Abstract
Existence of adaptive response (AR) was previously demonstrated in C57BL/6J mice. Irradiations were performed by delivering a priming low dose of X-rays (0.50 Gy) in combination with a challenge high dose of accelerated carbon or neon ion particles. AR was characterized by significantly decreased mortality in the 30-day survival test. This mouse AR model (‘Yonezawa Effect’) was originally established by using X-rays as both the priming and challenge irradiations. The underlying mechanism was due to radio-resistance occurring in blood-forming tissues. In this study, we verified the existence of AR and further investigated residual damage in the hematopoietic system in surviving animals. Results showed that the priming low dose of X-rays could relieve the detrimental effects on the hematopoietic system. We observed both an improvement in the blood platelet count and the ratio of polychromatic erythrocytes (PCEs) to the sum of PCEs and normochromatic erythrocytes (NCEs) and a marked reduction of the incidences of micronucleated PCEs and micronucleated NCEs. These findings suggest that the priming low dose of low linear energy transfer (LET) X-rays induced a protective effect on the hematopoietic system, which may play an important role in both rescue from acute lethal damage (mouse killing) and prevention of late detrimental consequences (residual anhematopoiesis and delayed genotoxic effects) caused by exposure to a high challenge dose from low-LET (X-ray) or high-LET (carbon and neon ion) irradiations. These findings provide new knowledge of the characterization of the Yonezawa Effect by providing new insight into the mechanistic study of AR in vivo.
Keywords: low dose, radioadaptive response, bone marrow micronucleated erythrocytes, mice
INTRODUCTION
Radiation-induced adaptive response (AR) is a phenomenon manifesting as a priming low-dose-induced resistance to a subsequent challenge exposure at higher doses. Investigations of AR are expected to provide an important scientific basis for radiation risk estimates, protection and practical applications, which are of great interest for both public health and academic research [1]. AR has been studied for nearly three decades since the editio princeps of the concept was introduced into radiation biology [2] and demonstrated in a variety of in vitro and in vivo systems [3–5]. Among the documented in vivo AR models, the mouse model for rescue from bone marrow death reported by Yonezawa and colleagues [3, 6–8] was repeatedly verified [9, 10]. This model was originally established by using low linear energy transfer (LET) X-rays for the delivery of both the priming and challenge doses. The underlying mechanism was mainly the induction of radio-resistance by the priming low dose in blood-forming tissues, which rescued the bone marrow from death caused by the challenge high dose exposure. This resulted in a significant decrease in mortality in the 30-day survival test.
We previously showed that AR in this model can be observed when high-LET accelerated heavy ions are used for delivering the challenge dose. A low-LET X-ray-induced AR against the high-LET challenge heavy carbon, neon and silicon ions was successfully demonstrated [11, 12]. In the present work, residual damage in the hematopoietic system was investigated further in the surviving animals receiving challenge carbon or neon ion irradiations with or without priming irradiations from X-rays. By verifying the residual damage in the hematopoietic system in the mouse survivors from the AR group (receiving both the priming and challenge irradiations) and the non-AR group (receiving only the challenge irradiations), the present investigation aimed to study whether the priming irradiations could relieve the detrimental late effect induced by the challenge irradiations in this mouse model of AR.
MATERIALS AND METHODS
Animals
C57BL/6J Jms strain female mice aged 5 weeks were purchased from SLC, Inc. (Japan). The mice were maintained in a conventional animal facility under a 12-h light/12-h dark photoperiod (lights on from 8:00 a.m. to 8:00 p.m.). Animals were housed in autoclaved cages with sterilized wood chips and allowed free access to standard laboratory chow (MB-1, Funabashi Farm Co., Japan) and acidified water ad libitum. Animals were acclimatized to the laboratory conditions for 1 week before use. To avoid possible effects from the developmental condition of the animals, 6-week-old mice with a significantly different body weight (more or less than the mean ± 2 SD) were omitted from this study. On the basis of our previous studies, at least 30 mice in the present study were used in each experimental group, and all experiments were repeated at least once. All experimental protocols involving mice were reviewed and approved (Experimental Animal Research Plan Nos. 07-2023, 08-2023 and 09-2023) by The Institutional Animal Care and Use Committee of the National Institute of Radiological Sciences (NIRS). The experiments were performed in strict accordance with the NIRS Guidelines for the Care and Use of Laboratory Animals.
