Abstract
The purpose of this study is to assess the efficacy of alternating chemoradiation in patients with nasopharyngeal cancer. From 1990–2006, 100 patients with nasopharyngeal cancer were treated with alternating chemoradiation at the Aichi Cancer Center. Of these, 4, 2, 23, 34, 13 and 23 patients were staged as I, IIA, IIB, III, IVA and IVB, respectively. The median radiation doses for primary tumors and metastatic lymph nodes were 66.6 Gy (range, 50.4–80.2 Gy) and 66 Gy (range, 40.4–82.2 Gy), respectively. A total of 82 patients received chemotherapy with both cisplatin and 5-fluorouracil (5-FU), while 14 patients received nedaplatin (CDGP) and 5-FU. With a median follow-up of 65.9 months, the 5-year rates of overall survival (OAS) and progression-free survival (PFS) were 78.1% and 68.3%, respectively. On multivariate analysis (MVA), elderly age, N3, and WHO type I histology proved to be significantly unfavorable prognostic factors of OAS. As for PFS, there were T4, N3, and WHO type I histology in MVA. Acute toxicities of hematologic and mucositis/dermatitis ≥ Grade 3 were relatively high (32%); however, they were well-managed. Late toxicities of ≥ Grade 3 were three (3%) mandibular osteomyelitis and one (1%) lethal mucosal bleeding. Results for alternating chemoradiation for nasopharyngeal carcinoma are promising. In order to improve outcomes, usage of intensity-modulated radiation therapy and application of active anticancer agents are hopeful treatments, especially for groups with poor prognosis factors with WHO type I histopathology, T4 and/or N3 disease.
Keywords: nasopharyngeal carcinoma, alternating chemoradiation, WHO type I histopathology
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a common disease among Southern Chinese, Southeast Asian, Northern African and Inuit populations. In Japan, the USA and Western European countries it is relatively rare. Because of anatomical characteristics, surgical treatment is very difficult. In addition, the majority of NPC patients revealed undifferentiated carcinoma, which is relatively sensitive to radiation therapy. Therefore, radiotherapy is widely accepted as the first choice of therapy for NPC. In recent years, by randomized-control trials, chemoradiotherapy has shown significant survival benefits over radiotherapy alone, improving both local and distant control [1–4]. In addition, meta-analysis of eight randomized trials showed significant benefits for OAS and event-free survival [5]. The pooled hazard ratio of death was 0.82 (95% confidence interval, 0.71–0.94; P = 0.006), corresponding to an absolute survival benefit of 6% at 5 y from the addition of chemotherapy. Thus, the standard treatment for locally advanced NPC is now believed to be concurrent chemoradiotherapy. However, several key factors need further clarification. Firstly, the chemotherapy used in the Intergroup 0099 study (IGS) consisted of three courses each of concurrent administration of cisplatin (CDDP) and adjuvant chemotherapy with both CDDP and 5-fluorouracil (5-FU). However, about two thirds (63%) of patients could receive concurrent chemotherapy, and about half (55%) could receive the full course of adjuvant chemotherapy. Secondly, a higher incidence of adverse events ≥ Grade 3 was observed in the chemoradiation group than in the radiation alone group (59% vs 34%). Finally, chemoradiation reduced distant metastasis; however, it did not reach sufficient levels. Of the 18 patients with recurrence in the chemoradiation arm, 10 (56%) developed distant metastasis (DM) in the IGS. A considerable incidence of DM still developed in the IGS due to insufficient dose intensities of chemotherapy, instead of increasing adverse events.
In the Aichi Cancer Center, we conducted alternating chemoradiotherapy for advanced NPC patients from 1987 and reported promising results with sufficiently better compliance (94%), of which the 5-year OAS and PFS rates were 75% and 63%, respectively [6]. In the present study, we analysed the efficacy of alternating chemoradiotherapy for NPC with relatively longer follow-up and sought to refine our treatment strategy according to data regarding failure patterns.
MATERIALS AND METHODS
Patient characteristics
Between 1990 and 2006, a total of 100 consecutive patients with newly diagnosed histology-proven nasopharyngeal carcinoma underwent definitive chemoradiotherapy (CRT) in the Aichi Cancer Center. All patients underwent fiberoptic nasopharyngoscopy and magnetic resonance imaging (MRI) to assess the extent of primary and cervical lymph nodes. Evaluation of distant metastasis was done by chest X-ray, computed tomography (CT), liver ultrasonography, and bone scintigraphy. After 2002, positron emission tomography (PET) or PET-CT was also used to evaluate the extent of the disease. In addition, laboratory data, electrocardiograms, and 24-h creatinine clearance were evaluated to assess general condition. For this analysis, all patients were restaged according to the 6th edition of the American Joint Committee on Cancer (AJCC) staging system [6].
