eosin for histologic analysis by a gastrointestinal pathologist. Tumor progression was based on invasiveness and glandular and epithelial organization. For immunohistochemistry, sections were deparaffinized in xylene and rehydrated in ethanol gradient. After antigen retrieval with citrate buffer and blocking with 5% normal goat serum, sections were incubated with an antibody for COX2 (Cell Signaling Technology, Danvers, MA), followed by detection with Alexa Fluor 488 goat antirabbit immunoglobulin G (Life Technologies Corporation, Grand Island, NY).
Quantitative real-time reverse transcriptase–PCR
RNA was isolated from frozen tissue using Isol-RNA lysis reagent (3 Prime, Gaithersburg, MD) and quantitated using the NanoDrop 2000 (NanoDrop products, Wilmington, DE). RNA with a purity (260/280 ratio) of approximately 2.0 was reverse transcribed using M-MLV reverse transcriptase (Fisher Scientific, Waltham, MD). Messenger RNA expression was measured by real-time reverse transcriptase–PCR using SYBR green (Life Technologies, Carlsbad, CA) (primers are listed in Supplementary Table 1, available at Carcinogenesis Online). Ct values were normalized to β-actin and expressed as fold difference from controls.
Luciferase assay
HCT116 cells were maintained at 37°C in 5% carbon dioxide and 21% oxygen. Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic (1 unit/ml of penicillin, 1mg/ml of streptomycin and 2.5ng/ml of amphotericin B; Life Technologies). COX2 promoter luciferase-reporter constructs were kind gifts from Dr Hiroyasu Inoue, Nara Women’s University (22). Super-repressor form of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (SR-IκBα) plasmid construct was kind gift from Dr Albert S.Baldwin Jr from The University of North Carolina at Chapel Hill (23). mPGES-1 promoter luciferase constructs were generated using primers listed in Supplementary Table 1, available at Carcinogenesis Online. The luciferase reporters were co-transfected with HIF-2α, SR-IκBα or empty vector into HCT116 cells with Fugene 6 transfection reagent (Roche, Indianapolis, IN). Standard dual luciferase assay was performed as described previously (21).
ChIP assay
ChIP assays were performed as described previously (21). Briefly, colon epithelium scrapings from VhlF/F and VhlΔIE mice were crosslinked and nuclei were isolated. Sheared soluble chromatin was immunoprecipitated with primary antibody for HIF-2α (Novus Biologicals), and 1 µl of sample was used for quantitative real-time reverse transcriptase–PCR (qPCR) using primers listed in Supplementary Table 1, available at Carcinogenesis Online. The data are expressed as fold enrichment over control IgG and normalized to input.
PGE2 detection by liquid chromatography-mass spectrometry
Colon epithelial cells were homogenized in 1× phosphate-buffered saline, and two equivalent volumes of acetone were added and incubated with shaking for 10min. The samples were centrifuged and supernatants were dried under vacuum centrifugation. The samples and purified PGE2 (Cayman Chemical, Ann Arbor, MI) were resuspended in 60% acetonitrile. Mass spectrometric analysis was performed on an Applied Biosystems (Foster City, CA) triple quadrupole mass spectrometer. PGE2 concentrations were determined by the integration of peak area and quantitated using standard curve with linear range from 20 to 1000nM of PGE2.
Statistics
Results are expressed as mean ± SD. P values were calculated by independent t-test, one-way analysis of variance, Dunnett’s t-test, Mann–Whitney test and two-way analysis of variance. P < 0.05 was considered significant.
Results
COX2 expression is dependent on HIF-2α in the Apcmin/+ intestinal tumor model
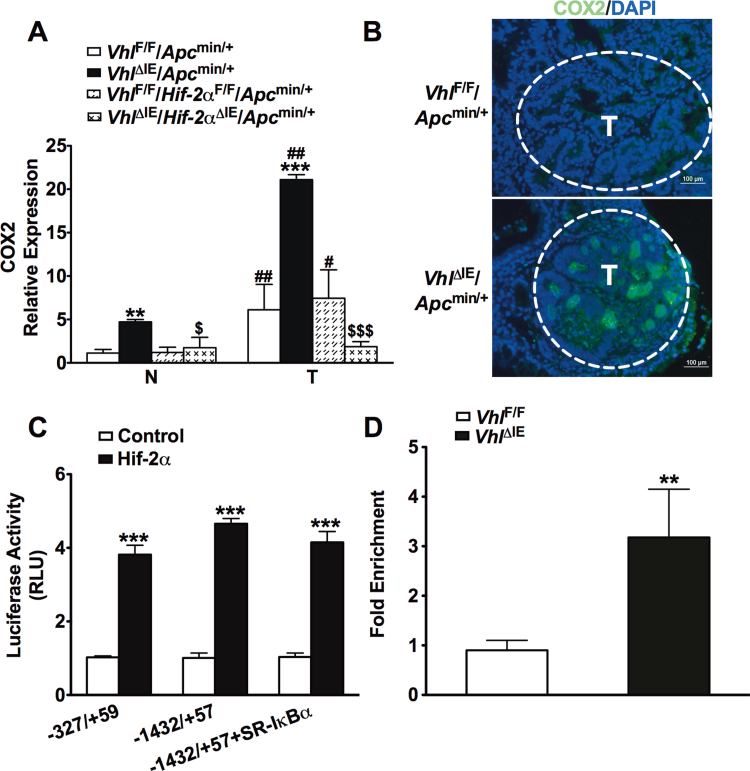
