Abstract
Granulocyte colony-stimulating factor (G-CSF) is one of the most critical cytokines used for the treatment of acute radiation syndrome (ARS). In addition to the hematopoietic effects of G-CSF on the differentiation and proliferation of myeloid progenitor cells, G-CSF is also known to have immunomodulatory effects. The aim of the present study was to investigate whether G-CSF could accelerate central and peripheral T lymphocyte recovery after a sublethal dose of irradiation. Female BALB/c mice were subjected to 6 Gy of total body irradiation and then were treated with either 100 μg/kg G-CSF or an equal volume of PBS once daily for 14 days. Percentages of thymocyte subpopulations including CD4 − CD8 − , CD4 + CD8 + , CD4 + CD8− and CD4 − CD8+ T cells, peripheral CD3 + , CD4+ and CD8+ cells were analyzed by flow cytometry. Recent thymic emigrants (RTEs) were assessed by real-time polymerase chain reaction (PCR) using primers specific to the 257-bp T cell receptor rearrangement excision circles (sjTRECs). The proliferative capacity of splenic mononuclear cells upon exposure to ConA was measured by using the Cell Count Kit-8 (CCK-8). G-CSF treatment promoted thymocyte regeneration, accelerated the recovery of CD4 + CD8+ cells and increased the frequency of thymocyte sjTRECs. These effects were more prominent at early time points (Day 28) after irradiation. G-CSF also increased the rate of recovery of peripheral CD3 + , CD4+ and CD8+ cells and shortened the period of severe lymphopenia following irradiation. G-CSF also increased the splenic mononuclear cell mitotic responsiveness to ConA more than control-treated cells. Our results show that G-CSF accelerates T cell recovery through both thymic-dependent and thymic-independent pathways, which could be used to increase the rate of immune reconstitution after sublethal irradiation.
Keywords: Irradiation, recent thymic emigrants, immune reconstitution, recombinant human granulocyte colony-stimulating factor
INTRODUCTION
Acute radiation syndrome (ARS), also known as radiation sickness, is defined by the signs and symptoms that occur after exposure to radiation. These symptoms arise following total body or partial body irradiation at a relatively high dose. Cells that have a rapid turnover rate such as hematopoietic cells are particularly sensitive to the effects of radiation. The hematopoietic cells include the immune cells, which can explain the observed impairment of the immune system in patients exposed to radiation. Bone marrow (BM) suppression and delayed immune reconstitution are the two primary causes of death after accidental or intentional exposure to moderate or high dose total body irradiation (TBI).
Management of acute myelosuppression has been significantly improved in recent years by the use of various hematopoietic growth factors (HGFs) such as granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage colony-stimulating factor and erythropoietin [1]. G-CSF remains the gold standard growth factor to counteract myelosuppression. Consensus treatment guidelines have been established in Europe and the United States [2, 3], which recommend that for moderate to severe myelosuppression corresponding to level 3 or 4 hematotoxicity [2] or grades H2 and H3 of the METROPOL clinical response scoring system [3], G-CSF should be administered soon after exposure.
Following immunosuppression, T cells appear to be regenerated more slowly than other cell types [4]. Furthermore, two pathways of T cell regeneration have been identified: thymic-dependent maturation of new T cells and thymic-independent expansion of mature peripheral T cells termed ‘homeostatic peripheral expansion’ (HPE) [5]. Defects in thymic production and HPE have been shown to impair effective T cell regeneration [6]. Several different mechanisms participate in the homeostatic control of T cell numbers and distribution, of which the cytokines IL-7 and IL-15 appear to be especially important [7, 8]. In addition to its hematopoietic properties, G-CSF is also known to exert pleiotropic effects on various immune cells [9–11]. Previous studies have shown that G-CSF alters the cytotoxic ability of natural killer (NK) cells [9] as well as the cytotoxic effectors activated by interleukin-2 (IL-2) [10] and the alloantigen-presenting capability of the immune accessory cells [11]. In the context of progenitor cell mobilization, G-CSF significantly increases the absolute number of peripheral T cells and profoundly influences their function [12], but little is known about whether G-CSF can stimulate T cell reconstitution after acute irradiation.