Treatment schedule
Chemotherapy
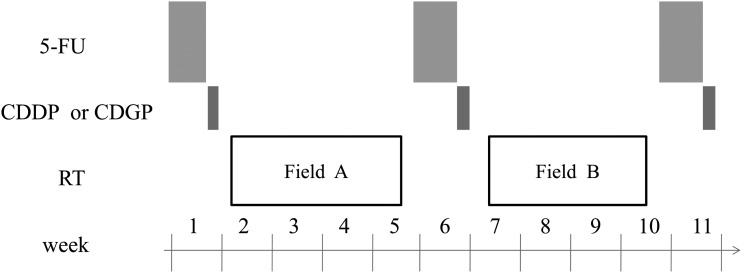
The treatment scheme is shown in Fig. 1. Details of the treatment regimen have been reported in another article [7]. Chemotherapy regimens were a combination of CDDP and 5-FU (FP) or nedaplatin (CDGP) and 5-FU (FN) regimens. In the FP regimen, 5-FU was administered continuously at a dose of 800 mg/m2 on Days 1–5 and CDDP at a dose of 50 mg/m2 on Days 6–7. In the FN regimen, 5-FU was administered continuously at a dose of 800 mg/m2 on Days 1–5 and CDGP at a dose of 130 mg/m2 on Day 6. Chemotherapy was performed in principal three times at 4-week intervals. However, when a WBC count <3000/mm2 or a platelet count <100 000/mm2 was obtained at the scheduled date of drug administration, chemotherapy was postponed and radiation therapy was alternately prescribed. When hematological data obtained two weeks after radiotherapy did not meet the inclusion criteria (WBC count >3000/mm2 and platelet count >100 000/mm2), the next cycle of chemotherapy was withdrawn. When the WBC count decreased to <1000/mm2 or the platelet count decreased to <25 000/mm2 after chemotherapy, doses of both 5-FU and CDDP were decreased by 25% at the next cycle. In addition, the dose of CDDP only was decreased by 25% when serum creatinine levels >1.5 mg/dl were noted.
Fig. 1.
Study design of alternating chemoradiotherapy. 5-FU = 5-fluorouracil 800 mg/m2 on Days 1–5 continuous infusion, CDDP = cisplatin 50 mg/m2 Day 6–7, CDGP = nedapatin 130 mg/m2 on Day 6, RT = radiotherapy, Field A = large field including from the skull base to supraclavicular fossa, Field B = boost field including the nasopharynx and metastatic lymph nodes.
Radiotherapy
Using a 6–10 MV photon beam by linear accelerator, external beam radiotherapy commenced 2–3 d after the completion of previous chemotherapy. At simulation and daily treatment, the head, neck and shoulder were immobilized in a hyperextended position using a thermoplastic mask. Radiotherapy was performed with a daily fraction of 1.8–2.0 Gy. The initial radiation field covered the nasopharynx and upper and middle cervical regions using bilateral opposing portals and lower cervical, and supraclavicular region using anterior single field irradiation at a dose of 36–40 Gy. Then, a shrinking field of 26–30 Gy was boosted to the nasopharynx and involved lymph nodes using the dynamic conformal rotational technique. In the shrinking field, we kept enough margins of primary tumors and involved lymph nodes from the edge of field. Those margins were mainly decided dependent on proximity to critical structures such as the brain-stem, spinal cord, optic pathway and temporal lobes. During the second period of chemotherapy, radiotherapy was temporarily interrupted to spare the increasingly acute toxicity of 5-FU. Additional boosts of up to 10 Gy with stereotactic multiple arc treatment were also permitted, if residual tumors existed at primary sites.
Follow-up and statistical consideration
Toxicities of CRT were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 [8]. During the treatment period, complete blood counts and biochemical examinations were performed at least once a week. After completion of CRT, the treatment response was assessed by fiberoptic nasopharyngoscopy, MRI and/or PET/CT. The frequency of follow-up was every month for the first year, once every two months between the second and third post-treatment year, and once every three months after the third post-treatment year. Fiberoptic nasopharyngoscopy was performed at every visit, and post-treatment MRI scans were obtained every three months for the first year and then every six months thereafter. The survival period was calculated from the start of treatment to death or the last follow-up examination, and progression-free survival was defined as the period from the start of treatment to the progression of tumors or death by any cause. Overall survival and progression-free survival curves were calculated by the Kaplan-Meier method [9]. The log-rank test was used to compare survival curves. A Cox-proportional hazard model was used for multivariate analysis. Differences in the ratios between the two groups were assessed by the chi-square test.
