Abstract
Cigarette smoke (CS) is convincingly carcinogenic in mice when exposure starts at birth. We investigated the induction and modulation of alterations in the kidney and urinary bladder of CS-exposed mice. A total of 484 strain H Swiss mice were either sham-exposed or exposed since birth to mainstream CS (MCS) for 4 months. Dietary agents, including myo-inositol, suberoylanilide hydroxamic acid, bexarotene, pioglitazone and a combination of bexarotene and pioglitazone, were administered after weaning. Comet analyses showed that, after 2 and 4 months, MCS causes DNA damage in exfoliated urothelial cells, which can be prevented by myo-inositol and the peroxisome proliferator-activated receptor-γ ligand pioglitazone. After 7 months, the 17.6% of MCS-exposed male mice exhibited lesions of the urinary tract versus the 6.1% of sham-exposed mice, which emphasizes the role of sex hormones in urinary tract carcinogenesis. Myo-inositol and the RXR-specific retinoid bexarotene did not affect these alterations. The histone deacetylase inhibitor suberoylanilide hydroxamic acid (Vorinostat) increased the incidence of kidney epithelium hyperplasia. Pioglitazone significantly enhanced the incidence of kidney lesions as compared with mice exposed to MCS only, indicating possible adverse effects of this antidiabetic drug, which were lost upon combination with bexarotene according to a combined chemoprevention strategy. RXR is a heterodymeric partner for peroxisome proliferator-activated receptor-γ, thereby modulating the expression of multiple target genes. In conclusion, there is contrast between the ability of pioglitazone to inhibit DNA damage in exfoliated cells and the alterations induced in the urinary tract of MCS-exposed mice, suggesting the occurrence of non-genotoxic mechanisms for this drug.
Introduction
With 138 280 new cases and 28 450 estimated deaths for 2012 in the USA, urinary bladder, kidney and renal pelvis cancers represent, cumulatively, the fourth cause of both cancer cases (after prostate, breast and lung cancers) and cancer deaths (after lung, colon and breast cancers (1)). Based on the GLOBOCAN estimates, 463 500 new cases and 184 326 deaths for kidney and bladder cancers occurred worldwide in 2008 (2). Epidemiological studies provide evidence that tobacco smoking is a major cause of bladder, ureter, renal pelvis and renal cell carcinomas (3–5). In particular, two-thirds of urothelial bladder cancers in men and one-third in women may be related to cigarette smoke (CS), and the risk correlates with the number of cigarettes smoked, the duration of smoking and the degree of smoke inhalation (6). In addition, failure to quit smoking once a diagnosis is made is associated with a worse outcome, even in patients initially diagnosed with non-invasive cancers (7).
Although the most obvious approach to avoid CS-induced lesions is to refrain from smoking or to quit smoking, chemoprevention provides a valuable complementary strategy for their prevention. In fact, urothelial cancer frequently recurs, even when the primary cancer is completely removed. Moreover, smoking cessation significantly reduces the bladder cancer risk but never reaches the baseline risk level of non smokers (8). It should be also taken into account that potential chemopreventive agents administered with the diet are excreted with the urines and accordingly benefit of favorable pharmacokinetic properties by remaining in close prolonged contact with the urothelium (9).
Animal models of bladder cancer have extensively been used by testing individual carcinogens. However, the tumorigenicity of CS as a complex mixture is difficult to be reproduced in rodents. We discovered that mainstream CS (MCS) becomes a potent pulmonary carcinogen when exposure starts soon after birth. In addition, under these conditions, preneoplastic and neoplastic lesions of the urinary tract could be induced in mice exposed to MCS since birth (10). The MCS-induced hyperplasia of the urinary bladder epithelium in neonatally exposed mice was inhibited by the prenatal administration of the antioxidant N-acetylcysteine (11). Likewise, exposure of mice to environmental CS since birth induced adenomas in renal pelvis and kidney, papillary hyperplasia of urothelium and urinary bladder papillomas (12).
Based on these premises, in the framework of broader studies investigating the ability of chemopreventive agents to modulate MCS-induced cancer in mice, we implemented a study aimed at evaluating (i) DNA damage in exfoliated urothelial cells and (ii) histopathological alterations in the urinary tract of mice, as related to exposure to MCS and administration of chemopreventive agents with the diet. In particular, the agents under scrutiny included myo-inositol, suberoylanilide hydroxamic acid (SAHA), bexarotene, pioglitazone and a combination of bexarotene and pioglitazone.
Myo-inositol or cis-1,2,3,5-trans-4,6-cyclohexanehexol, a constituent of a variety of foods such as whole grains, seeds and fruits, is formed by dephosphorylation of isonitol hexaphosphate in the gastrointestinal tract. Myo-inositol was shown to inhibit, either alone or in combination with dexamethasone, the yield of lung tumors induced by various carcinogens, such as benzo(a)pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and environmental CS (13–16). However, myo-inositol failed to reduce the yield of lung tumors induced by vinyl carbamate (17). SAHA (Vorinostat, Zolinza®) is an inhibitor of histone deacetylases, which control the acetylation state of lysine side chains of the histone proteins of chromatin. The catalytic activity of histone deacetylases remodels chromatin to control gene expression without altering gene sequence. Histone deacetylase inhibitors are among the most promising targeted anticancer agents and are potent inducers of growth arrest, differentiation and/or apoptotic cell death of transformed cells (18). SAHA has significant anticancer activity against both hematologic and solid tumors and has been approved for treatment of cutaneous T-cell lymphoma (19). Bexarotene (9cUAB30, Targretin®) is a retinoid X receptor (RXR)-specific retinoid, which is used to treat cutaneous T-cell lymphoma. It shares a variety of mechanisms, such as block of cell-cycle progression, induction of apoptosis and differentiation, prevention of multidrug resistance and inhibition of angiogenesis and metastasis, that make this agent a promising cancer chemopreventive agent (20). It has been evaluated in a model of N-methyl-N-nitrosourea-induced (MNU) mammary carcinogenesis in rats (21,22). Moreover, this agent showed both preventive and therapeutic activities with respect to lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and vinyl carbamate in strain A mice (23) and prevented lung tumor progression in vinyl carbamate–treated A/J mice bearing genetic alterations of p53 or K-ras (24). Pioglitazone is a synthetic ligand of peroxisome proliferator-activated receptor-γ (PPARγ), one of the three PPAR isotypes identified to date in vertebrates (25). Pioglitazione was approved for the therapy of type 2 diabetes mellitus, as an insulin-sensitizing drug, and is expected to have a role in other diseases as well, such as cancer, atherosclerosis and inflammation- and oxidative stress-related conditions (26). In particular, this thiazolidinedione compound exhibits antitumor activity on the basis of nuclear receptor modulation that unfolds pleiotropic biological effects, such as antistromal, antiangiogenic and immunomodulating activities (27). Moreover, PPARγ agonists induce G0/G1 arrest and apoptosis of malignant cells (28). Pioglitazone inhibited pancreatic carcinogenesis induced by N-nitrosobis(2-oxopropyl)amine in hamsters (29) and colon carcinogenesis induced by azoxymethane in mice (30). It downregulated COX-2 and cyclin D1 and inhibited colon cancer proliferation and liver metastasis in immunodeficient mice (31), as well as early carcinogenic transformation in rat liver (32).