Intestine-specific disruption of Vhl (VhlΔIE) activates HIF signaling, and when these mice are crossed to the Apcmin/+ intestinal tumor model (VhlΔIE/Apcmin/+), colon tumorigenesis is robustly increased compared with littermate control mice (VhlF/F/Apcmin/+ (18)). COX2 plays an important role in colon carcinogenesis (24), and hypoxia can activate COX2 gene expression (6,7,25). Therefore, COX2 expression was assessed in VhlΔIE/Apcmin/+ and VhlF/F/Apcmin/+ mice by qPCR analysis. Consistent with other reports (26), tumors isolated from littermate control VhlF/F/Apcmin/+ mice demonstrate an increase in COX2 gene expression compared with their adjacent normal tissue. COX2 gene expression was further potentiated in colon tumors and adjacent normal tissue from VhlΔIE/Apcmin/+ mice compared with VhlF/F/Apcmin/+ mice (Figure 1A). The increase in colon tumor multiplicity, incidence and progression observed in the VhlΔIE/Apcmin/+ mice is completely dependent on HIF-2α expression (18). To assess if COX2 gene expression is HIF-2α dependent, a double knockout mouse model of Vhl and Hif-2α on an Apcmin/+ background (VhlΔIE/Hif-2αΔIE/Apcmin/+) was compared with their littermate controls (VhlF/F/Hif-2αF/F/Apcmin/+). The compound disruption of Vhl and Hif-2α demonstrated that the increase in COX2 observed in tumor tissue compared with normal tissue in the VhlF/F/Apcmin/+ mice and the potentiation of this expression observed in the VhlΔIE/Apcmin/+ mice was due to HIF-2α (Figure 1A). The induction of COX2 in VhlΔIE/Apcmin/+ tumors was confirmed by immunohistochemistry (Figure 1B). These data demonstrate that HIF-2α is essential in the increase of COX2 observed in colon tumors. COX2 is a HIF-1α direct target gene in vitro (6,7). To investigate whether COX2 is a target gene of HIF-2α, luciferase-reporter assay and in vivo ChIP assay were performed. The COX2 promoter contains several HIF response elements (HRE) and the −327/+59 and −1432/+57 COX2 promoter luciferase constructs demonstrated that HIF-2α is capable of activating the COX2 promoter at proximal and distal HREs (Figure 1C). The nuclear factor-kappaB (NF-κB) p65 transcription factor is reported to mediate the hypoxic induction of COX2 (5). Repressing the nuclear translocation of NF-κB with a super-repressor form of IκBα (SR-IκBα (23)) did not reverse HIF-2α-mediated activation of the COX2 promoter (Figure 1C), demonstrating a NF-κB independent pathway is involved. Furthermore, in vivo ChIP assay from colons of VhlΔIE and VhlF/F mice demonstrates direct HIF-2α binding to the mouse COX2 promoter (Figure 1D). These results provide evidence that HIF-2α directly regulates COX2 expression independent of NF-κB signaling pathway. Together, these data demonstrate the importance of HIF-2α signaling in COX2 expression in colon tumors.
Fig. 1.
COX2 expression is dependent on HIF-2α in colon tumors. (A) qPCR analysis of COX2 in normal and tumor colon tissues from VhlF/F/Apcmin/+, VhlΔIE/Apcmin/+, VhlF/F/Hif-2αF/F/Apcmin/+ and VhlΔIE/Hif-2αΔIE/Apcmin/+ mice. Expression was normalized to β-actin. (B) COX2 staining in colon tissues from VhlF/F/Apcmin/+ and VhlΔIE/Apcmin/+ mice. (C) Luciferase-reporter constructs under the control of the proximal 5′-flanking region of the human COX2 gene (−327/+59 or −1432/+57). HCT116 cells transiently transfected with the luciferase construct and co-transfected with empty vector (control), HIF-2α or SR-IκBα expression plasmids. Standard dual luciferase assays were performed on cell extracts as described in the Materials and methods. (D) In vivo ChIP assays were performed on colon extracts from VhlF/F and VhlΔIE mice using primers amplifying the proximal 5′-flanking region of the mouse COX2 gene. Each bar represents the mean value ± SD. **P < 0.01 and ***P < 0.001, compared with empty transfection, VhlF/F mice or VhlF/F/Apcmin/+ mice; #P < 0.05, ##P < 0.01 versus normal tissue; $P < 0.05, $$$P < 0.001 versus VhlΔIE/Apcmin/+. N, normal tissue; T, tumor tissue.