RESULTS
Patient characteristics
Between June 1990 and March 2005, 100 patients with NPC received definitive CRT in the Aichi Cancer Center. Table 1 shows patient characteristics in this cohort. We analysed all patients who were treated with CRT. The median age was 55 years old (range, 28–80). Performance status was distributed as 2 of 0, 93 of 1, 3 of 2, and 2 of 3, respectively. Of these, 8 patients (8%) had histopathology with keratinizing squamous cell carcinoma (WHO type I), and 70 patients (70%) had Stage III–IVB disease. During this period the number of patients with NPC who were treated with radiotherapy alone was 13. The common reasons for radiotherapy alone were advanced age or poor general condition.
Table 1.
Patient characteristics
| Characteristics | n | ||||
|---|---|---|---|---|---|
| Age, years: median (range) | 55 (28–80) | ||||
| Gender: | |||||
| Male | 72 | ||||
| Female | 28 | ||||
| Performance status | |||||
| 0 | 2 | ||||
| 1 | 93 | ||||
| 2 | 3 | ||||
| 3 | 2 | ||||
| Histology | |||||
| type I | 8 | ||||
| non type I | 90 | ||||
| others | 2 | ||||
| T stage | |||||
| 1 | 37 | ||||
| 2a | 15 | ||||
| 2b | 15 | ||||
| 3 | 15 | ||||
| 4 | 18 | ||||
| N stage | |||||
| 0 | 11 | ||||
| 1 | 31 | ||||
| 2 | 34 | ||||
| 3a | 9 | ||||
| 3b | 15 | ||||
| Stage | |||||
| I | 4 | ||||
| IIA | 2 | ||||
| IIB | 24 | ||||
| III | 34 | ||||
| IVA | 12 | ||||
| IVB | 24 |
Treatment contents
The median dose to the primary site was 66.6 Gy (range, 50.4–80.2 Gy), and the median dose to involved lymph nodes was 66 Gy (range, 40.4–82.2 Gy), respectively. The median period of the whole course of alternating CRT was 85 days (range, 47–147 days), and the median period of overall treatment time of radiation therapy (OTT) was 69 days (range, 42–110 days).
Treatment outcomes
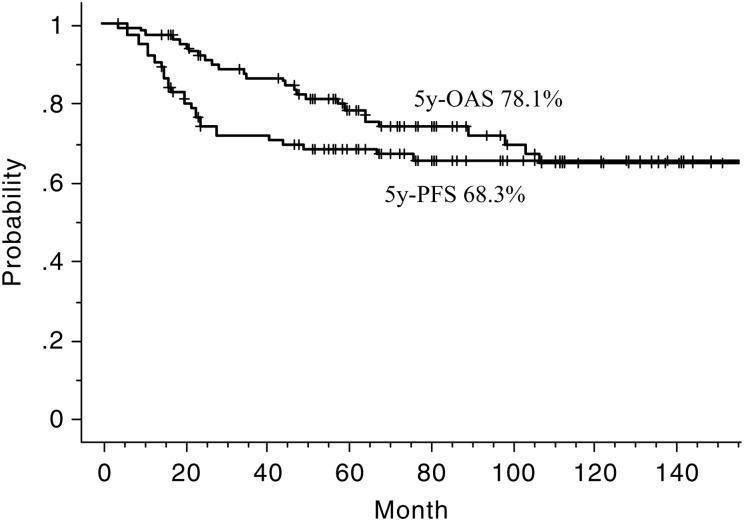
The 5-year rates of OAS and PFS were 78.1% and 68.3%, respectively (Fig. 2). The 5-year rates of OAS of the group divided by stage were 100, 100, 86.1, 77.6, 91.7 and 60.3% for Stage I, IIA, IIB, III, IVA and IVB, respectively. The 5-year rates of OAS and PFS of 96 patients who received alternating CRT were 78.2% and 68%, respectively. As for initial response after completion of CRT, complete remission (CR) rates of primary and nodal lesions were 86% and 83%, respectively. At a median follow-up of 65.9 months (range, 3.9–22.9 months), 62 were alive without disease, 11 were alive with disease, 18 died from the disease, 2 died from other diseases (both esophagus carcinoma) and 7 died from unknown reasons.
Fig. 2.
Overall survival (OAS) and progression-free survival (PFS) curves.
The 5-year rates of loco-regional progression-free survival (LRPFS) and distant metastasis-free survival (DMFS) were 77.9% and 87.8%, respectively.
A total of 32 patients (32%) developed treatment failure at one or more sites. Disease progression developed in 19 for primary, 9 for regional and 11 for distant sites at the last follow-up. Among 11 patients with distant failure, the most frequent site was the lung in 8, followed by bone in 4 and the liver in 2.