Irradiation
X-rays were generated with an X-ray machine (Pantak-320S, Shimadzu, Japan) operated at 200 kVp and 20 mA, using a 0.50-mm Al + 0.50-mm Cu filter. An exposure rate meter (AE-1321M, Applied Engineering Inc., Japan) was used for the dosimetry. The dose rate for delivering the priming dose at 0.50 Gy was about 0.30 Gy/min. For high-LET heavy-ion irradiations, a monoenergetic ion beam of carbon particles was generated and accelerated by a synchrotron, the Heavy Ion Medical Accelerator in Chiba (HIMAC), at NIRS, Japan. The beam energy was 290 MeV/nucleon for carbon ions and 400 MeV/nucleon for neon ions, corresponding to average LET values of about 15 keV/μm and 30 keV/μm, respectively. The dose rate was at about 0.70 Gy/min for delivery of a challenge dose at 7.50 Gy for X-rays. The dose rate was at about 1.50 Gy/min for delivery of a challenge dose at 5.75 Gy or 6.50 Gy for carbon ions and at 5.50 Gy for neon ions. The mice were held in acryl containers and were exposed to whole-body irradiation at room temperature.
Mouse model for induction of adaptive response
The mouse AR model for rescue from bone marrow death established by Yonezawa et al. [8] was adopted, verified and confirmed under the experimental conditions in our research facilities and was finally applied to our study. In brief, the efficient priming dose of X-rays was 0.50 Gy. The priming dose and challenge dose were given to mice at postnatal ages of 6 and 8 weeks, respectively. The challenge doses for the heavy carbon ion irradiations depended on the endpoint. A challenge dose of 6.50 Gy was used for verification and confirmation of the experimental conditions ensuring the successful induction of AR with priming low-dose X-rays in combination with the challenge high dose from accelerated carbon ions, and a challenge dose of 5.75 Gy was used to obtain more surviving animals in the 30-day survival test for investigations of residual damage in the hematopoietic system. The challenge dose for the heavy neon ion irradiations was 5.50 Gy.
Biological endpoints
The 30-day survival test
The number of deaths occurring within the 30-day period after delivery of the challenge dose was recorded. When the priming dose induced a significant suppression of mortality caused by the challenge dose alone, AR was considered to be successfully induced.
Peripheral blood hemogram
Animals surviving the 30-day survival test were anesthetized by CO2 inhalation, their peripheral blood was collected from a femoral artery, and the animals were killed by cervical dislocation. A differential blood cell count was done using a blood cell differential automatic analyzer (SYSMEX K-4500, Sysmex Corporation, Japan). The data for each experimental group were from at least five mouse survivors.
Micronucleus test
A bone marrow micronucleus test was carried out according to Schmid [13] with minor modifications [14]. Bone marrow smears prepared from both femurs were processed for the enumeration of micronucleated polychromatic erythrocytes (PCEs) and micronucleated normochromatic erythrocytes (NCEs). The slides were coded to avoid any observer bias. The micronuclei were scored using a light microscope at a magnification of 1000×. At least 5000 cells per mouse were counted, and the data for each experimental point were from at least five mice.
Statistical analysis
Statistical evaluation of the data was done with the χ2 test and Student t-test, as appropriate. Statistical significance was assigned to P < 0.05.