COX2 inhibition reduces HIF-2α-induced colon tumor formation
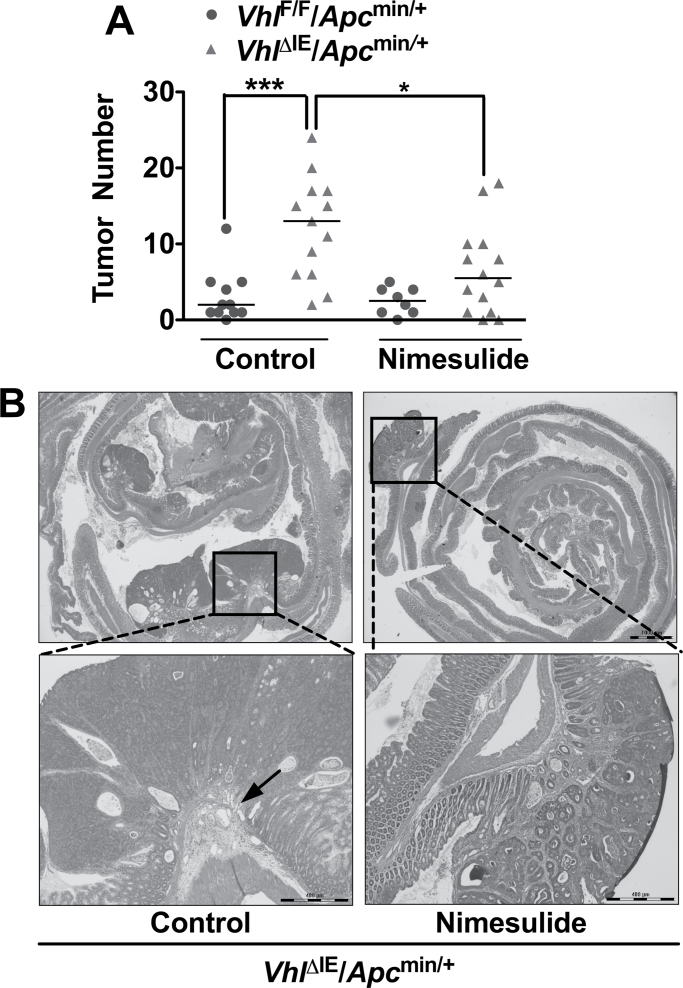
To determine if the increased COX2 expression contributes to the increase in colon carcinogenesis in VhlΔIE/Apcmin/+ mice, diet containing nimesulide or matched control diet was fed to VhlΔIE/Apcmin/+ and VhlF/F/Apcmin/+ mice for 8 weeks. As expected, the VhlΔIE/Apcmin/+ mice on control diet demonstrated increased colon tumor numbers compared with littermate controls (P < 0.001) (Figure 2A). The increase in colon tumors is attenuated in VhlΔIE/Apcmin/+ mice fed with the nimesulide diet for 2 months (P < 0.05) (Figure 2A). Similar to our previous report (18), all tumors assessed in the colon from 3-month-old VhlF/F/Apcmin/+ mice on control or nimesulide diet demonstrated well-organized glandular structures and was classified as adenomas. However, histological analysis revealed that 2 out of 11 tumors from 3-month-old VhlΔIE/Apcmin/+ mice on control diet demonstrated early signs of carcinoma formation, whereas no carcinomas were observed in VhlΔIE/Apcmin/+ mice fed with nimesulide diet (Figure 2B). These results demonstrate that COX2 induction by HIF-2α signaling pathway contributes to colon tumorigenesis and cancer progression.
Fig. 2.
COX2 inhibition decreases HIF-2α-mediated intestinal tumorigenesis. (A) Tumor numbers were counted in the colons from VhlF/F/Apcmin/+ (n = 19) and VhlΔIE/Apcmin/+ mice (n = 27) treated with control or nimesulide diet for 2 months. *P < 0.05, ***P < 0.001. (B) Hematoxylin and eosin staining of representative colon from 3-month-old VhlΔIE/Apcmin/+ mice treated with control or nimesulide diet for 2 months. The arrow indicates tumor invading into submucosa.
COX2 inhibition reduces inflammatory responses in the HIF-2α-induced colon tumor model
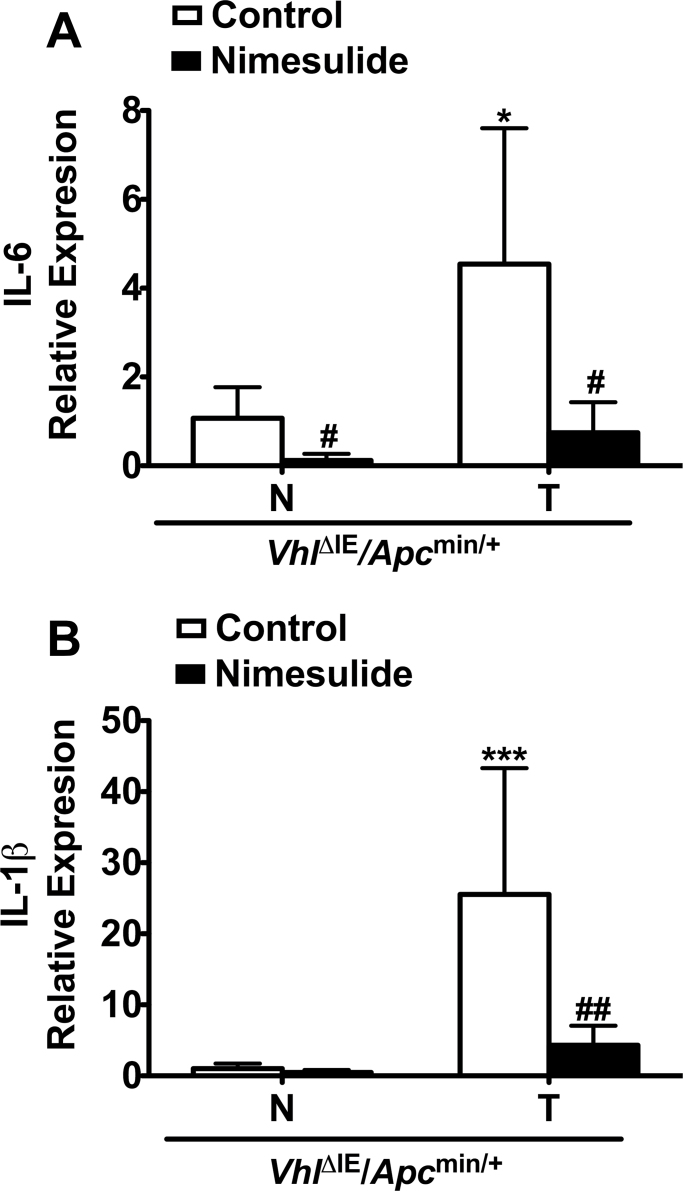
Nimesulide decreased tumors in VhlΔIE/Apcmin/+ mice compared with control diet–treated VhlΔIE/Apcmin/+ mice. However, COX2 inhibition did not completely inhibit tumorigenesis in the VhlΔIE/Apcmin/+ mice because colon tumors remained elevated as compared with nimesulide-treated VhlF/F/Apcmin/+ mice. This suggests that HIF-2α might activate pathways in addition to those inhibited by non-steroidal anti-inflammatory drugs. As inflammation is a major risk factor for colon carcinogenesis and COX2 inhibitors are anti-inflammatory agents, critical proinflammatory mediators were assessed by qPCR analysis in VhlΔIE/Apcmin/+ mice treated with control or nimesulide diet. Interleukin (IL)-1β and IL-6 expression were