The results of the present study show that exposure of mice to MCS results both in DNA damage in exfoliated urothelial cells and in histopathological alterations in the urinary tract, which are only detectable in males. The most remarkable effect of chemopreventive agents was that pioglitazone significantly enhances the MCS-related preneoplastic and neoplastic lesions in kidney and bladder. These adverse effects were lost when pioglitazone was combined with bexarotene.
Material and methods
Mice
We used 50 pregnant H mice, originated from Swiss albino mice, aged 14–16 weeks and weighing 27–30g. Housing at the Animal Laboratory of the National Center of Oncology (Sofia, Bulgaria), breeding and treatment of mice were in accordance with national and institutional guidelines. The mice were kept in Makrolon cages on sawdust bedding and maintained on rodent chow (Kostinbrod, Sofia, Bulgaria) and tap water ad libitum, at a temperature of 23±2°C, relative humidity of 55% and a 12h day–night cycle.
Chemopreventive agents
Myo-inositol, SAHA, bexarotene and pioglitazone were supplied by Fisher Biosciences (Germantown, MD). The doses to be used in the chemoprevention study were selected based on a preliminary subchronic toxicity study, in which a total of 340 postweanling mice (170 males and 170 females) were either untreated or treated with four doses of each agent with the diet. Survival for 6 weeks, body weight and general appearance of the mice were monitored for 6 weeks (data not shown). All agents were not toxic at the maximum dose tested (myo-inositol 8000mg/kg diet, SAHA 1000mg/kg diet and bexarotene 240mg/kg diet), except pioglitazone, which caused a more than 10% body weight loss at 240mg/kg diet and was accordingly used at 120mg/kg diet.
Experimental groups
The pregnant mice generated a total of 484 newborns, which were divided into the following experimental groups: Group A, control mice kept in filtered air and killed at 7 months of life; Group B, mice exposed to MCS for 4 months, starting within 12h after birth, and then kept in filtered air until sacrifice, at 7 months of life; Group C, MCS-exposed mice receiving, after weaning (4–5 weeks), myo-inositol at 8g/kg diet; Group D, MCS-exposed mice receiving, after weaning, SAHA at 1g/kg diet; Group E, MCS-exposed mice receiving, after weaning, bexarotene at 240mg/kg diet; Group F, MCS-exposed mice receiving, after weaning, pioglitazone at 120mg/kg diet; Group G, MCS-exposed mice receiving, after weaning, a combination of bexarotene (240mg/kg diet) and pioglitazone (120mg/kg diet).
Exposure of neonatal mice to MCS
Exposure of the mice belonging to Groups B–G started within 12h after birth and continued daily for 4 months. A whole-body exposure of mice was obtained using commercially available cigarettes (Melnik King Size, Bulgartabac), having a declared content of 9mg tar and 0.8mg nicotine and delivering 10mg CO each. The mice of each litter and their dam were placed in a 22.5 l sealed Makrolon chamber that was subsequently filled with MCS, as described previously (10). Briefly, MCS was generated by drawing 15 consecutive puffs, each of 60ml and lasting 6 s. Each MCS treatment lasted 60min, involving six exposures of 10min each with 1min intervals, during which a total air change was made. The concentration of total particulate matter in the exposure chamber was, on average, 684mg/m3.
Collection and analysis of urines and exfoliated cells
At 2 and 4 months of age, each group of mice was housed in metabolic cages for 2–3h in a dark room to prevent additional DNA damage. The pH of each urine sample was measured before being filtered through 50 µm nylon mesh (Partec, Münster Germany), then centrifuged at 300g for 10min and resuspended in 3ml of ice-cold phosphate-buffered saline, pH 7.4. After centrifugation at 250g for 10min, the urine pellet was suspended in 60 µl phosphate-buffered saline.
For evaluating the cellularity of urines, 20 µl of suspended cells from each sample were smeared on slide and air dried, fixed for 10min at 4°C in methanol (80% v/v) and stored in dry air up to the end of sampling. The slides were stained with 10% Giemsa solution for 10min and scored at a ×400 magnification.
DNA damage in exfoliated urothelial cells
In preliminary assays, transition cell viability was measured using the trypan blue exclusion technique. Because each sample had a very low number of cells, viability was thereafter evaluated indirectly using the comet images after electrophoresis by counting DNA ‘clouds’ (DNA clouds/comet (33)). These clouds are made of very low–molecular weight DNA fragments, and the images are referred to as typical of dead cells (34,35). The samples with a high proportion of DNA clouds were excluded from DNA damage assessment (36).
The alkaline comet assay was performed according to Tice et al. (37) with some modifications. Briefly, suspension of 40 µl of urine cells was resuspended in 75 µl of 0.5% of low–melting point agarose and layered onto coded slides precoated with a thin layer of normal melting point agarose, and immediately covered with a coverslip, and allowed to solidify at 4°C. After gently removing the coverslip, a final layer of low–melting point agarose was added to the slide and covered with a second coverslip. After agarose solidification, the slides were immersed in freshly prepared, cold lysing solution (2.5M NaCl, 100mM ethylenediaminetetraacetic acid , 10mM Tris, pH 10, 1% Triton X-100 and 10% dimethyl sulfoxide ) for 1h at 4°C. The slides were removed from the lysis solution, rinsed for 40min in two changes of an alkaline solution (0.3M NaOH, 1mM ethylenediaminetetraacetic acid, pH 13) and placed in a horizontal gel electrophoresis tank that was filled with an ice-cold fresh alkaline solution. Electrophoresis was conducted for 30min at 25V (0.66V/cm) adjusted to 300 mA by raising or lowering the buffer level in the tank. After a neutralization step (0.4M Tris–HCl, pH 7.5), the slides were dehydrated with absolute ethanol and stored in dry air up to the end of sampling. The slides were rinsed and stained with ethidium bromide (2 μg/ml in H2O). The analyses were carried out using a fluorescence microscope equipped with a digital camera at a ×400 magnification. For each coded spot, with exclusion of the outer area, images of at least 100 randomly selected nuclei were acquired and submitted to the automated image analysis system CASP (Comet assay software project, http://www.casp.sourceforge.net). The results were expressed as tail moment, which is obtained by multiplying the percent of DNA in the tail by the tail length. The analyses were performed in quadruplicate.