RESULTS
Verification of the adaptive response mouse model (Yonezawa Effect)
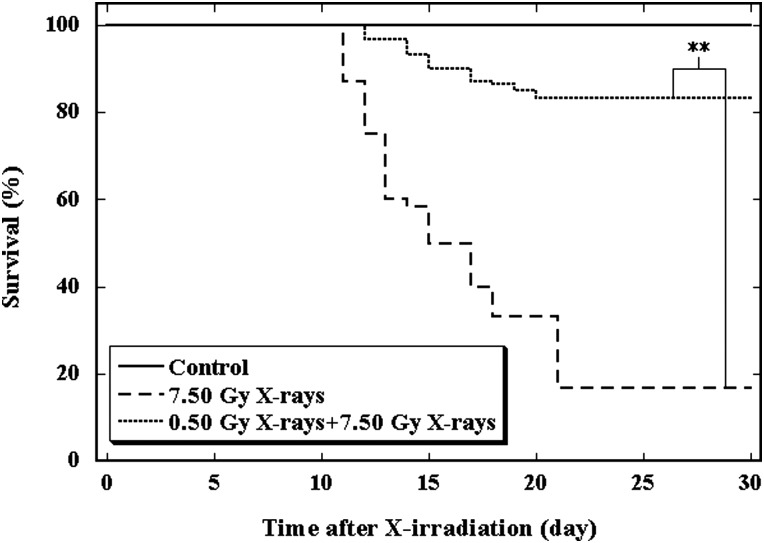
Reproducibility of the Yonezawa Effect, the model for induction of AR in young adult female mice, was verified and confirmed by using low-LET X-rays for delivery of both the priming low dose at 0.50 Gy and challenge high dose at 7.50 Gy. The 30-day survival test was applied to evaluate the induction of AR (Fig. 1). Administration of the priming dose markedly increased the survival rate from 16.67% to 83.33%. Results clearly indicated that AR was induced with efficient reliability and reproducibility in our experimental setup. Serving also as a positive control, the verification work was performed in parallel with the following investigations using heavy ion irradiations as the challenge doses.
Fig. 1.
Effect of a priming dose of 0.50-Gy X-rays on a challenge dose of 7.50-Gy X-rays on 30-day survival of mice.
The control group (solid line) received no irradiation, the 7.50-Gy X-rays group (broken line) received only the challenge irradiation, and the 0.50-Gy X-rays + 7.50-Gy X-rays (dotted line) received both the priming and the challenge irradiations. **Indicates statistically significant differences (P < 0.01) between the two groups that were compared.
Verification of previous in vivo adaptive response studies by the priming dose of 0.50 Gy X-rays against challenge doses from heavy ion irradiations
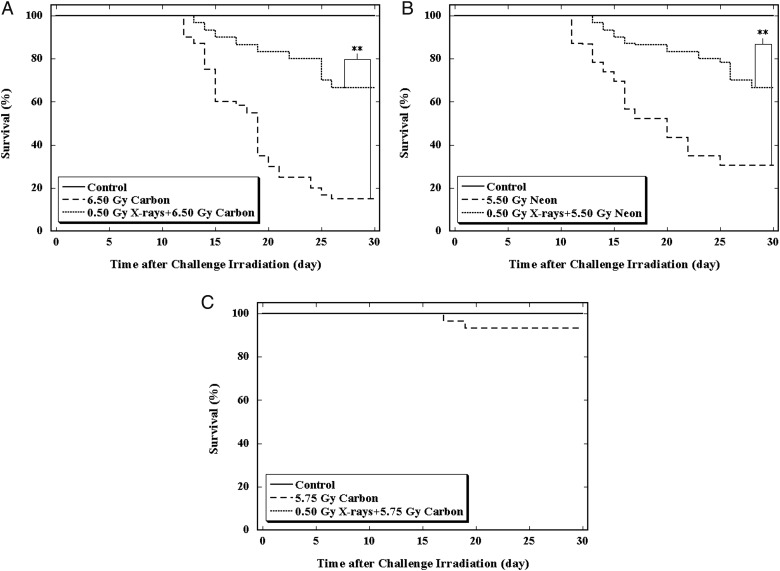
A rescue effect on bone marrow death in the 30-day survival test by the priming dose of 0.50 Gy X-rays against a challenge dose of 6.50 Gy from carbon ion irradiation or 5.50 Gy from neon ion irradiations was confirmed. Representative survival graphs showing that the priming low dose of X-rays could relieve the mouse killing effect caused by the challenge dose from carbon and neon ion irradiations are illustrated in Fig. 2A and B, respectively. In the following studies with carbon ion irradiations, a challenge dose of 5.75 Gy was used instead of 6.50 Gy to obtain more survivors in the 30-day survival test for further investigations of residual damage in the hematopoietic system. As shown in Fig. 2C, the challenge dose of 5.75 Gy alone resulted in mortality of 6.67%, whereas with a priming dose of 0.50 Gy from X-rays, no deaths occurred in the 30-day survival test.