increased in tumor tissue compared with normal colon tissue (Figure 3). Nimesulide treatment suppressed the expression of proinflammatory mediators in colon tumor tissues from VhlΔIE/Apcmin/+ mice (Figure 3). These results show that inflammation is significantly suppressed by nimesulide, and other pathways downstream or in addition to COX2 are involved in the HIF-2α-mediated tumorigenesis. Ido1 is a downstream effector of COX2 that facilitates immune tolerance in tumor cells (27–29). Crosstalk between the COX2 and Ido1 pathways is suggested (6,30). Ido1 gene expression was found to be highly elevated in normal and tumor tissue from VhlΔIE/Apcmin/+ mice compared with VhlF/F/Apcmin/+ mice (Supplementary Figure 1A, available at Carcinogenesis Online). However, the Ido1 inhibitor, 1-methyl-L-tryptophan, did not reduce colon tumors in the VhlΔIE/Apcmin/+ mice (Supplementary Figure 1B, available at Carcinogenesis Online (28,31,32)). These results indicate that Ido1 is not involved in HIF-2α-induced colon tumorigenesis.
Fig. 3.
COX2 inhibition reduces proinflammatory gene expression in HIF-2α-induced colon tumors. qPCR analysis of IL-6 (A) and IL-1β (B) in control– or nimesulide diet–treated normal and tumor colon tissues from VhlΔIE/Apcmin/+ mice. Each bar represents the mean value ± SD. (n = 4). Expression was normalized to β-actin. N, normal tissue; T, tumor tissue; *P < 0.05, ***P < 0.001 versus normal tissue; #P < 0.05, ##P < 0.01 versus control diet–treated mice.
COX2 inhibition reduces PGE2 levels in HIF-2α-induced colon tumors
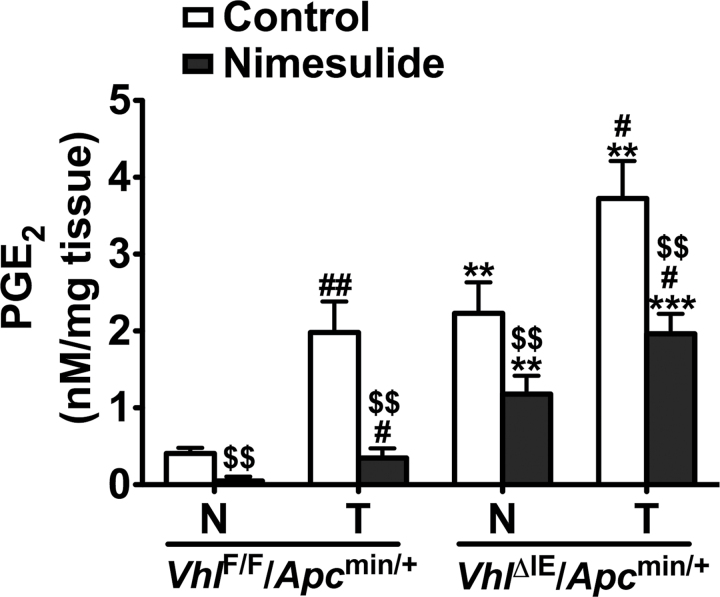
Because Ido1 inhibition could not reduce the growth of HIF-2α-induced colon tumors, other pathways downstream of COX2 were assessed. As COX2-derived PGE2 promotes the progression of colon cancer (33), we assessed the PGE2 levels in VhlΔIE/Apcmin/+ and VhlF/F/Apcmin/+ mice treated with control or nimesulide diet. Compared with VhlF/F/Apcmin/+ mice, PGE2 levels in normal colon tissues were significantly increased in VhlΔIE/Apcmin/+ mice, and the increase is further potentiated in colon tumor tissues (Figure 4). Nimesulide treatment suppressed PGE2 levels in both the normal and tumor tissues from both genotypes compared with control diet–treated mice. However, after nimesulide treatment, PGE2 levels remained elevated in both normal and tumor colon tissues from VhlΔIE/Apcmin/+ mice compared with those from VhlF/F/Apcmin/+ mice (Figure 4). These results suggest that enzymes downstream of COX2 may also be important for the HIF-2α-mediated PGE2 increase.