Of 21 patients who developed locoregional recurrence, 13 were treated with additional chemoradiation. Of the remainder, 2 patients were re-treated with radiotherapy alone, and 4 with only chemotherapy. One patient received neck dissection for regional failure, and another did not receive any treatment because of the patient's refusal for treatment.
Out of 11 patients who developed distant metastasis, 9 were treated by chemotherapy, and 2 patients received palliative radiotherapy only.
Univariate analysis
Univariate analysis (UVA) results are listed in Table 2.
Table 2.
Univariate analyses for overall survival and progression-free survival
| Factors | No. | 5-year OAS (%) | P-value | 5-year PFS (%) | P-value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 28 | 88.7 | 0.017 | 77.9 | 0.15 |
| Male | 72 | 73.8 | 64.4 | ||
| Age (years) | |||||
| <51 | 48 | 93.4 | 0.0006 | 73.6 | 0.26 |
| ≥51 | 52 | 64.2 | 63.4 | ||
| PS | |||||
| 0, 1 | 95 | 79.1 | 0.148 | 69.9 | 0.1 |
| 2, 3 | 5 | 60 | 30 | ||
| Histology | |||||
| WHO non type I | 90 | 81.6 | P < 0.0001 | 72.1 | P < 0.0001 |
| type I | 8 | 33.3 | 14.3 | ||
| T stage | |||||
| T1–3 | 82 | 78.2 | 0.79 | 71.4 | 0.014 |
| ≥T4 | 18 | 77.4 | 54.5 | ||
| N stage | |||||
| N0–2 | 76 | 84 | 0.001 | 76.5 | 0.001 |
| N3 | 24 | 60.3 | 41.5 | ||
| Total treatment duration (day) | |||||
| <85 | 48 | 69 | 0.0615 | 62.3 | 0.135 |
| ≥85 | 52 | 85.6 | 73.8 | ||
| OTT (day) | |||||
| <69 | 49 | 78.2 | 0.884 | 72.2 | 0.36 |
| ≥69 | 51 | 78.2 | 64.8 | ||
| Dose for primary site (Gy) | |||||
| <66 | 30 | 76.7 | 0.712 | 70 | 0.7 |
| ≥66 | 70 | 78.7 | 67.5 | ||
| Dose for metastatic LN (Gy) | |||||
| <66 | 35 | 77.5 | 0.683 | 71.8 | 0.78 |
| ≥66 | 54 | 74.8 | 65.1 |
OAS = overall survival, PFS = progression-free survival, PS = performance status, WHO = World Health Organization, OTT = overall treatment time of radiotherapy, LN = lymph node.
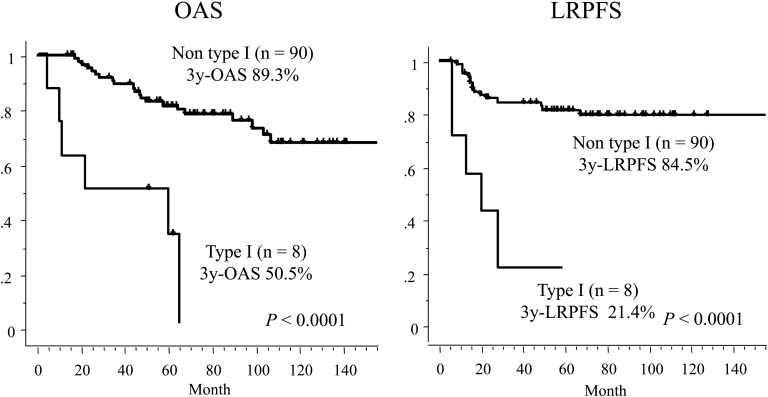
Elderly age, male, WHO type I histology, and N3 were revealed as significant unfavorable prognostic factors of OAS. The 5-year rate of OAS of the group with WHO type I histology was significantly lower than that with non-type I histology (33.3% vs 81.6%, P < 0.0001, Fig. 3). The group with N3 lesions had significantly worse 5-year OAS (60.3%) than that with N0–2 (84%; P = 0.0017). The 5-year rates of OAS of patients who received reduced dose and planned dose chemotherapy were 76.6% and 78.6%, respectively (P = 0.75).
Fig. 3.
Overall survival (OAS) and locoregional progression-free survival (LRPFS) curves of groups divided by WHO histopathological types.
As for PFS, significantly unfavorable factors were revealed as WHO type I histology, T4 and N3.
The 5-year PFS rate of the group with N3 was significantly lower than that with N0–2 (41.5% vs 76.5%, P = 0.001). The 5-year PFS rate of the group with T4 was significantly lower than that with T1–3 (54.5% vs 71.4%, P = 0.014). The 5-year rates of PFS of patients who received reduced dose and planned dose chemotherapy were 69.7% and 66.7%, respectively (P = 0.59).