Fig. 2.
Effect of a priming dose of 0.50-Gy X-rays on a challenge dose of 6.50-Gy carbon, 5.50-Gy neon or 5.75-Gy carbon on 30-day survival of mice.
(A) The control group (solid line) received no irradiation, the 6.50-Gy carbon group (broken line) received only the challenge irradiation and the 0.50-Gy X-rays + 6.50-Gy carbon (dotted line) received both the priming and the challenge irradiations. (B) The control group (solid line) received no irradiation, the 5.50-Gy neon group (broken line) received only the challenge irradiation and the 0.50-Gy X-rays + 5.50-Gy neon (dotted line) received both the priming and the challenge irradiations. (C) The control group (solid line) received no irradiation, the 5.75-Gy carbon group (broken line) received only the challenge irradiation and the 0.50-Gy X-rays + 5.75 Gy carbon (dotted line) group received both the priming and the challenge irradiations. **Indicates statistically significant differences (P < 0.01) between the two groups that were compared.
Residual damage in bone marrow erythrocytes in the surviving animals
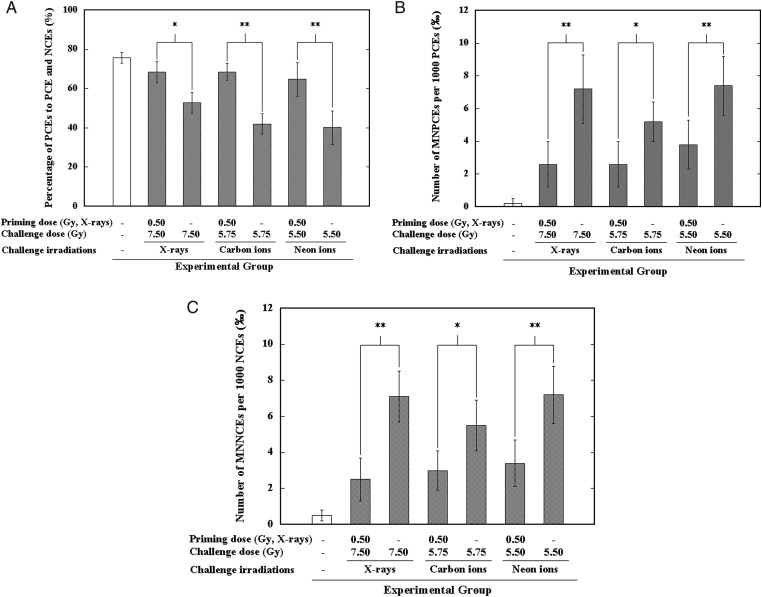
Because bone marrow failure was the main cause of animal death in this AR mouse model, we measured residual damage in the bone marrow cells of surviving animals 1 day after the 30-day survival test. The ratio of PCEs to the to the sum of PCEs and NCEs is an indicator for bone marrow proliferation, and the micronucleus test is a tool for genotoxic assessment. Results obtained in surviving animals from the positive control group (receiving both priming and challenge doses from X-rays), the carbon ion group (receiving priming dose from X-rays and challenge dose from carbon ions), and the neon ion group (receiving priming dose from X-rays and challenge dose from neon ions) are shown in Fig. 3. The micronucleus test study showed that independently of the nature of the challenge dose (X-rays or heavy ion), the priming dose markedly improved the ratio of PCEs to the sum of PCEs and NCEs (Fig. 3A) and significantly reduced the occurrences of both micronucleated PCEs per 1000 PCEs (Fig. 3B) and micronucleated NCEs per 1000 NCEs (Fig. 3C) in the femur bone marrow when respectively compared with that receiving the challenge dose alone.
Fig. 3.
Effect of a priming dose of 0.50-Gy X-rays on a challenge dose of 7.50-Gy X-rays, 6.50-Gy carbon, 5.50-Gy neon or 5.75-Gy carbon on the femur bone marrow of mice.