Fig. 4.
COX2 inhibition reduces PGE2 levels in HIF-2α-induced colon tumors. Liquid chromatography-mass spectrometry analysis of PGE2 levels in control– or nimesulide diet–treated normal and tumor colon tissues from VhlF/F/Apcmin/+ and VhlΔIE/Apcmin/+ mice. Each bar represents the mean value ± SD. (n = 3). N, normal tissue; T, tumor tissue; **P < 0.01, ***P < 0.001 versus VhlF/F/Apcmin/+ mice; #P < 0.05, ##P < 0.01 versus normal tissue; $$P < 0.01 versus control diet–treated mice.
mPGES-1 expression is dependent on HIF-2α in the Apcmin/+ intestinal tumor model
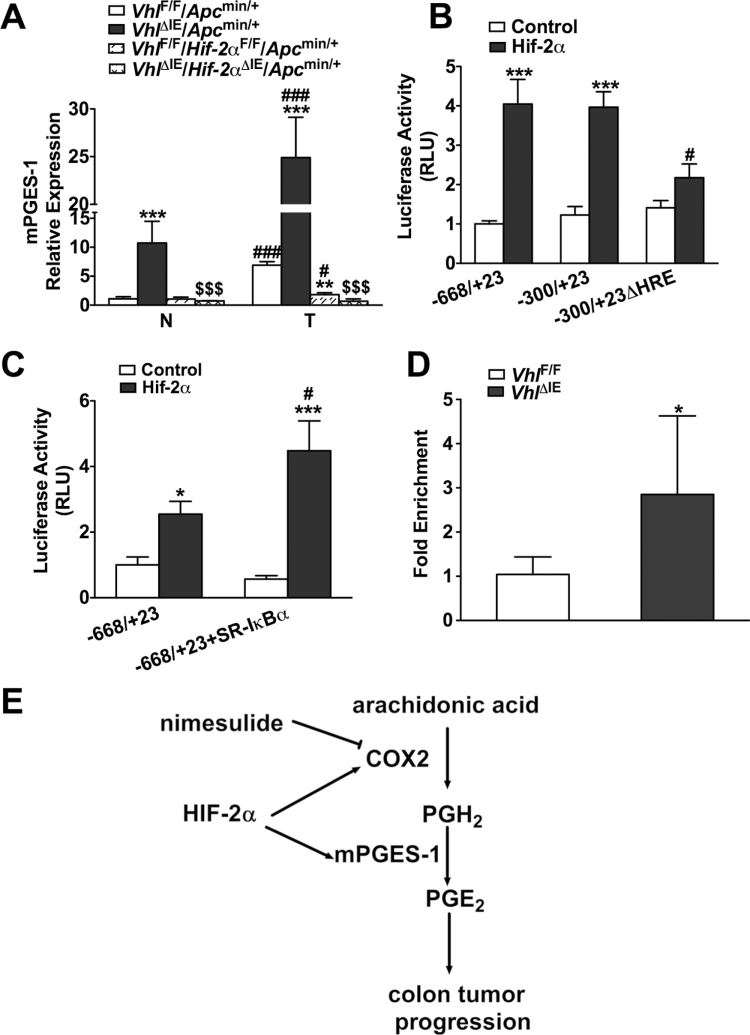
mPGES-1 is the enzyme that converts PGH2 to PGE2 (3). It is an essential enzyme that functions downstream of COX2 and is activated by proinflammatory stimuli and hypoxia (25). Similar to COX2, mPGES-1 is highly induced in tumors compared with normal tissue from the littermate control VhlF/F/Apcmin/+ mice, and the expression is further potentiated in colon tumors and adjacent normal tissue from VhlΔIE/Apcmin/+ mice. The compound disruption of Vhl and Hif-2α demonstrated a very similar gene expression pattern to COX2. The compound disruption of Vhl and Hif-2α demonstrated that the increase in mPGES-1 observed in tumor tissue compared with normal tissue in the VhlF/F/Apcmin/+ mice and the potentiation of this expression observed in the VhlΔIE/Apcmin/+ mice was due to HIF-2α (Figure 5A). To assess whether mPGES-1 is a direct target gene of HIF-2α, luciferase reporter assays were performed using the luciferase constructs containing the mPGES-1 promoter (−668/+23 and −300/+23). HCT116 cells co-transfected with the promoter luciferase constructs, and HIF-2α demonstrated increased luciferase activity for both constructs. Demonstrating that HIF-2α regulates the promoter of mPGES-1, a single consensus HRE was identified within the −300/+23 promoter region, and deletion of this HRE (−300/+23ΔHRE) decreased HIF-2α-induced promoter activity (Figure 5B). Inhibiting the nuclear translocation of NF-κB with SR-IκBα could not repress the activation of the mPGES-1 promoter by HIF-2α (Figure 5C), thus demonstrating that NF-κB signaling pathway was not involved. Furthermore, in vivo ChIP assay from colons of VhlΔIE and VhlF/F mice demonstrates direct HIF-2α binding to the mouse mPGES-1 promoter (Figure 5D). These results provide evidence that HIF-2α directly regulates mPGES-1 expression. Taken together, the results demonstrate that HIF-2α is a critical mediator of the COX2/mPGES-1/PGE2 pathway in colon tumors (Figure 5E).
