Abstract
The use of mercurated "strong stop" complementary DNA (complementary to the 5'-terminal 101 nucleotides of Rous sarcoma virus RNA) in the isolation of virus-specific RNA from infected chicken embryo fibroblasts is described. Strong stop Rous sarcoma virus complementary DNA was mercurated chemically, and, as a result of the low complexity of this DNA, short hybridization times (up to 15 min) and heating in the absence of formamide were found to be adequate conditions for the isolation of virus-specific RNA. The purity of the isolated RNA was demonstrated by analysis of labeled RNase T1-resistant oligonucleotides by two-dimensional polyacrylamide gel electrophoresis. The isolated RNA could be translated in the in vitro protein synthesis system derived from rabbit reticulocytes, and an analysis of polypeptides programmed by isolated RNA before and after immunoprecipitation further demonstrated both the purity of the isolated mRNA and the quantitative nature of the isolation procedure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genes responsible for transformation by avian RNA tumor viruses. Cancer Res. 1976 Nov;36(11 Pt 2):4282–4288. [PubMed] [Google Scholar]
- Coffin J. M., Parsons J. T., Rymo L., Haroz R. K., Weissmann C. A new approach to the isolation of RNA-DNA hybrids and its application to the quantitative determination of labeled tumor virus RNA. J Mol Biol. 1974 Jun 25;86(2):373–396. doi: 10.1016/0022-2836(74)90026-6. [DOI] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Levray M., Bromley P. A., Spahr P. F. Characterization of the genomic RNA from a Rous sarcoma virus mutant temperature sensitive for cell transformation. Nucleic Acids Res. 1979 Feb;6(2):471–485. doi: 10.1093/nar/6.