The percentage of polychromatic erythrocytes (PCEs) to PCEs and normochromatic erythrocytes (NCEs), the number of micronucleated PCEs (MNPCEs) per 1000 PCEs, and the number of micronucleated NCEs (MNNCEs) per 1000 NCEs are shown in A, B and C, respectively. *Indicates statistically significant differences (P < 0.05) between the two groups that were compared. **Indicates statistically significant differences (P < 0.01) between the two groups that were compared.
Residual damage in the peripheral blood hemogram in the surviving animals
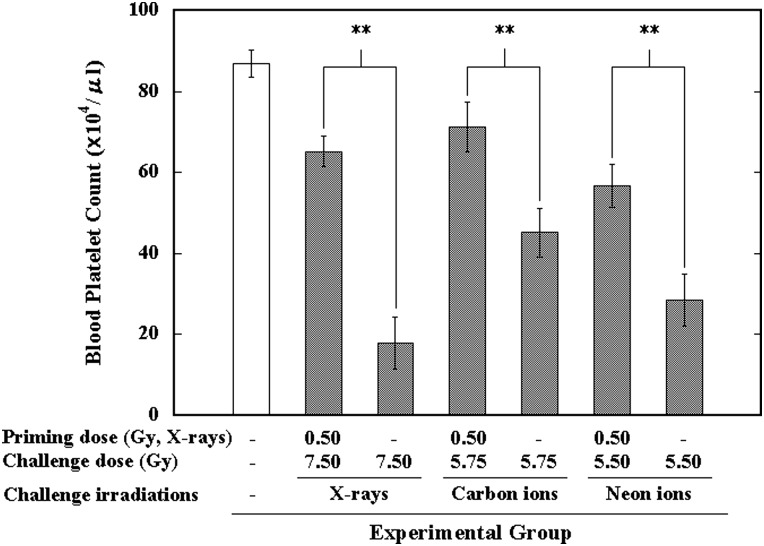
Residual damage in the hematopoietic system was also studied in the peripheral blood hemogram in the surviving animals 1 day after the 30-day survival test. The priming dose of X-rays significantly reduced the decrease in peripheral blood platelet count induced by the challenge exposure (X-rays or heavy ion) (Fig. 4). Although a tendency for improved erythrocyte count and leukocyte count was observed in the surviving animals from each AR group compared with their counterparts that received only the challenge irradiation, no statistical significance was found (data not shown). These data indicated that the priming low dose of 0.50 Gy low-LET X-rays could relieve, to a certain extent, the detrimental effects from the challenge high dose from either low-LET X-rays or high-LET accelerated ion irradiations in the hematopoietic system.
Fig. 4.
Effect of a priming dose of 0.50-Gy X-rays on a challenge dose of 7.50-Gy X-rays, 6.50-Gy carbon, 5.50-Gy neon or 5.75-Gy carbon on the blood platelet count in the peripheral blood of mice. **Indicates statistically significant differences (P < 0.01) between the two groups that were compared.
DISCUSSION
To understand to what extent low-dose irradiations might be beneficial in humans, a better understanding of AR and other non-targeted effects is needed [15]. The ‘Yonezawa Effect’ summarized a series of studies on radiation-induced AR at whole-body level in mice. In a series of comprehensive investigations, Yonezawa and colleagues verified the existence of AR under a variety of experimental conditions (varying doses of priming and challenge irradiations, varying intervals between priming and challenge exposures, and varying age and strain of the experimental mice) [7]. These efforts helped to lay the cornerstone for an in vivo AR model in mice. Based on the priming dose and on the interval between priming and challenge exposures, two different phenotypes of AR were observed that involved different mechanisms: the first phenotype was induced 2 weeks after a 0.3–0.5-Gy priming irradiation and was due to Trp53-dependent radioresistance in blood-forming tissues [10, 16]; the second phenotype was observed 2 months after a 0.05–0.1-Gy priming exposure and resulted from the interaction between blood-forming tissue and the central nervous system [17, 18]. The model for the first phenotype was applied to the present study.