Evaluation of histopathological alterations in kidney and urinary bladder
The ‘spontaneously’ moribund mice, which were housed separately, and all those mice that survived after 7 months were deeply anesthesized and killed using diethyl ether and subjected to complete necropsy. In addition to lungs and other organs, whose histopathological analysis is in progress, we collected the kidneys (three sections/kidney) and the urinary bladders (1 section each), which were processed by standard histopathological analysis.
Statistical analysis
Data of body weights were expressed as means ± standard errors of the mice composing each experimental group, and comparisons between groups were made by Student’s t-test for unpaired data. Comparisons between groups regarding survival and incidence data were made by Fisher’s exact test. Comet assay data were expressed as means ± standard errors of quadruplicate analyses, and comparisons between groups were made by Mann–Whitney test. All analyses used the StatView Program.
Results
Survival and body weights of mice
Table I summarizes the data relative to survival after 4 and 7 months and body weights (i) at weaning, when the mice started to receive the chemopreventive agents with the diet; (ii) after 4 months, when exposure to MCS was discontinued and (iii) after 7 months, when the mice were killed. Survival of all MCS-exposed mice was high after 4 months, with the exception of the MCS-exposed mice treated either with SAHA or with the combination of pioglitazone and bexarotene. At the end of the experiment (7 months), survival of MCS-exposed mice was poor. Treatment of MCS-exposed mice with either bexarotene or pioglitazone resulted in a significant protection toward MCS lethality, whereas the combination of these two agents and administration of either myo-inositol or SAHA did not affect MCS lethality.
Table I.
Survival and body weights of Swiss H mice exposed for 4 months to MCS, since birth, and treated with chemopreventive agents after weaning, until the end of the experiment (7 months)
| Experimental group | Gender | Initial no. of mice | Surviving mice | Body weights (g) | |||
|---|---|---|---|---|---|---|---|
| 4 months | 7 months | At weaning | 4 months | 7 months | |||
| Sham | M | 33 | 33 (100 %) | 31 (93.9%) | 20.9±0.79 | 37.9±0.78 | 42.2±1.18 |
| F | 31 | 31 (100%) | 28 (90.3%) | 19.3±0.86 | 34.4±0.74 | 38.0±1.27 | |
| MCS | M | 34 | 34 (100%) | 24 (70.6%)a | 17.9±0.54a | 29.4±0.65b | 40.1±0.91 |
| F | 38 | 34 (89.5%) | 17 (44.7%)b | 16.6±0.58c | 23.5±0.96b | 35.5±1.36 | |
| MCS + myo-inositol | M | 34 | 31 (91.2%) | 23 (67.6%)a | 17.4±0.55b | 27.8±0.91b | 40.2±0.94 |
| F | 37 | 33 (89.2%) | 20 (54.1%)b | 15.5±0.43b | 23.6±0.90b | 33.1±1.33a | |
| MCS + SAHA | M | 35 | 29 (82.9%)c | 24 (68.6%)a | 17.5±0.38b | 23.3±0.82b,d | 32.8±1.22b,d |
| F | 35 | 29 (82.9%)c | 16 (45.7%)b | 16.4±0.30a | 22.4±0.77b | 32.5±1.21b | |
| MCS + bexarotene | M | 35 | 33 (94.3%) | 31 (88.6%)e | 17.8±0.55a | 27.1±0.68b | 37.4±0.94 |
| F | 40 | 37 (92.5%) | 31 (77.5%)f | 16.7±0.54c | 24.0±0.92b | 32.9±1.15a | |
| MCS + pioglitazone | M | 34 | 32 (94.1%) | 32 (94.1%)f | 16.7±0.32b | 29.9±0.64b | 40.6±1.03 |
| F | 35 | 31 (88.6%) | 31 (88.6%)d | 15.8±0.41b | 27.5±0.52b,d | 36.4±0.86 | |
| MCS + pioglitazone + bexarotene | M | 31 | 27 (87.1%)c | 22 (71.0%)b | 15.5±0.56b | 27.6±1.20b | 36.7±0.89b,f |
| F | 34 | 23 (67.6%)b | 16 (47.1%)b | 14.6±0.66b,e | 26.0±1.20b | 28.9±1.75b,f | |
Body weight data are means ± standard errors within each experimental group. M, male. F, female.
a P < 0.01, as compared with sham-exposed mice of the same gender.
b P < 0.001, as compared with sham-exposed mice of the same gender.
c P < 0.05, as compared with sham-exposed mice of the same gender.
d P < 0.001, as compared with MCS-exposed mice of the same gender.
e P < 0.05, as compared with MCS-exposed mice of the same gender.
f P < 0.01, as compared with MCS-exposed mice of the same gender.
MCS caused a significant loss of body weight at weaning and at 4 months of age in both male and female mice, whereas 3 months later, after discontinuation of exposure to MCS, the body weights were no longer different from those of sham-exposed mice. Pioglitazone significantly counteracted the MCS-induced body weight loss in 4-month old females. On the contrary, SAHA further worsened the MCS-related body weight loss in males at both 4 and 7 months, and the combination of pioglitazone and bexarotene significantly attenuated the body weight recovery after discontinuation of exposure to MCS in both males and females.
Physical and cytological alterations of urines and DNA damage in exfoliated urothelial cells
Table II shows that, irrespective of gender, exposure to MCS significantly decreased the pH of urines in mice, both at 2 months and 4 months of age. Administration of myo-inositol prevented this effect, whereas administration of pioglitazone, bexarotene or SAHA did not affect the MCS-induced urine acidification. The cells recovered from the urines were mainly transitional urothelial cells, which accounted for a proportion of cells in the 87.9–98.2% range. The other cells were leukocytes, which accounted for a proportion of cells in the 1.8–12.1% range. The percent of dead cells was relatively low in sham-exposed mice, MCS-exposed mice and MCS-exposed mice treated with either myo-inositol or pioglitazone. Conversely, dead cells were ≥30% in the urines of MCS-exposed mice treated with either bexarotene or SAHA, which precluded evaluation of DNA damage in these urine samples.