Fig. 5.
mPGES-1 expression is dependent on HIF-2α in colon tumors. (A) qPCR analysis of mPGES-1 in normal and tumor colon tissues from VhlF/F/Apcmin/+, VhlΔIE/Apcmin/+, VhlF/F/Hif-2αF/F/Apcmin/+ and VhlΔIE/Hif-2αΔIE/Apcmin/+ mice. Expression was normalized to β-actin. N, normal tissue; T, tumor tissue. (B and C) Luciferase-reporter constructs under the control of the proximal promoter of the human Ptges gene (−668/+23 and −300/+23 or −300/+23ΔHRE). HCT116 cells transiently transfected with the luciferase construct and co-transfected with empty vector (control), HIF-2α or SR-IκBα plasmids. Standard dual luciferase assays were performed on cell extracts as described in the Materials and methods. (D) In vivo ChIP assays on colon extracts from VhlF/F and VhlΔIE mice using primers amplifying the proximal promoter of the mouse mPGES-1. Each bar represents the mean value ± SD. *P < 0.05, **P < 0.01 and ***P < 0.001, compared with empty transfection, VhlF/F mice or VhlF/F/Apcmin/+ mice; #P < 0.05, ###P < 0.001 versus −668/+23 or normal tissue; $$$P < 0.001 versus VhlΔIE/Apcmin/+. (E) Schematic diagram of COX2 and mPGES-1 expression in colon tumors. COX2 is the rate-limiting enzyme in the conversion of arachidonic acid into PGH2, which is converted into PGE2 by mPGES-1. PGE2 promotes the progression of colon cancer. Nimesulide is a selective COX2 inhibitor, whereas HIF-2α induces the expression of both COX2 and mPGES-1 in colon tumors.
Discussion
The COX2/mPGES-1/PGE2 signaling axis is aberrantly activated in the majority of colon cancers and has a critical role in promoting carcinogenesis (34). COX2 is overexpressed in human colon cancers but not in adjacent normal mucosa (1,35–37). Transgenic overexpression of COX2 in colon epithelial cells promotes colon tumor progression following colon carcinogen initiation (38). Genetic disruption or pharmacologic inhibition of COX2 dramatically suppresses the number and size of intestinal polyps in different mouse models (39,40). mPGES-1 is a downstream enzyme of COX2 in the PGE2-biosynthetic pathway and is also overexpressed in human colon cancers but not in normal tissues (41,42). Genetic deletion of mPGES-1 significantly reduces the number and size of intestinal tumor in the chemical carcinogen-induced colon carcinogenesis mouse model, whereas transgenic overexpression of mPGES-1 enhances colon tumorigenesis (43,44). The mechanisms that drive COX2 and mPGES-1 expression in colon cancer are unclear. This study demonstrates that COX2 and mPGES-1 are regulated by HIF-2α and activated in tumors through a HIF-2α-dependent pathway.
HIF-2α facilitates colon carcinogenesis by inducing COX2 expression
HIF-1α and HIF-2α are overexpressed in a variety of tumor tissues and play overlapping and distinct functions (17). In renal carcinomas, HIF-1α and HIF-2α have opposing roles in cell proliferation. HIF-1α decreases cell proliferation, whereas HIF-2α induces proliferation via an increase in c-Myc activity (45). Both HIF-1α and HIF-2α are overexpressed in colon cancer (46,47). However, the role of HIF-α in colon carcinogenesis remains unclear. HIF-1α was reported to directly up-regulate COX2 and promote colon cancer cell line survival under hypoxic conditions (7). Here, we found that HIF-2α activation in vivo could also directly up-regulate COX2 expression and facilitate colon tumorigenesis. The present data demonstrate that HIF-2α has a central role in the regulation of COX2 expression in tumors because its genetic ablation markedly represses COX2 expression in colon tumors.