The 5-year rate of LRPFS of the group with WHO type I histology was significantly lower than that with non-type I histology (21.4 % vs 84.5 %, P < 0.0001).
The 5-year rate of DMFS of patients with N3 was significantly lower than that with N0–2 (62.8% vs 95.1%, P < 0.0001). The 5-year LRPFS of patients with T4 was significantly lower than that with T1–3 (63.3% vs 81.1%, P = 0.027).
Multivariate analysis
Multivariate analysis (MVA) results are listed in Table 3. On MVA, significantly unfavorable prognostic factors of OAS were elderly age, WHO type I histology and N3, respectively. As for PFS, they were WHO type I histology, T4 and N3, respectively.
Table 3.
Multivariate analyses for overall survival and progression-free survival
| OAS | PFS | ||||
|---|---|---|---|---|---|
| Factors | No. | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Gender | |||||
| Female | 28 | 0.109 | 0.5 | ||
| Male | 72 | 2.76 (0.104–1.257) | 1.36 (0.291–1.836) | ||
| Age (years) | |||||
| <51 | 48 | 0.0018 | 0.198 | ||
| ≥51 | 52 | 4.92 (0.074–0.551) | 1.62 (0.294–1.290) | ||
| Histology | |||||
| WHO non type I | 90 | 0.0034 | 0.0004 | ||
| type I | 8 | 4.62 (0.077–0.603) | 5.747 (0.067–0.454) | ||
| T stage | |||||
| T1–3 | 82 | 0.555 | 0.023 | ||
| T4 | 18 | 1.36 (0.264–2.047) | 2.5 (0.181–0.881) | ||
| N stage | |||||
| N0–2 | 76 | 0.0076 | 0.0025 | ||
| N3 | 24 | 3.03 (0.147–0.745) | 3.012 (0.163–0.680) | ||
| OTT (day) | |||||
| <69 | 49 | 1.10 (0.395–2.065) | 0.8092 | 0.605 | |
| ≥69 | 51 | 1.215 (0.393–1.724) |
HR = hazard ratio, CI = confidence intervals, OAS = overall survival, PFS = progression-free survival, WHO = World Health Organization, OTT = overall treatment time of radiotherapy.
Treatment compliance
Regarding the contents of chemotherapy, 82 patients received FP, while 14 received FN. Four patients had other chemotherapy regimens, as described below. One patient with Stage I (cT1N0M0) received two courses of CDDP/5-FU followed by definitive radiotherapy. One patient received six courses of weekly docetaxel (TXT) because of elderly age and poor medical condition. One patient received chemotherapy with both CDGP and TXT because 5-FU was inappropriate due to a past history of myocardial infarction. One patient received concurrent administration with decreased doses of CDGP and 5-FU due to elderly age. Chemotherapy compliance is shown in Table 4. In 96 patients who received alternating CRT, over 90% of patients received three courses of chemotherapy and 70% of patients received the planned dose of three courses. In detail, 29 patients received reduced dose chemotherapy while 67 patients received the planned dose of three courses. The most common reason for dose reductions was renal dysfunction (47%), followed by severe mucositis (20%). The median total dose of CDDP was 300 mg/m2 (range, 150–340 mg/m2), CDGP was 375 mg/m2 (range, 80–400 mg/m2), and for 5-FU was 12 000 mg/m2 (range, 3050–12 000 mg/m2). In the cohort of patients who received reduced dose chemotherapy, the median total doses of CDDP, CDGP and 5FU were 250 mg/m2, 330 mg/m2 and 9400mg/m2, respectively. Unplanned interruption of RT was experienced in 14 patients (14%), and 2 out of 14 patients required a break in RT over seven days. Severe mucositis (36%) was the most common reason for interruption of RT, followed by infection of the hyperalimentation catheter (29%).
Table 4.
Compliance of chemotherapy
| n | median (range) | |
|---|---|---|
| Total cycles given | ||
| 1 | 2 | |
| 2 | 7 | |
| ≥3 | 87 | |
| Total dose given | ||
| Cisplatin (mg/m2) | 300 (150–340) | |
| Nedaplatin (mg/m2) | 375 (80–400) | |
| 5-fluorouracil (mg/m2) | 12 000 (3050–12 000) |
Treatment toxicity
Acute toxicities observed during treatment are listed in Table 5. The most common toxicity was leukopenia. Grade 3 or higher leukopenia, neutropenia, thrombocytopenia and anemia occurred in 37, 22, 11 and 18 patients, respectively. Grade 3 or higher mucositis and dermatitis developed in 20 and 18 patients, respectively.
Table 5.