Table II.
Physical, cytological, and genotoxicity end-points in mouse urines, as related to exposure to MCS and treatment with chemopreventive agents
| End-point | Sham | MCS | MCS + myo-inositol | MCS + pioglitazone | MCS + bexarotene | MCS + SAHA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | |
| After 2 months | ||||||||||||
| Urine pH | 7.2±0.2 | 7.2±0.2 | 6.2±0.2a | 6.3±0.3a | 7.0±0.1b | 7.0±0.6c | 6.5±0.2a | 6.3±0.1a | 6.5±0.5 | 6.3±0.3a | 6.3±0.4a | 6.3±0.3a |
| Transitional cells (%) | 97.5 | 98.2 | 96.2 | 97.8 | 93.0 | 96.9 | 92.8 | 92.1 | 94.8 | 93.2 | 90.2 | 87.9 |
| Leukocytes (%) | 2.5 | 1.8 | 3.8 | 2.2 | 7.0 | 3.1 | 7.2 | 7.9 | 5.2 | 6.8 | 9.8 | 12.1 |
| Dead cells (%) | 4.6 | 4.1 | 18.0 | 6.9 | 2.9 | 2.5 | 3.2 | 3.9 | 32.1 | 34.6 | 34.5 | 30.0 |
| DNA damage in exfoliated cells (TM) | 22.9±1.6 | 15.8±1.8 | 78.0±5.2a | 34.4±2.7a | 18.8±2.1c | 11.4±0.7c | 17.5±1.3c | 13.8±3.2c | NT | NT | NT | NT |
| After 4 months | ||||||||||||
| Urine pH | 7.1±0.3 | 7.2±0.1 | 6.0±0.3a | 6.2±0.2a | 7.1±0.2b | 7.0±0.5c | 6.4±0.2a | 6.2±0.2a | 6.2±0.4d | 6.4±0.1a | 6.2±0.2a | 6.3±0.2a |
| Transitional cells (%) | 95.4 | 96.7 | 95.5 | 98.4 | 99.5 | 96.9 | 93.4 | 94.1 | 91.2 | 89.3 | 87.9 | 79.3 |
| Leukocytes (%) | 4.6 | 3.3 | 4.5 | 1.6 | 0.5 | 3.1 | 6.6 | 5.9 | 9.8 | 10.7 | 12.1 | 20.7 |
| Dead cells (%) | 18.1 | 15.1 | 21.4 | 28.0 | 13.8 | 2.0 | 14.4 | 10.9 | 35.6 | 42.1 | 36.2 | 31.5 |
| DNA damage in exfoliated cells (TM) | 42.4±13.6 | 31.4±8.0 | 125.5±14.1a | 118.8±7.6d | 94.2±11.8 | 69.8±7.6c | 52.2±1.9c | 72.9±11.1c | NT | NT | NT | NT |
Comet data are means ± standard error of quadruplicate analyses. NT, not tested.
a P < 0.05, as compared with sham-exposed mice of the same gender.
b P < 0.01, as compared with MCS-exposed mice of the same gender.
c P < 0.05, as compared with MCS-exposed mice of the same gender.
d P < 0.01, as compared with sham-exposed mice of the same gender.
As shown in Table II, the results of the alkaline comet assay showed a significant increase of DNA damage in the urothelial exfoliated cells of both male and female MCS-exposed mice, both at 2 months and 4 months of age. Myo-inositol and pioglitazone attenuated the MCS-induced DNA damage. At 2 months, this protective effect was total, tail moment values in MCS-exposed mice treated with either myo-inositol or pioglitazone being reverted at the same levels as in MCS-exposed mice in the absence of chemopreventive treatment. At 4 months, the protective effect was partial but still statistically significant in myo-inositol-treated female mice and pioglitazone-treated mice, both males and females.
Histopathological alterations in mouse kidney and urinary bladder
The data relative to the histopathological analyses in the kidney and urinary bladder of the variously treated 438 mice are summarized in Table III. The number of mice in each experimental group, reported between brackets, includes all mice killed after 7 months plus the mice that prematurely died in which it was possible to collect the biological samples for histopathological analyses.
Table III.
Histopathological alterations detected, after 7 months, in the kidney and urinary bladder of mice, as related to exposure to MCS for 4 months, starting at birth, and treatment with chemopreventive agents, starting after weaning until the end of the experiment
| Tissues histopathological alterations | Sham | MCS | MCS + myo-inositol | MCS + pioglitazone | MCS + bexarotene | MCS + pioglitazone + bexarotene | MCS + SAHA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (33) | F (28) | M (34) | F (38) | M (31) | F (37) | M (32) | F (35) | M (34) | F (36) | M (31) | F (34) | M (33) | F (35) | |
| Kidney | ||||||||||||||
| Tubular epithelium hyperplasia | 0 | 0 | 2 (5.9%) | 0 | 3 (9.7%) | 3 (8.1%) | 5 (15.6%)a | 8 (22.9%)b,c | 0 | 0 | 0d | 2 (5.9%)d | 3 (9.1%) | 5 (16.7%)a,e |
| Adenomas | 0 | 0 | 0 | 0 | 1 (3.2%) | 2 (5.4%) | 3 (9.4%) | 5 (14.3%)a,e | 0 | 0 | 0 | 0 | 1 (3.0%) | 3 (11.7%) |
| Renal cell carcinoma | 0 | 0 | 1 (2.9%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mice with kidney lesions | 0 | 0 | 3 (8.8%) | 0 | 3 (9.7%) | 3 (8.1%) | 7 (21.9%)b | 9 (25.7%)b | 0 | 0 | 0f | 2 (5.9%)d | 3 (9.1%) | 6 (17.1%) |
| Urinary bladder | ||||||||||||||
| Papillary epithelium hyperplasia | 2 (6.1%) | 0 | 5 (14.7%) | 0 | 5 (16.1%) | 0 | 6 (18.8%) | 0 | 1 (2.9%) | 0 | 2 (6.5%) | 1 (2.9%) | 1 (3.0%) | 1 (2.9%) |
| Papilloma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.9%) | 0 | 0 | 0 | 0 | 1 (2.9%) |
| Carcinoma in situ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.7%) | 0 | 0 | 0 | 0 |
| Mice with urinary bladder lesions | 2 (6.1%) | 0 | 5 (14.7%) | 0 | 5 (16.1%) | 0 | 6 (18.8%) | 0 | 2 (5.9%) | 1 (2.7%) | 2 (6.5%) | 1 (2.9%) | 1 (3.0%) | 1 (2.9%) |
| Mice with urinary tract lesions | 2 (6.1%) | 0 | 6 (17.6%) | 0 | 6 (19.4%) | 3 (8.1%) | 12 (37.5%)b | 9 (25.7%)b | 2 (5.9%) | 1 (2.7%) | 2 (6.5%)f | 2 (5.9%)d | 3 (9.1%) | 7 (20.0%)a,d |
a P < 0.05, as compared with sham-exposed mice.