HIF-2α-mediated mPGES-1 expression promotes PGE2 production under COX2 inhibition
Selective COX2 inhibition by nimesulide reduced colon tumor formation compared with control treatment in the VhlΔIE/Apcmin/+ mice, demonstrating that COX2 is an important protein involved in hypoxic tumor progression. However, the numbers of colon tumor in nimesulide-treated VhlΔIE/Apcmin/+ mice were still elevated compared with nimesulide-treated VhlF/F/Apcmin/+ mice. These data suggest that HIF-2α may also activate pathways downstream of COX2 that are important in maintaining colon cancer growth following COX2 inhibition. PGE2 is elevated in colon cancer (48). PGE2 promotes intestinal tumor growth (49) and ablates non-steroidal anti-inflammatory drug-induced intestinal tumor regression in Apcmin/+ mice (50). Furthermore, genetic deletion of mPGES-1 suppressed intestinal tumor growth in Apcmin/+ mice (44). The levels of PGE2 in normal and tumor tissues from the VhlΔIEApcmin/+ mice were reduced by nimesulide, but they were still significantly higher compared with normal and tumor tissues from nimesulide-treated VhlF/FApcmin/+ mice. This suggests that HIF-2α activates pathways downstream of COX2 important for PGE2 production. Indeed, an increase in mPGES-1 is observed in VhlΔIEApcmin/+ mice. The mPGES-1 gene is induced by hypoxia and HIF-1α in cancer-derived cell lines (25,51). However, the mechanism by which HIF regulates mPGES-1 is not clear. This study identifies a functional HRE in the mPGES-1 promoter, which is bound by HIF-2α in vivo. Moreover, the data in this study clearly demonstrate that HIF-2α is critical for mPGES-1 expression in colon tumors.
HIF-2α is a therapeutic target for colon cancer
The findings demonstrate that tumor hypoxia is a critical mediator of aberrant COX2 and mPGES-1 expression in colon cancer. COX2 and mPGES-1 are considered attractive therapeutic targets for anticancer drug discovery. However, targeting the COX2/mPGES-1/PGE2 pathways is challenging (52–56). Long-term COX2 inhibition leads to deleterious side effects. COX2 inhibitors promote hypertension, thrombosis and increase cardiovascular risk (52–54). Whereas compounds found to inhibit mPGES-1 in cancer-derived cell lines, rarely exhibit potent inhibitor function in vivo (55,56). Thus, HIF-2α inhibition may provide an alternative target for the prevention and treatment of colon cancer. In conclusion, our findings demonstrate that HIF-2α activates COX2/mPGES-1/PGE2 signaling pathway to facilitate colon tumorigenesis.
Supplementary material
Supplementary Table 1 and Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (grant CA148828 to Y.M.S.); The University of Michigan Gastrointestinal Peptide Center; Jeffrey A.Colby Colon Cancer Research and the Tom Liu Memorial Funds of the University of Michigan Comprehensive Cancer Center.
Supplementary Material
Acknowledgments
We appreciate Dr Bishr Omary and members of the Shah lab for critical discussion of the manuscript. We would like to thank Dr Hiroyasu Inoue for providing COX2 promoter luciferase-reporter constructs, and Dr Albert S.Baldwin Jr for providing SR-IκBα plasmid construct.
Conflicts of Interest: none declared.
Glossary
Abbreviations:
- Apc
adenomatous polyposis coli
- ChIP
chromatin immunoprecipitation
- COX2
cyclooxygenase 2
- HIF
hypoxia-inducible factor
- HRE
HIF response elements
- Ido1
indoleamine 2,3-dioxygenase
- IL
interleukin
- mPGES-1
prostaglandin E synthase 1
- NF-κB
nuclear factor-kappaB
- PGE2
prostaglandin E2
- qPCR
quantitative real-time reverse transcriptase–PCR
- VHL
von Hippel–Lindau tumor suppressor protein.
References
- 1.Eberhart C.E., et al. (1994)Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1071183–1188 [DOI] [PubMed] [Google Scholar]
- 2.Jakobsson P.J., et al. (1999)Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U.S.A. 967220–7225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami M., et al. (2006)Prostaglandin E synthase: a novel drug target for inflammation and cancer. Curr. Pharm. Des. 12943–954 [DOI] [PubMed] [Google Scholar]
- 4.Steinbach G., et al. (2000)The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 3421946–1952 [DOI] [PubMed] [Google Scholar]
- 5.Schmedtje J.F., Jr, et al. (1997)Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 272601–608 [DOI] [PubMed] [Google Scholar]
- 6.Csiki I., et al. (2006)Thioredoxin-1 modulates transcription of cyclooxygenase-2 via hypoxia-inducible factor-1alpha in non-small cell lung cancer. Cancer Res. 66143–150 [DOI] [PubMed] [Google Scholar]
- 7.Kaidi A., et al. (2006)Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 666683–6691 [DOI] [PubMed] [Google Scholar]
- 8.Eltzschig H.K., et al. (2011)Hypoxia and inflammation. N. Engl. J. Med. 364656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertout J.A., et al. (2008)The impact of O2 availability on human cancer. Nat. Rev. Cancer 8967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza G.L., et al. (1992)A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 125447–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G.L., et al. (1993)Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 26821513–21518 [PubMed] [Google Scholar]
- 12.Ivan M., et al. (2001)HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292464–468 [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola P., et al. (2001)Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292468–472 [DOI] [PubMed] [Google Scholar]
- 14.Wang G.L., et al. (1993)General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. U.S.A. 904304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G.L.v.(1995)Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 925510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian H., et al. (1997)Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1172–82 [DOI] [PubMed] [Google Scholar]
- 17.Loboda A., et al. (2010)HIF-1 and HIF-2 transcription factors–similar but not identical. Mol. Cells 29435–442 [DOI] [PubMed] [Google Scholar]