Low doses of low-LET irradiations induce protective effects through mechanisms such as enhancement of antioxidative capacities, increase in cellular DNA double-strand break repair capacity leading to reduction of initial DNA damage in AR in mice in vivo [19, 20], and reduction of cell death, chromosomal aberrations, mutations and malignant transformation in vitro [5, 21]. These induced responses have been tightly conserved throughout evolution, suggesting that they are basic responses critical to life [4]. In addition to the acute mouse killing effect in the AR animal model, examination of residual damage and late detrimental effects on the hematopoietic system, such as myelosuppression and delayed genotoxic effects, is of importance from the point of view of AR study and radiation protection. Well-designed animal experiments over extended periods of time have made studies on late effects possible. Because the biological effects produced by high-LET particulate irradiations were of some qualitative difference from those produced by low-LET photon exposures [22], the existence of AR induced by low-LET irradiations against high-LET irradiations provides new clues for mechanistic studies. In the present study, the residual damage in surviving animals was studied in the mouse AR model after the 30-day survival test. Induction of AR in mice was performed using X-rays as priming irradiations in combination with challenge irradiations from either X-rays or accelerated carbon or neon ions with LET values of about 15 and 30 keV/μm, respectively. Results showed that in the surviving animals rescued by AR, the ratio of PCEs to the sum of PCEs and NCEs was significantly higher and the incidences of micronucleated PCEs and micronucleated NCEs were markedly lower than those in the survivors that received only the challenge dose. These findings indicated that a priming low dose of X-rays at 0.5 Gy significantly relieved myelosuppression and reduced residual damage in bone marrow cells induced by the challenge irradiations. In addition, the priming low dose of X-rays also markedly improved the blood platelet count in the surviving mice. Our blood platelet count results were consistent with previous results showing that rescue from bone marrow death was mainly due to priming irradiation-induced radio-resistance to challenge irradiation-induced Trp53-dependent apoptosis in hematopoietic stem cells [16, 23] and that the recovery of blood platelet count after exposure was one of the most important factors for restoration from bone marrow death [24]. The results of blood platelet counts obtained in the present study were consistent with these previous studies.
The recovery ratio from potentially lethal damage is known to depend on the quality of radiation [25]; cellular radio-sensitivity correlates with the frequency of residual chromatin breaks [26], and high-LET irradiations have been shown to induce higher rates of residual chromatin breaks [27]. Although high-LET heavy ions generally induce qualitatively different DNA damage (such as clustered DNA damage) in comparison with that of low-LET irradiations, the results of the present study suggested that the X-ray-induced biological defense mechanisms may be considered as effective countermeasures, being sufficient enough against the damage caused by high-LET challenge doses. In fact, mechanistic studies using cultured human fibroblasts reported that gene expression profiles following gamma radiation and decays of high-LET-like 125I shared the majority of genes in common, indicating that both kinds of radiation elicited similar signal transduction pathways [28]; thus, DNA double-strand breaks may not be the major factor modulating changes in gene expression following irradiation [29]. In vitro studies also showed that low doses of low-LET X-ray irradiations were effective in reducing chromosomal aberrations and mutation frequency induced by high-LET irradiations [21, 30]. These findings suggest that it may share at least to a large extent the same mechanisms against the damage caused by the high dose of challenge irradiations from low-LET X-rays and high-LET heavy ions.
Taken together, our results showed that a priming low dose of low-LET irradiations could relieve detrimental late effects (anhematopoiesis and delayed genotoxic effects) occurring in bone marrow cells. Results indicated the significance and possible application of AR to the reduction of genomic instability induced by high-dose irradiations from either low- or high-LET irradiations. These findings give new knowledge of the characterization of the ‘Yonezawa Effect’ by providing new insight into the mechanistic study of low-LET X-ray-induced AR against the detrimental effects of high-LET accelerated heavy ions in the mouse model of AR in vivo.