b P < 0.01, as compared with sham-exposed mice.
c P < 0.01, as compared with MCS-exposed mice.
d P < 0.05, as compared with MCS-exposed mice treated with pioglitazone.
e P < 0.05, as compared with MCS-exposed mice.
f P < 0.01, as compared with MCS-exposed mice treated with pioglitazone.
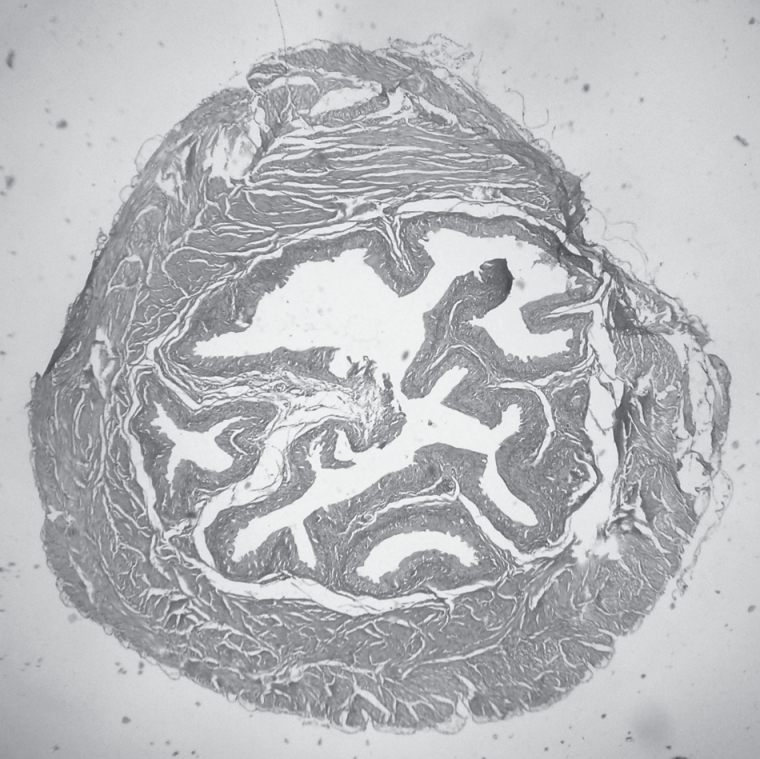
No lesion was detected in sham-exposed mice, except two males with papillary hyperplasia of the urinary bladder epithelium. Figure 1 shows an example of a papillary focal hyperplasia of the urinary bladder with an initial papilloma. Exposure to MCS had no effect in females, whereas among males there were two cases of hyperplasia of the kidney tubular epithelium, one case of renal cell carcinoma and five cases of papillary epithelium hyperplasia of the urinary bladder. On the whole, the 17.6% of MCS-exposed males suffered from lesions in the urinary tract. However, these differences were not statistically significant as compared with sham-exposed mice of the same gender. Of the chemopreventive regimens tested, myo-inositol and bexarotene did not significantly affect the incidence of renal and urinary bladder lesions as compared with either sham-exposed or MCS-exposed mice. SAHA produced an increase of hyperplasias of the kidney tubular epithelium, which was statistically significant in females as compared with both sham-exposed and MCS-exposed mice of the same gender. The most striking effects were produced by treatment with pioglitazone. In fact, this PPARγ agonist enhanced the incidence of kidney tubular epithelium hyperplasias and adenomas. A total of 21 mice (12 males and 9 females) had lesions in the urinary tract of MCS-exposed mice treated with pioglitazone, as compared with 6 mice, all of them in males, in MCS-exposed males in the absence of chemopreventive agents (P < 0.01). The histopathological alterations produced by pioglitazone in the urinary tract of MCS-exposed mice were prevented when this agent was combined with bexarotene.
Fig. 1.
Example of histopathological analysis showing areas of papillary hyperplasia of the urinary bladder epithelium with initial papilloma.
Discussion
The results of the present study deserve a series of comments inherent to the effects of cigarette smoking in the urinary tract and to their prevention. They include (i) the ability of smoke to induce preneoplastic and neoplastic lesions in the urinary tract in animal models; (ii) the intergender differences in both smoke-related DNA damage in urothelial cells and histopathological changes in the urinary tract; (iii) the possibility of attenuating these health hazards using chemopreventive agents; (iv) the feasibility of using non-invasive intermediate biomarkers in kidney and urinary bladder carcinogenesis; (v) the risk that certain pharmacological agents may increase the yield of preneoplastic lesions in smoke-exposed mice; (vi) the opportunity of using mechanistically designed combinations of chemopreventive agents to avoid adverse effects; (vii) the possible inconsistency between the indications of genotoxicity biomarkers and the histopathological alterations induced by non-genotoxic carcinogens.
Consistently with our previous studies (11), exposure of neonatal male mice to MCS for 4 months, starting since birth, resulted in histopathological alterations in the kidney and in the urinary bladder. These alterations did not reach the statistical significance threshold, but it is noteworthy that the 17.6% of MCS-exposed male mice developed lesions in the urinary tract, as compared with the 6.1% only of sham-exposed mice of the same gender. Interestingly, no such lesion was detected in 66 female mice, either sham-exposed or exposed to MCS. Several studies support a role for sex hormones in modulating the growth and the development of bladder cancer (38) and point out the antiestrogenic capacity of CS (39). For instance, a higher incidence of bladder cancer was observed in nitrosamine-exposed rats treated with testosterone as compared with rats treated with either estradiol or estriol (40–42). Keeping in mind the prominent role of chronic inflammation in CS-related carcinogenesis (43,44), an important mechanism explaining the lower susceptibility of females, at least toward urinary tract lesions, is that estrogens are able to reduce chronic bladder inflammation (45).