Acute, severe and life-threatening toxicities due to chemoradiotherapy
| Toxicity | Gr 0 | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 5 | unknown | ≥ Gr 3 |
|---|---|---|---|---|---|---|---|---|
| Leukopenia | 4 | 12 | 43 | 32 | 5 | 0 | 4 | 37 |
| Granulocytopenia | 18 | 27 | 28 | 17 | 5 | 0 | 5 | 22 |
| Anemia | 6 | 33 | 39 | 14 | 4 | 0 | 4 | 18 |
| Thrombocytopenia | 28 | 37 | 10 | 8 | 3 | 0 | 4 | 11 |
| Liver dysfunction | 71 | 20 | 5 | 1 | 0 | 0 | 1 | 1 |
| Renal dysfunction | 71 | 28 | 0 | 0 | 0 | 0 | 1 | 0 |
| Vomiting | 33 | 14 | 50 | 3 | 0 | 0 | 0 | 3 |
| Mucositis | 0 | 13 | 67 | 19 | 1 | 0 | 0 | 20 |
| Dermatitis | 0 | 37 | 45 | 17 | 1 | 0 | 0 | 18 |
| Salivary gland changes | 1 | 13 | 86 | 0 | 0 | 0 | 0 | 0 |
Late toxicities are listed in Table 6. Three Grade 3 osteomyelitis of the mandible occurred in this series. One patient died because of late toxicity due to lethal mucosal bleeding. The patient diagnosed as cT3N1M0 with histology of Type I received 80 Gy to the primary site including additional SRT boosts of 10 Gy due to an insufficient response at the planned 70 Gy. The patient developed active mucosal bleeding in the nasopharynx, and died five years later. We experienced no Grade 3 or higher late toxicity of brain necrosis, visual disturbance or swallowing disturbance.
Table 6.
Late, severe and life-threatening toxicities due to chemoradiotherapy
| Toxicity | Gr 0 | Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 5 | ≥ Gr 3 |
|---|---|---|---|---|---|---|---|
| Swallowing dysfunction | 95 | 4 | 1 | 0 | 0 | 0 | 0 |
| Visual dysfunction | 99 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hearing impairment | 81 | 5 | 14 | 0 | 0 | 0 | 0 |
| Osteomyelitis | 96 | 0 | 1 | 3 | 0 | 0 | 3 |
| Brain necrosis | 99 | 1 | 0 | 0 | 0 | 0 | 0 |
| Bleeding | 99 | 1 | 0 | 0 | 0 | 1 | 1 |
DISCUSSION
A randomized control trial showed survival advantages of concurrent chemoradiotherapy over radiation alone, thus it is believed to be the standard treatment for locally advanced NPC. In the IGS, Stage III–IVB patients with NPC were randomized to CRT or RT, and the combined CRT group was treated with radiation and concurrent triweekly CDDP followed by three adjuvant cycles of FP [1]. The 3-year rate of OAS of the RT-only group was significantly lower than that of the CRT group (46% vs 76%; P < 0.001), and the same results were noted for the 3-year rate of PFS (24% vs 69%; P < 0.001). However, some problems with the results from the IGS were identified. Firstly, results of the RT arm in the IGS seem to be unacceptably bad because the reported 3-year rates of OAS for the same stages were over 70%. One of the reasons for this discrepancy is that the rate of WHO type I histology in the IGS series (24%) is larger than that of endemic regions, which is believed to have adversely impacted on clinical results. Secondly, the compliance of chemotherapy was insufficient in the IGS. The completion rates of planned chemotherapy of concurrent and adjuvant series were reported as 63% and 55%, respectively. In order to confirm this result, the IGS should be extrapolated in endemic regions [4]. In Hong Kong, the NPC-9901 trial on patients with T1-4N2-3M0 disease was designed to confirm the therapeutic ratio achieved by the IGS regimen. Regarding the compliance of chemotherapy, 65% of patients completed all six cycles, and 79% had five cycles. The CRT arm achieved significantly higher failure-free survival (72% vs 62% at 3 years, P = 0.027), mostly as a result of improvements in locoregional control. However, DMFS did not improve significantly (76% vs 73%, P = 0.47) and OAS was identical (78% vs 78%, P = 0.97). In other RCTs reported by Lin and Chen, the CRT arm significantly improved PFS and OAS [2, 3].
There is also evidence by meta-analysis dealing with eight randomized trials of 1753 patients regarding locally advanced NPC. In this analysis, the pooled hazard ratio of death for adding chemotherapy was 0.82 (95% confidence interval, 0.71–0.94; P = 0.006), corresponding to an absolute survival benefit of 6% at 5 years (56% vs 62%). A significant interaction was observed between the timing of chemotherapy and overall survival (P = 0.005), with the highest benefit resulting from concomitant chemotherapy [5]. However, increasing acute toxicities caused by administration of chemotherapy were also reported in this analysis. In the IGS, acute toxicities of ≥ Grade 3 were reported as 50% and 76% for RT and CRT arms, respectively. Similarly, in the NPC-9901 trial, toxicities of ≥ Grade 3 were observed as 53% and 84% for RT and CRT arms, respectively (P < 0.01). The 3-year actuarial rate of late toxicity was slightly higher in the CRT arm than in that of the RT arm, although it was not significant (28% vs 13%, P = 0.24).