ACKNOWLEDGEMENTS
This work was partially supported both by a Grant-in-Aid for Scientific Research (C), 21510060, 2009-2011 from the Ministry of Education, Culture, Sports, and Science Culture, and two Research Project Grants (19B-258 and 22B-258) with Heavy Ions at NIRS-HIMAC. The authors thank all the staff of the Experiment Support Group, Accelerator Division, Accelerator Engineering Corporation, for performing heavy-ion irradiations. The expert technical assistance and administrative support of Ms Mikiko Nakajima, Ms Nobuko Tsutsumi, Ms Yasuko Morimoto, Ms Satoko Idohara, Ms Ichika Kishi, Ms Kaori Tateno, Mr Tatsuo Hayao, Dr Toshiaki Kokubo, Ms Maki Asano and Ms Hiromi Arai are gratefully acknowledged. The authors also thank Dr Yi Shang for her critical and constructive comments on the experimental design, performance, data analysis and manuscript preparation. We are also deeply grateful to Dr Takeshi Murakami for his continual support that made this study possible. Great appreciation is especially given to Dr Takeo Ohnishi, Professor Emeritus, Nara Medical University School of Medicine, for his constructive comments and continual encouragement throughout the study. Thanks are also due to the anonymous peer reviewers for providing the constructive comments that strengthened the presentation of this work. This paper is dedicated to the late Ms Remmy Wang, who devoted her whole life as an atmosphere maker in supporting the lead author's research work.
REFERENCES
- 1.UNSCEAR; UNSCEAR (ed) UNSCEAR 1994 Report to the General Assembly, with Scientific Annexes: Sources and Effects of Ionizing Radiation. Annex B. New York: United Nations: 1994. Adaptive responses to radiation in cells and organisms; pp. 185–272. [Google Scholar]
- 2.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–7. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi A, Ohnishi T. Molecular mechanisms involved in adaptive responses to radiation, UV light, and heat. J Radiat Res. 2009;50:385–93. doi: 10.1269/jrr.09048s. [DOI] [PubMed] [Google Scholar]
- 4.Mitchel RE. Low doses of radiation are protective in vitro and in vivo: evolutionary origins. Dose Response. 2006;4:75–90. doi: 10.2203/dose-response.04-002.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varès G, Bing W, Tanaka K, et al. Radiation-induced adaptive response with reference to evidence and significance: a review. Indian J Radiat Res. 2006;3:16–34. [Google Scholar]
- 6.Yonezawa M, Misonoh J, Hosokawa Y. Acquired radioresistance after small dose X-irradiation in mice. J Radiat Res. 1990;31:256–62. doi: 10.1269/jrr.31.256. [DOI] [PubMed] [Google Scholar]
- 7.Yonezawa M. Induction of radio-resistance by low dose X-irradiation. Yakugaku Zasshi. 2006;126:833–40. doi: 10.1248/yakushi.126.833. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 8.Yonezawa M, Misonoh J, Hosokawa Y. Two types of X-ray-induced radioresistance in mice: Presence of 4 dose ranges with distinct biological effects. Mutat Res. 1996;358:237–43. doi: 10.1016/s0027-5107(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 9.Nose M, Wang B, Itsukaichi H, et al. Rescue of lethally irradiated mice from hematopoietic death by pre-exposure to 0.5 Gy X rays without recovery from peripheral blood cell depletion and its modification by OK432. Radiat Res. 2001;156:195–204. doi: 10.1667/0033-7587(2001)156[0195:rolimf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka K, Koana T, Tomita M, et al. Rapid myeloid recovery as a possible mechanism of whole-body radioadaptive response. Radiat Res. 2008;170:307–15. doi: 10.1667/RR1146.1. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Tanaka K, Varès G, et al. X-rays-induced radioresistance against high LET irradiations from accelerated heavy ions in mice. Radiat Res. 2010;174:532–6. doi: 10.1667/RR2133.1. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Tanaka K, Ninomiya Y, et al. X-ray-induced radioresistance against high-LET radiations from accelerated neon-ion beams in mice. In: Nenoi M, editor. Current Topics in Ionizing Radiation Research. Rijeka: Intech-Open Access Publisher; 2012. pp. 199–214. [Google Scholar]