A common drawback affecting animal studies is that high doses of both carcinogens and protective factors are needed to obtain significant results using reasonable numbers of animals. All tested chemopreventive agents were used at doses lower than the maximum tolerated doses in smoke-free mice. The CS dose tested in the present study is comparable with the one used in previous studies evaluating the pulmonary tumorigenicity of MCS in the same mouse strain (10,11). A transient decrease of body weight has been reported to occur during the period of exposure to CS also in studies evaluating the tumorigenicity of environmental CS in other mouse strains, such as A/J mice (45) and ICR(CD-1) mice (12).
Of the chemopreventive agents tested, myo-inositol and bexarotene failed to attenuate the incidence of MCS-induced urinary tract lesions, whereas SAHA increased the incidence of tubular epithelium hyperplasia in the kidney as compared with MCS-exposed mice in the absence of chemopreventive agents. The dietary administration of pioglitazone even enhanced the incidence of urinary tract lesions in MCS-exposed mice, especially in the kidney. In fact, 16 out of 67 pioglitazone-treated mice (23.9%) suffered from kidney lesions as compared with 3 out of 72 MCS-exposed mice in the absence of chemopreventive agents (4.2%), a difference that is statistically significant. Note that PPAR agonists have been reported to be tumorigenic in one or more species of rodents at multiple sites, and in particular urothelial changes have been observed in rats and monkeys (46). For instance, a 2 year study in male rats treated with the diet with pioglitazone for 85 weeks showed the formation of advanced proliferative changes in the urinary bladder of 11 of 82 rats (13.4%), including papillary hyperplasia, nodular hyperplasia, papilloma or carcinoma (47). In addition, some data suggest a possible increase of bladder cancer among diabetic patients treated with this drug, although this issue is controversial (48). Furthermore, PPARγ is expressed in various human cancers, including renal cell carcinoma (49) and bladder cancer (50).
Evaluation of intermediate biomarkers is useful to assess both efficacy and safety of cancer chemopreventive agents. Collection of urines is the most commonly used noninvasive approach and remains the gold standard for bladder screening (51) and for evaluating cytogenetical damage in exfoliated cells (52,53). The comet assay has been found to detect an increased DNA damage in bladder washing cells from patients suffering from urothelial carcinoma in comparison with a matched control group (54). In the present study, a significant increase of DNA damage was detected in mice of both genders exposed to MCS for either 2 or 4 months since birth, prior to appearance of any histopathological alteration. This finding is consistent with the notion that CS contains a variety of chemicals, such as aromatic amines, heterocyclic amines, polycyclic aromatic hydrocarbons etc., which can be metabolically activated in the liver and then eliminated via the urinary tract (3,55). In fact, the urines from smokers are invariably mutagenic, to such an extent that this end-point has been used in cancer chemoprevention trials (56), and DNA adducts are detected in bladder cells from both smoking humans (57) and smoke-exposed rats (58). The stronger genotoxic effect observed in the urothelial cells of male mice after 2 months of exposure to MCS correlates with the greater susceptibility of males toward the induction of histopathological alterations in the urinary tract, as discussed previously.
Of the chemopreventive agents under study, bexarotene and SAHA could not be evaluated for modulation of CS-related DNA damage because a high proportion of exfoliated cells were dead at the doses tested. In contrast, both myo-inositol and pioglitazone were successful in completely preventing DNA damage in urothelial cells after 2 months of exposure to MCS and in significantly attenuating DNA damage after 4 months. Female mice responded more efficiently than males to the protective effect of myo-inositol, which strengthens the view that estrogens play a direct role in the etiology of bladder cancer. In fact, the administration of oral myo-inositol decreases total androgens and increases estradiol (59).
It is thus apparent that there is a discrepancy between the ability of pioglitazone to inhibit the DNA damage induced by MCS in exfoliated urothelial cells and induction by the same compound of histopathological changes in the urinary tract at 7 months of age. This inconsistency is presumably due to the fact that pioglitazone damages the kidney and bladder of MCS-exposed mice via non-genotoxic mechanisms, which after discontinuation of exposure to MCS prevail on its antigenotoxic properties. One of the possible mechanisms for this effect is probably to be related to our finding that the urine samples had a lower pH in MCS-exposed mice of both genders and, at variance with myo-inositol, pioglitazone failed to restore a neutral pH. Workers exposed to aromatic amines whose urines had a pH lower than 6.0 were found to have 10-fold higher DNA adduct levels than subjects with a neutral pH (60). Moreover, in a case–control study, a dose-response relationship in bladder cancer risk was observed with increasing acidity of urines among current smokers (61).
It is noteworthy that the renal tumorigenicity of pioglitazone in MCS-exposed mice was lost when this agent was combined with bexarotene. This finding emphasizes the convenience of pursuing a combined chemoprevention strategy when the combination is based on mechanistic premises. In fact, the rexinoid bexarotene is an RXR agonist, whereas pioglitazone is a synthetic ligand of PPARγ. Both RXRs and PPARs belong to the nuclear hormone receptor superfamily and can control the expression of multiple target genes, thereby integrating upstream signals into coordinated systemic and programmatic responses. RXR is an obligate heterodymeric partner for other nuclear receptors, including PPARs (62,63). Our data suggest that the potential tumorigenicity of pioglitazone in the urinary tract of MCS-exposed mice is lost when PPARγ is bound to RXR.
In conclusion, the results of the present study provide evidence that exposure of mice to MCS for 4 months, starting at birth, results both in DNA damage detectable in exfoliated urothelial cells after 2 and 4 months and in histopathological alterations in the urinary tract detectable after 7 months. Both effects selectively occur in males but not in females, due to the hormonal regulation of kidney and bladder carcinogenesis. Of the chemopreventive agents tested, both myo-inositol and pioglitazone were successful to inhibit DNA damage in urothelial cells, but pioglitazone significantly enhanced the MCS-related induction of histopathological alterations in kidney and bladder, which suggests a non-genotoxic mechanism for this PPARγ agonist. In any case, potential adverse effects in the urinary tract should be taken into account when using pioglitazone either in the treatment of diabetes or in cancer prevention. The renal tumorigenicity of pioglitazone in MCS-exposed mice was lost when this agent was combined with the RXR agonist bexarotene, which emphasizes the convenience of adopting mechanistically designed combination strategies in cancer chemoprevention.
Funding
US National Cancer Institute (contract N01-CN53301); Bulgarian Ministry of Youth and Science (National Science Fund); Hasumi International Foundation.