In our institute, we adopted alternating CRT for NPC from 1987. In a previous report, 32 patients with NPC received alternating CRT, and the 5-year rates of OAS and PFS were 75% and 63%, respectively. A Phase II study of alternating chemoradiotherapy for patients with NPC was performed in four medical institutions including our institution from 1997 and reported promising results with high compliance (91%), of which the 2-year OAS and PFS rates were 94% and 83%, respectively [10]. In the present study with longer follow-up and a larger cohort, the 5-year rates of OAS and PFS were 78.1% and 68.3%, respectively. We think these data are comparable with previous series. In addition, we believe that acute and late complication rates were sufficiently low according to longer follow-up with 65.9 months.
We believe alternating chemoradiotherapy has several advantages in CRT for NPC. Because the radiation field has to be large, severe mucositis and dermatitis sometimes develops and leads to a treatment break. In addition, late complications, such as disturbances in swallowing or hearing sometimes become significant problems. Alternating chemoradiotherapy has the potential benefit in reducing acute toxicities. As for reported data of the NPC-9901 trial, acute mucositis and skin reactions over Grade 3 were observed in 62% and 20% patients in the CRT arm, respectively. In the present study, acute mucositis or dermatitis of ≥ Grade 3 developed in 20% and 18%, respectively. By alternating chemotherapy and radiotherapy, we could also use intensive multi-agent chemotherapy regimens such as FP or FN without increasing acute and late complications. Although our data is a retrospective analysis in a single institute, the 5-year rate of OAS in the present study (78.1%) was more promising than that of the IGS trial (67%). Regarding the compliance of chemotherapy, over 90% patients in the present study could receive three courses of chemotherapy and 70% of our cohort had completed planned full doses. As a result the total dose of chemotherapy in patients who received a reduced dose was still about 80% of the planned dose. Our data is thought to be more encouraging than that of the IGS, in which only 55% patients completed the planned chemotherapy. Failure patterns in CRT for NPC patients are thought to be both loco-regional, but also in distant sites. In the present study, DMFS at 5-years was 87.8%, which was higher than that of the reported series. The 3-year DMFS rate of the NPC-9901 study was reported as 76%. We believe that it was caused by the advantages of intensive chemotherapy in the present study. An unexpected RT break was needed in 14 patients (14%), of which only 2 patients needed RT breaks longer than one week.
The argument against alternating CRT is that planned RT interruptions may lead to sacrifices in treatment efficacy. In many studies, it is well known that prolongation of overall treatment time negatively influences clinical outcomes. In vitro, accelerated repopulation occurred 28 days after the start of RT; thus, prolongation of treatment time led to the development of radiation resistance. In the present study, OTT was not significantly related to clinical outcome. One of the reasons is that the high compliance of the present study would have helped avoid essential prolongation of OTT in our cohort.
In the present series, WHO type I histopathology was a significantly unfavorable factor of both OAS and PFS. The incidence of WHO type I histology in Western countries is very different from East Asian countries. In the IGS series conducted in North America, the rate of WHO type I histology was 22%, which was higher than the rates in studies conducted in endemic regions. WHO type I histopathology, keratinizing squamous cell carcinoma, was reported to be much less related to EBV infection than non-keratinizing carcinoma. It was also reported to be less sensitive to RT [11]. However, there are not so many reports regarding clinical results. One of the reasons is that the proportion of type I histopathology is very low in endemic regions. In Japan, the proportion of type I histopathology is about 20%, which was similar to North America. Kawashima et al. reported a Japanese multi-institutional survey of 333 NPC patients, in which the proportion of type I histopathology was 19% [12]. In that series, type I histopathology proved to be a significantly worse prognostic factor of OAS and PFS on both UVA and MVA. In the present study, the population of type I histopathology was 8%; however, these eight patients had remarkably poor prognosis. Six of the eight patients developed treatment failure. In our series, WHO type I histopathology was a significantly worse factor of both OAS (3-year rates; 50.5% vs 89.3%; P < 0.0001) and LRPFS (3-year rates; 21.4% vs 84.5%, P < 0.0001). The majority of failure patterns of these patients were in loco-regional sites. In order to improve treatment outcomes of these patients, dose escalation without increasing adverse events is believed to be promising. In recent years, intensity-modulated radiation therapy (IMRT) is widely used for head and neck cancer because of its dose conformity ability for PTV, reducing doses to normal tissue. RTOG 0225, a multi-institutional Phase II trial was conducted to test the feasibility of IMRT with or without chemotherapy for NPC. A 90% LRPF rate was reported as well as an acceptably low incidence of Grade 3 adverse events without xerostomia of Grade 4 [13]. In our institution, we started IMRT for NPC patients using Helical Tomotherapy until June 2006, and we have reported our preliminary clinical results [14]. In the future, dose escalation for patients with type I histopathology using IMRT will be helpful for improving clinical results.