- 13.Schmid M. The micronucleus test. Mutat Res. 1975;31:9–153. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 14.Chaubey RC, Bhilwade HN, Joshi BN, et al. Studies on the migration of micronucleated erythrocytes from bone marrow to the peripheral blood in irradiated Swiss mice. Int J Radiat Biol. 1993;63:239–45. doi: 10.1080/09553009314550311. [DOI] [PubMed] [Google Scholar]
- 15.Tapio S, Jacob V. Radioadaptive response revisited. Radiat Environ Biophys. 2007;46:1–12. doi: 10.1007/s00411-006-0078-8. [DOI] [PubMed] [Google Scholar]
- 16.Horie K, Kubo K, Yonezawa M. p53 dependency of radio-adaptive responses in endogenous spleen colonies and peripheral blood-cell counts in C57BL mice. J Radiat Res. 2002;43:353–60. doi: 10.1269/jrr.43.353. [DOI] [PubMed] [Google Scholar]
- 17.Yonezawa M. Radioadaptive survival response in mice. In: Yamada T, Mothersill C, Michael BD, et al., editors. Biological Effects of Low Dose Radiaiton. Amsterdam: Elsevier Sciences; 2000. pp. 93–9. [Google Scholar]
- 18.Misonoh J, Yonezawa M British Nuclear Energy Society. Health Effects of Low Dose Radiation: Challenges of the 21st Century. London: Thomas Telford Services Ltd; 1997. Dose ranges for radioadaptive response in mice on the viewpoint of aquired radio-resistance after low dose irradiation; pp. 169–74. [Google Scholar]
- 19.Otsuka K, Koana T, Tauchi H, et al. Activation of antioxidative enzymes induced by low-dose-rate whole-body gamma irradiation: adaptive response in terms of initial DNA damage. Radiat Res. 2006;166:474–8. doi: 10.1667/RR0561.1. [DOI] [PubMed] [Google Scholar]
- 20.Phan N, De Lisio M, Parise G, et al. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res. 2012;177:164–75. doi: 10.1667/rr2532.1. [DOI] [PubMed] [Google Scholar]
- 21.Varès G, Wang B, Tanaka K, et al. Mutagenic adaptive response to high-LET radiation in human lymphoblastoid cells exposed to X-rays. Mutat Res. 2011;706:46–52. doi: 10.1016/j.mrfmmm.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Held KD. Effects of low fluences of radiations found in space on cellular systems. Int J Radiat Biol. 2009;85:379–90. doi: 10.1080/09553000902838558. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki R, Ootsuyama A, Norimura T. TP53 and TP53-related genes associated with protection from apoptosis in the radioadaptive response. Radiat Res. 2007;167:51–7. doi: 10.1667/RR0623.1. [DOI] [PubMed] [Google Scholar]
- 24.Takeda A, Yonezawa M, Katoh N. Restoration of radiation injury by Ginseng. I. J Radiat Res. 1981;22:323–35. doi: 10.1269/jrr.22.323. Responses of X-irradiated mice to Ginseng extract. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Kase Y, Kanai T, et al. Change in radiosensitivity with fractionated-dose irradiation of carbon-ion beams in five different human cell lines. Int J Radiat Oncol Biol Phys. 2000;48:251–8. doi: 10.1016/s0360-3016(00)00606-4. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki M, Kase Y, Kanai T, et al. Correlation between cell killing and residual chromatin breaks measured by PCC in six human cell lines irradiated with different radiation types. Int J Radiat Biol. 2000;76:1189–96. doi: 10.1080/09553000050134429. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M, Kase Y, Nakano T, et al. Residual chromatin breaks as biodosimetry for cell killing by carbon ions. Adv Space Res. 1998;22:1663–71. doi: 10.1016/s0273-1177(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 28.Sokolov M, Panyutin IG, Neumann R. Genome-wide gene expression changes in normal human fibroblasts in response to low-LET gamma-radiation and high-LET-like 125IUdR exposures. Radiat Prot Dosimetry. 2006;122:195–201. doi: 10.1093/rpd/ncl423. [DOI] [PubMed] [Google Scholar]
- 29.Sokolov MV, Smirnova NA, Camerini-Otero RD, et al. Microarray analysis of differentially expressed genes after exposure of normal human fibroblasts to ionizing radiation from an external source and from DNA-incorporated iodine-125 radionuclide. Gene. 2006;382:47–56. doi: 10.1016/j.gene.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Wolff S, Jostes R, Cross FT, et al. Adaptive response of human lymphocytes for the repair of radon-induced chromosomal damage. Mutat Res. 1991;250:299–306. doi: 10.1016/0027-5107(91)90185-q. [DOI] [PubMed] [Google Scholar]