Acknowledgement
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CS
cigarette smoke
- MCS
mainstream CS
- PPARγ
peroxisome proliferator-activated receptor-γ
- RXR
retinoid X receptor
- SAHA
suberoylanilide hydroxamic acid.
References
- 1. American Cancer Society (2012). Cancer facts and figures. www.cancer.org/Research/CancerFactsFigures/ACSPC-031941
- 2. International Agency for Research on Cancer GLOBOCAN 2008. Cancer incidence, mortality and prevalence worldwide in 2008. http://globocan.iarc.fr/
- 3. International Agency for Research on Cancer (2004). Tobacco smoke and involuntary smoking. IARC monographs on the evaluation of carcinogenic risks to humans. IARC, Lyon, 83 [PMC free article] [PubMed] [Google Scholar]
- 4. Stewart S.L, et al. (2008). Surveillance for cancers associated with tobacco use–United States, 1999-2004. MMWR. Surveill. Summ., 57, 1–33 [PubMed] [Google Scholar]
- 5. Ray G, et al. (2010). Cigarette smoking as a cause of cancers other than lung cancer: an exploratory study using the Surveillance, Epidemiology, and End Results Program. Chest, 138, 491–499 [DOI] [PubMed] [Google Scholar]
- 6. Brennan P, et al. (2001). The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes Control, 12, 411–417 [DOI] [PubMed] [Google Scholar]
- 7. Fleshner N, et al. (1999). Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer, 86, 2337–2345 [PubMed] [Google Scholar]
- 8. Gandini S, et al. (2008). Tobacco smoking and cancer: a meta-analysis. Int. J. Cancer, 122, 155–164 [DOI] [PubMed] [Google Scholar]
- 9. Tanaka T, et al. (2011). Pathobiology and chemoprevention of bladder cancer. J. Oncol., 2011, 528353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balansky R, et al. (2007). Potent carcinogenicity of cigarette smoke in mice exposed early in life. Carcinogenesis, 28, 2236–2243 [DOI] [PubMed] [Google Scholar]
- 11. Balansky R, et al. (2009). Prenatal N-acetylcysteine prevents cigarette smoke-induced lung cancer in neonatal mice. Carcinogenesis, 30, 1398–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D’Agostini F, et al. (2008). Preneoplastic and neoplastic lesions in the lung, liver and urinary tract of mice exposed to environmental cigarette smoke and UV light since birth. Int. J. Cancer, 123, 2497–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wattenberg L.W. (1999). Chemoprevention of pulmonary carcinogenesis by myo-inositol. Anticancer Res., 19(5A)3659–3661 [PubMed] [Google Scholar]
- 14. Zhang Z, et al. (2000). A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res., 60, 901–907 [PubMed] [Google Scholar]
- 15. Witschi H. (2000). Successful and not so successful chemoprevention of tobacco smoke-induced lung tumors. Exp. Lung Res., 26, 743–755 [DOI] [PubMed] [Google Scholar]
- 16. Hecht S.S, et al. (2002). Inhibition of lung tumorigenesis in A/J mice by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis, 23, 1455–1461 [DOI] [PubMed] [Google Scholar]
- 17. Gunning W.T, et al. (2000). Chemoprevention of vinyl carbamate-induced lung tumors in strain A mice. Exp. Lung Res., 26, 757–772 [DOI] [PubMed] [Google Scholar]
- 18. Long J, et al. (2009). Antitumor effects of a novel sulfur-containing hydroxamate histone deacetylase inhibitor H40. Int. J. Cancer, 124, 1235–1244 [DOI] [PubMed] [Google Scholar]
- 19. Marks P.A. (2007). Discovery and development of SAHA as an anticancer agent. Oncogene, 26, 1351–1356 [DOI] [PubMed] [Google Scholar]
- 20. Qu L, et al. (2010). Bexarotene: a promising anticancer agent. Cancer Chemother. Pharmacol., 65, 201–205 [DOI] [PubMed] [Google Scholar]
- 21. Grubbs C.J, et al. (2003). 9cUAB30, an RXR specific retinoid, and/or tamoxifen in the prevention of methylnitrosourea-induced mammary cancers. Cancer Lett., 201, 17–24 [DOI] [PubMed] [Google Scholar]
- 22. Lubet R.A, et al. (2005). Efficacy of Targretin on methylnitrosourea-induced mammary cancers: prevention and therapy dose-response curves and effects on proliferation and apoptosis. Carcinogenesis, 26, 441–448 [DOI] [PubMed] [Google Scholar]
- 23. Pereira M.A, et al. (2006). Prevention of mouse lung tumors by targretin. Int. J. Cancer, 118, 2359–2362 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, et al. (2006). Organ-specific expression profiles of rat mammary gland, liver, and lung tissues treated with targretin, 9-cis retinoic acid, and 4-hydroxyphenylretinamide. Mol. Cancer Ther., 5, 1060–1072 [DOI] [PubMed] [Google Scholar]
- 25. Zieleniak A, et al. (2008). Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch. Immunol. Ther. Exp. (Warsz.), 56, 331–345 [DOI] [PubMed] [Google Scholar]
- 26. Burgermeister E, et al. (2008). PPARgamma and MEK Interactions in Cancer. PPAR Res., 2008, 309469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bundscherer A, et al. (2009). Targeting the tumor stroma with peroxisome proliferator activated receptor (PPAR) agonists. Anticancer. Agents Med. Chem., 9, 816–821 [DOI] [PubMed] [Google Scholar]
- 28. Mrówka P, et al. (2008). Pioglitazone, a PPAR-gamma ligand, exerts cytostatic/cytotoxic effects against cancer cells, that do not result from inhibition of proteasome. Acta Biochim. Pol., 55, 75–84 [PubMed] [Google Scholar]
- 29. Takeuchi Y, et al. (2007). Suppression of N-nitrosobis(2-oxopropyl)amine-induced pancreatic carcinogenesis in hamsters by pioglitazone, a ligand of peroxisome proliferator-activated receptor gamma. Carcinogenesis, 28, 1692–1696 [DOI] [PubMed] [Google Scholar]
- 30. Osawa E, et al. (2003). Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology, 124, 361–367 [DOI] [PubMed] [Google Scholar]
- 31. Takano S, et al. (2008). Pioglitazone, a ligand for peroxisome proliferator-activated receptor-gamma acts as an inhibitor of colon cancer liver metastasis. Anticancer Res., 28(6A)3593–3599 [PubMed] [Google Scholar]
- 32. Borbath I, et al. (2007). The PPARgamma agonist pioglitazone inhibits early neoplastic occurrence in the rat liver. Eur. J. Cancer, 43, 1755–1763 [DOI] [PubMed] [Google Scholar]