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F., Bromley P. A., Jaton J. C. In vitro, the major ribosome binding site on Rous sarcoma virus RNA does not contain the nucleotide sequence coding for the N-terminal amino acids of the gag gene product. J Virol. 1979 Feb;29(2):597–611. doi: 10.1128/jvi.29.2.597-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Collett M. S., Lau A. F., Perdue M. L., Leis J. P., Faras A. J. Evidence for splicing of avian sarcoma virus 5'-terminal genomic sequences into viral-specific RNA in infected cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1284–1288. doi: 10.1073/pnas.75.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
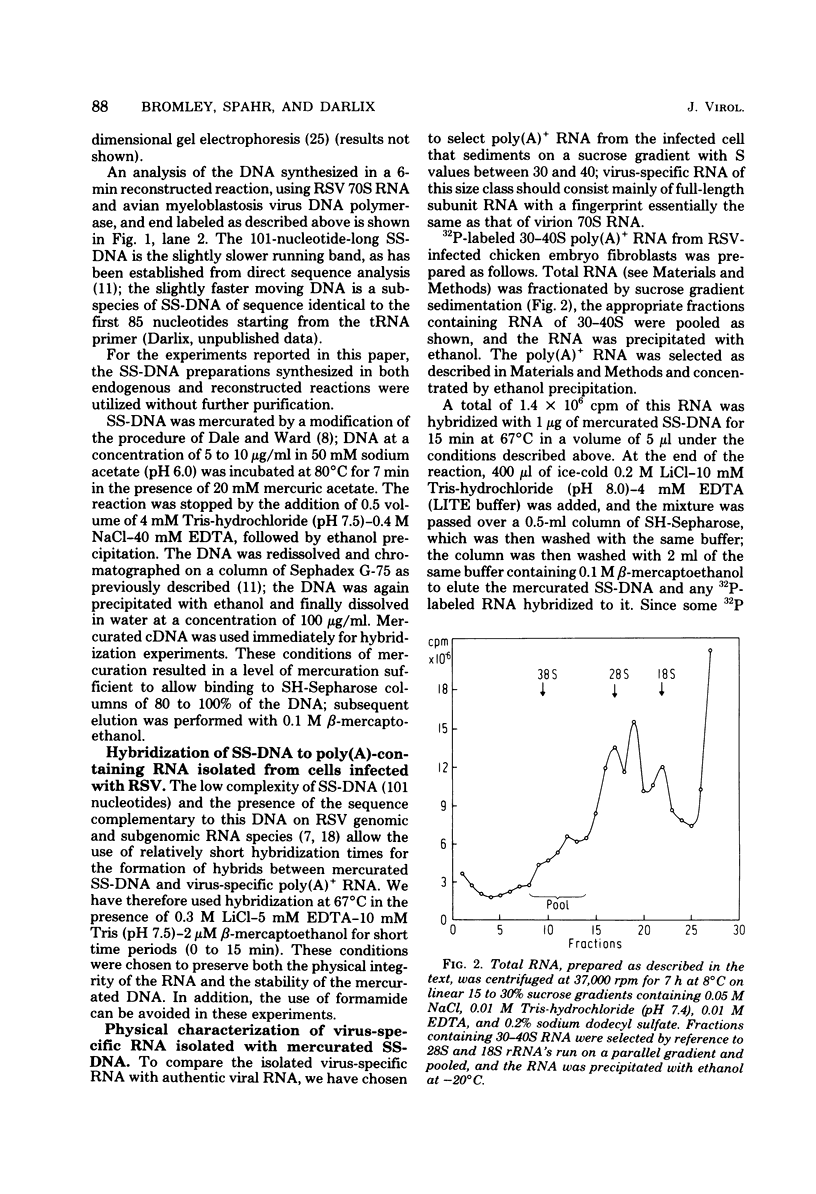
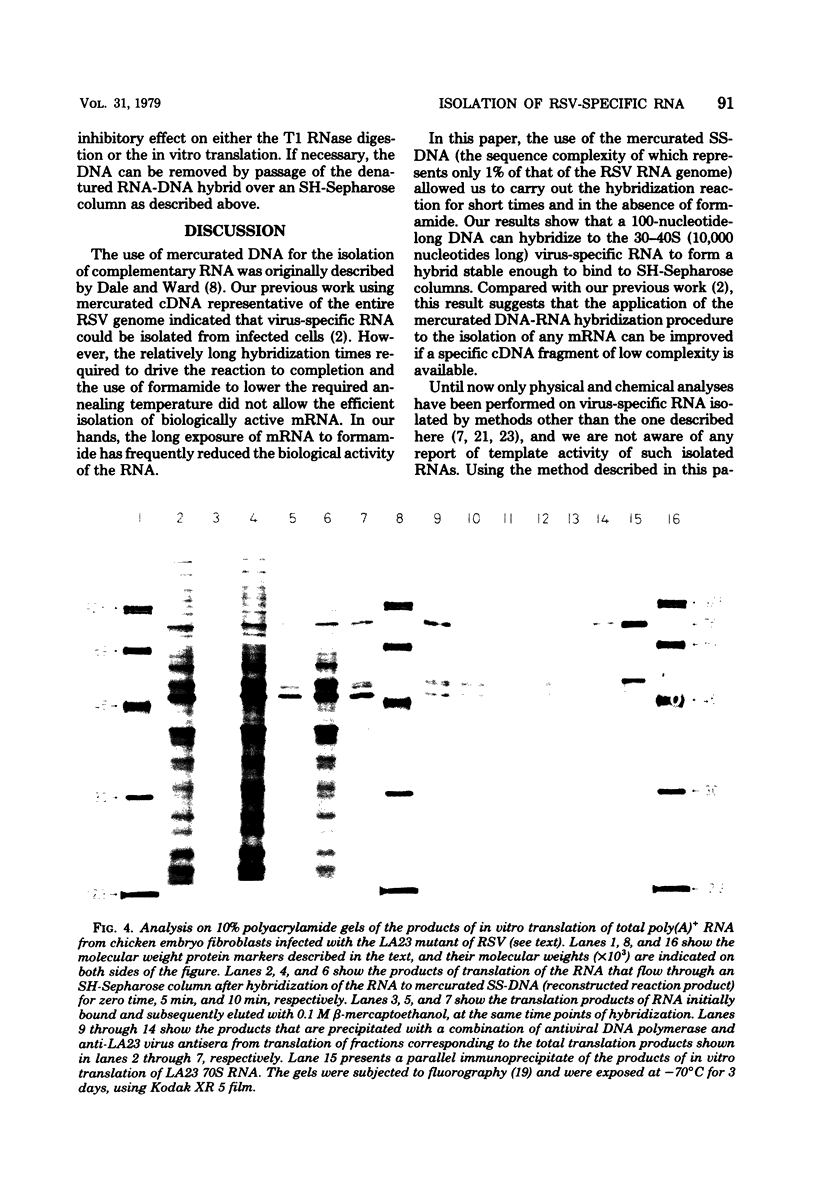
- Parsons J. T., Lewis P., Dierks P. Purification of virus-specific RNA from chicken cells infected with avian sarcoma virus: identification of genome-length and subgenome-leghth viral RNAs. J Virol. 1978 Jul;27(1):227–238. doi: 10.1128/jvi.27.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rensing U. F., Schoenmakers G. G. A sequence of 50 nucleotides from coliphage R17 RNA. Eur J Biochem. 1973 Feb 15;33(1):8–18. doi: 10.1111/j.1432-1033.1973.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Rymo L., Parsons J. T., Coffin J. M., Weissmann C. In vitro synthesis of Rous sarcoma virus-specific RNA is catalyzed by a DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2782–2786. doi: 10.1073/pnas.71.7.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]