- 18.Xue X., et al. (2012)Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 722285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah Y.M., et al. (2009)Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9152–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase V.H., et al. (2001)Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl. Acad. Sci. U.S.A. 981583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor M., et al. (2011)Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 1402044–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue H., et al. (1999)Glucocorticoid-mediated suppression of the promoter activity of the cyclooxygenase-2 gene is modulated by expression of its receptor in vascular endothelial cells. Biochem. Biophys. Res. Commun. 254292–298 [DOI] [PubMed] [Google Scholar]
- 23.Wang C.Y., et al. (1996)TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 274784–787 [DOI] [PubMed] [Google Scholar]
- 24.Brown J.R., et al. (2005)COX-2: a molecular target for colorectal cancer prevention. J. Clin. Oncol. 232840–2855 [DOI] [PubMed] [Google Scholar]
- 25.Lee J.J., et al. (2010)Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis 31427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan K.M., et al. (1999)The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 2821254–1257 [DOI] [PubMed] [Google Scholar]
- 27.Cesario A., et al. (2011)The interplay between indoleamine 2,3-dioxygenase 1 (IDO1) and cyclooxygenase (COX)-2 in chronic inflammation and cancer. Curr. Med. Chem. 182263–2271 [DOI] [PubMed] [Google Scholar]
- 28.Uyttenhove C., et al. (2003)Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 91269–1274 [DOI] [PubMed] [Google Scholar]
- 29.Muller A.J., et al. (2005)Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11312–319 [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee P., et al. (2009)Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J. Immunol. 182216–224 [PMC free article] [PubMed] [Google Scholar]
- 31.Koblish H.K., et al. (2010)Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol. Cancer Ther. 9489–498 [DOI] [PubMed] [Google Scholar]
- 32.Hou D.Y., et al. (2007)Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 67792–801 [DOI] [PubMed] [Google Scholar]
- 33.Wang D., et al. (2010)Eicosanoids and cancer. Nat. Rev. Cancer 10181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marnett L.J., et al. (2002)COX-2: a target for colon cancer prevention. Annu. Rev. Pharmacol. Toxicol. 4255–80 [DOI] [PubMed] [Google Scholar]
- 35.Cianchi F., et al. (2001)Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology 1211339–1347 [DOI] [PubMed] [Google Scholar]
- 36.Sano H., et al. (1995)Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 553785–3789 [PubMed] [Google Scholar]
- 37.Dimberg J., et al. (1999)Differential expression of cyclooxygenase 2 in human colorectal cancer. Gut 45730–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Salihi M.A., et al. (2009)Transgenic expression of cyclooxygenase-2 in mouse intestine epithelium is insufficient to initiate tumorigenesis but promotes tumor progression. Cancer Lett. 273225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshima M., et al. (1996)Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87803–809 [DOI] [PubMed] [Google Scholar]
- 40.Chulada P.C., et al. (2000)Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 604705–4708 [PubMed] [Google Scholar]
- 41.Kamei D., et al. (2003)Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J. Biol. Chem. 27819396–19405 [DOI] [PubMed] [Google Scholar]
- 42.Yoshimatsu K., et al. (2001)Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin. Cancer Res. 73971–3976 [PubMed] [Google Scholar]
- 43.Sasaki Y., et al. (2012)Microsomal prostaglandin E synthase-1 is involved in multiple steps of colon carcinogenesis. Oncogene 312943–2952 [DOI] [PubMed] [Google Scholar]
- 44.Nakanishi M., et al. (2008)Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 683251–3259 [DOI] [PubMed] [Google Scholar]
- 45.Gordan J.D., et al. (2007)HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 11 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talks K.L., et al. (2000)The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong H., et al. (1999)Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 595830–5835 [PubMed] [Google Scholar]
- 48.Rigas B., et al. (1993)Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med. 122518–523 [PubMed] [Google Scholar]
- 49.Xia D., et al. (2012)Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat. Med. 18224–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen-Petrik M.B., et al. (2002)Prostaglandin E(2) protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in Apc(Min/+) mice. Cancer Res. 62403–408 [PubMed] [Google Scholar]
- 51.Grimmer C., et al. (2007)Hypoxia-inducible factor 1alpha is involved in the prostaglandin metabolism of osteoarthritic cartilage through up-regulation of microsomal prostaglandin E synthase 1 in articular chondrocytes. Arthritis Rheum. 564084–4094 [DOI] [PubMed] [Google Scholar]
- 52.Bombardier C., et al. ; VIGOR Study Group (2000)Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N. Engl. J. Med. 3431520–8, 2 p following 1528 [DOI] [PubMed] [Google Scholar]
- 53.Drazen J.M.(2005)COX-2 inhibitors–a lesson in unexpected problems. N. Engl. J. Med. 3521131–1132 [DOI] [PubMed] [Google Scholar]
- 54.Cannon C.P., et al. (2012)Physiology. COX-2 inhibitors and cardiovascular risk. Science 3361386–1387 [DOI] [PubMed] [Google Scholar]
- 55.Chang H.H., et al. (2011)Identification and development of mPGES-1 inhibitors: where we are at? Future Med. Chem. 31909–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He S., et al. (2011)Molecular docking and competitive binding study discovered different binding modes of microsomal prostaglandin E synthase-1 inhibitors. J. Chem. Inf. Model. 513254–3261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.