The 5-year rates of PFS and LRPFS of patients with T4 were significantly inferior to those with T1–3, even though there was no significant difference in the 5-year rates of DMFS between these two groups. Because of the proximity of tumors to critical structures such as the brain-stem, spinal cord, optic pathway and temporal lobes, the radiation fields and dose coverages for primary tumors are often compromised. Preliminary results of radiation dose escalation for patients with T3–T4 NPC show good local control (2-year rate of locoregional control; 95.7%) and survival (2-year rate of OAS; 92.1%) [15]. For these patients, dose escalation using IMRT is also promising improved clinical results.
The 5-year rates of OAS and DMFS of patients with N3 were significantly inferior to those with N0–2 in the present series. On the other hand, N3 showed no apparent correlation with worsening LRPF. From this result, patients with N3 are expected to have a higher incidence of distant metastasis. Thus, a more effective regimen of chemotherapy should be considered to overcome limitations. In fact, TAX 324, a randomized Phase III trial, has shown the distinct survival advantages of multi-agent intensive chemotherapy including docetaxel and FP over PF for locally advanced head and neck cancer [16].
We believe that the present results for alternating chemoradiotherapy are promising compared to previously reported series of concurrent chemoradiotherapy. However, several subgroups with some risk factors proved to have insufficient outcomes. In order to refine clinical results without increasing adverse events, there is room for modification especially in patients with high-risk factors. Dose escalation using IMRT for type I histopathology and/or T4 disease and more intensive modifications of chemotherapy for N3 disease should be considered in future.
REFERENCES
- 1.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 2.Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–7. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Liu MZ, Liang SB, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. 2008;71:1356–64. doi: 10.1016/j.ijrobp.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–75. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 5.Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Greene FL, Page DL, Fleming ID, et al. 6th ed. New York: Springer: 2002. AJCC cancer staging handbook from the AJCC cancer staging manual. [Google Scholar]
- 7.Fuwa N, Ito Y, Kodaira T, et al. Therapeutic results of alternating chemoradiotherapy for nasopharyngeal cancer using cisplatin and 5-fluorouracil: its usefulness and controversial points. Jpn J Clin Oncol. 2001;31:589–95. doi: 10.1093/jjco/hye135. [DOI] [PubMed] [Google Scholar]
- 8.Bethesda: Chemoradiotherapy for hypopharyngeal cancer 9 National Cancer Institute; 2003. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events version 3.0 (CTCAE) http://ctep.cancer.gov/forms/CTCAEv3.pdf . [Google Scholar]
- 9.Kaplan E, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:475–81. [Google Scholar]
- 10.Fuwa N, Kano M, Toita T, et al. Alternating chemoradiotherapy for nasopharyngeal cancer using cisplatin and 5-fluorouracil: a preliminary report of phase II study. Radiother Oncol. 2001;61:257–60. doi: 10.1016/s0167-8140(01)00422-4. [DOI] [PubMed] [Google Scholar]
- 11.Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29–35. doi: 10.1093/annonc/mdl320. [DOI] [PubMed] [Google Scholar]
- 12.Kawashima M, Fuwa N, Myojin M, et al. A multi-institutional survey of the effectiveness of chemotherapy combined with radiotherapy for patients with nasopharyngeal carcinoma. Jpn J Clin Oncol. 2004;34:569–83. doi: 10.1093/jjco/hyh111. [DOI] [PubMed] [Google Scholar]
- 13.Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3784–90. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodaira T, Tomita N, Tachibana H, et al. Aichi Cancer Center initial experience of intensity modulated radiation therapy for nasopharyngeal cancer using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1129–34. doi: 10.1016/j.ijrobp.2008.06.1936. [DOI] [PubMed] [Google Scholar]
- 15.Kwong DL, Sham JS, Leung LH, et al. Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:374–81. doi: 10.1016/j.ijrobp.2005.07.968. [DOI] [PubMed] [Google Scholar]
- 16.Lorch JH, Goloubeva O, Haddad RI, et al. Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX324 randomised phase 3 trial. Lancet Oncol. 2011;12:153–9. doi: 10.1016/S1470-2045(10)70279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]