- 33. Gontijo A.M, et al. (2001). Single-cell gel (comet) assay detects primary DNA damage in nonneoplastic urothelial cells of smokers and ex-smokers. Cancer Epidemiol. Biomarkers Prev., 10, 987–993 [PubMed] [Google Scholar]
- 34. Fairbairn D.W, et al. (1995). The comet assay: a comprehensive review. Mutat. Res., 339, 37–59 [DOI] [PubMed] [Google Scholar]
- 35. Hellman B, et al. (1995). The concepts of tail moment and tail inertia in the single cell gel electrophoresis assay. Mutat. Res., 336, 123–131 [DOI] [PubMed] [Google Scholar]
- 36. Speit G, et al. (2006). The comet assay: a sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol. Biol., 314, 275–286 [DOI] [PubMed] [Google Scholar]
- 37. Tice R.R, et al. (2000). Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen., 35, 206–221 [DOI] [PubMed] [Google Scholar]
- 38. McGrath M, et al. (2006). Hormonal and reproductive factors and the risk of bladder cancer in women. Am. J. Epidemiol., 163, 236–244 [DOI] [PubMed] [Google Scholar]
- 39. Spangler J.G. (1999). Smoking and hormone-related disorders. Prim. Care, 26, 499–511 [DOI] [PubMed] [Google Scholar]
- 40. Okajima E, et al. (1975). Effects of sex hormones on development of urinary bladder tumours in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Urol. Res., 3, 73–79 [DOI] [PubMed] [Google Scholar]
- 41. Tanahashi N.K, et al. (1977). Effects of sex hormones on oncogenesis in rat urinary bladder by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Int. J. Clin. Pharmacol. Biopharm., 15, 101–105 [PubMed] [Google Scholar]
- 42. Terada S, et al. (1992). Effect of testosterone on the development of bladder tumors and calculi in female rats. Gynecol. Obstet. Invest., 34, 105–110 [DOI] [PubMed] [Google Scholar]
- 43. Malkinson A.M. (2004). Evidence that inflammation encourages pulmonary adenocarcinoma formation in mice: clinical implications. Chest, 125(5 Suppl)154S–155S [DOI] [PubMed] [Google Scholar]
- 44. Takahashi H, et al. (2010). Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell, 17, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martinez-Ferrer M, et al. (2008). Role of nicotinic and estrogen signaling during experimental acute and chronic bladder inflammation. Am. J. Pathol., 172, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hardisty J.F, et al. (2008). Histopathology of the urinary bladders of cynomolgus monkeys treated with PPAR agonists. Toxicol. Pathol., 36, 769–776 [DOI] [PubMed] [Google Scholar]
- 47. Sato K, et al. (2011). Suppressive effects of acid-forming diet against the tumorigenic potential of pioglitazone hydrochloride in the urinary bladder of male rats. Toxicol. Appl. Pharmacol., 251, 234–244 [DOI] [PubMed] [Google Scholar]
- 48. Lewis J.D, et al. (2011). Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care, 34, 916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Inoue K, et al. (2001). Expression of peroxisome proliferator-activated receptor gamma in renal cell carcinoma and growth inhibition by its agonists. Biochem. Biophys. Res. Commun., 287, 727–732 [DOI] [PubMed] [Google Scholar]
- 50. Guan Y.F, et al. (1999). Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in human transitional bladder cancer and its role in inducing cell death. Neoplasia, 1, 330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown F.M. (2000). Urine cytology. It is still the gold standard for screening?. Urol. Clin. North Am., 27, 25–37 [DOI] [PubMed] [Google Scholar]
- 52. Reali D, et al. (1987). Micronuclei in exfoliated urothelial cells and urine mutagenicity in smokers. Mutat. Res., 192, 145–149 [DOI] [PubMed] [Google Scholar]
- 53. Lehucher-Michel M.P, et al. (1995). The micronucleus assay in human exfoliated urothelial cells: effect of smoking. Mutagenesis, 10, 329–332 [DOI] [PubMed] [Google Scholar]
- 54. Fracasso M.E, et al. (2004). DNA breaks as measured by the alkaline comet assay in exfoliated cells as compared to voided urine cytology in the diagnosis of bladder cancer: a study of 105 subjects. Mutat. Res., 564, 57–64 [DOI] [PubMed] [Google Scholar]
- 55. Kadlubar F.F, et al. (1977). Hepatic microsomal N-glucuronidation and nucleic acid binding of N-hydroxy arylamines in relation to urinary bladder carcinogenesis. Cancer Res., 37, 805–814 [PubMed] [Google Scholar]
- 56. De Flora S, et al. (1996). Smokers and urinary genotoxins: implications for selection of cohorts and modulation of endpoints in chemoprevention trials. J. Cell. Biochem. Suppl., 25, 92–98 [PubMed] [Google Scholar]
- 57. Curigliano G, et al. (1996). Immunohistochemical quantitation of 4-aminobiphenyl-DNA adducts and p53 nuclear overexpression in T1 bladder cancer of smokers and nonsmokers. Carcinogenesis, 17, 911–916 [DOI] [PubMed] [Google Scholar]
- 58. Izzotti A, et al. (1999). Formation and persistence of nucleotide alterations in rats exposed whole-body to environmental cigarette smoke. Carcinogenesis, 20, 1499–1505 [DOI] [PubMed] [Google Scholar]
- 59. Minozzi M, et al. (2008). Treatment of hirsutism with myo-inositol: a prospective clinical study. Reprod. Biomed. Online, 17, 579–582 [DOI] [PubMed] [Google Scholar]
- 60. Rothman N, et al. (1997). Acidic urine pH is associated with elevated levels of free urinary benzidine and N-acetylbenzidine and urothelial cell DNA adducts in exposed workers. Cancer Epidemiol. Biomarkers Prev., 6, 1039–1042 [PubMed] [Google Scholar]
- 61. Alguacil J, et al. (2011). Urinary pH, cigarette smoking and bladder cancer risk. Carcinogenesis, 32, 843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ziouzenkova O, et al. (2008). Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett., 582, 32–38 [DOI] [PubMed] [Google Scholar]
- 63. Tachibana K, et al. (2008). The Role of PPARs in Cancer. PPAR Res., 2008, 102737 [DOI] [PMC free article] [PubMed] [Google Scholar]