In this study, a sublethally irradiated mouse model was used to explore whether G-CSF might have some effects on central and peripheral T cell reconstitution. Interestingly, we found that G-CSF accelerated thymocyte regeneration, increased recent thymic emigrant cells, and accelerated central and peripheral T lymphocyte subset reconstitution.
MATERIALS AND METHODS
Mice
Female BALB/c (H-2d/d) mice between 8 to 12 weeks of age were purchased from the Animal Center of the Chinese Academy of Military Medical Sciences (Beijing, China). Mice were housed in sterilized cages and were maintained in a temperature-controlled (21–23°C), specific pathogen-free environment with a relative humidity between 50% and 60%. Mice received sterilized, commercial rodent chow and filtered water ad libitum. All experiments were approved by the local review board of the Chinese Academy of Military Medical Sciences in Beijing, China and were performed in accordance with national and international guidelines for laboratory animal care.
Irradiation and G-CSF administration
Recombinant human G-CSF (Sihuan Inc., Beijing, China) was diluted in phosphate-buffered saline (PBS) at a concentration of 10 μg/ml immediately before injection. BALB/c mice were sublethally γ-irradiated with a single dose of 600 cGy administered at a rate of 187 cGy/min using a cobalt source (GWXJ80 Theratron; Nuclear Power Institute of China, Sichuan, China). Immediately after irradiation, mice were injected subcutaneously with G-CSF (100 μg/kg) or with a similar volume of PBS once daily for 14 days. For all data, Day 0 refers to the day on which the mice were irradiated and received their first treatment. This therapeutic regimen for G-CSF was determined based on previous human clinical trials and scaled accordingly [13].
Peripheral hematological analysis
Approximately 30 μl of peripheral blood was collected from the lateral tail vein and was mixed with the anticoagulant Na2-EDTA (Beijing Chemical Agent Co., Ltd, Beijing, China). Automated hematological analysis was performed on the blood samples using a Sysmex MEK-7222K automated hematology analyzer (Nihon Kohden, Japan) with settings appropriate for the analysis of mouse samples. The following blood components were determined: white blood cell (WBC) count, platelet count, hemoglobin (HGB) level and absolute number of neutrophils and lymphocytes.
Preparation of thymocyte suspension
Thymi were harvested from BALB/c mice at Days 7, 14, 21, 28 and 60 after irradiation. Thymocytes were gently passed through sterile stainless steel mesh strainers into cold RPMI 1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FCS). Thymocytes in suspension were centrifuged at 200 × g for 10 min and then were resuspended in cold PBS at a concentration of 5 × 106 cells/ml. The cells were stored at 4°C until further use.
Lymphocyte flow cytometry
Rat anti-mouse CD4-FITC (GK1.5), CD8-PE (53-6.7) and CD3-PerCP (145-2c11) antibody conjugates were used for peripheral T cell subset analysis by flow cytometry. Rat anti-mouse CD4-FITC (GK1.5) and CD8-PE (53-6.7) were used for thymocyte subset analysis. All fluorochrome-conjugated monoclonal antibodies (mAbs) and their appropriate isotype controls were purchased from eBioscience (San Diego, CA, USA). Fifty microliters of whole blood or 1 × 106 thymocytes were incubated with anti-mouse CD16/CD32 FcR (2.4G2) (eBioscience Inc.) for 20 min at 4°C to prevent nonspecific binding. The samples were treated with FACS lysis solution (Becton Dickinson, San Jose, CA, USA) to remove red blood cells and then were washed twice with PBS. Cells were stained as previously described [14] and then analyzed using a Coulter EPICS XL-MCL flow cytometer operated with System II software (Coulter). For each sample, a lymphocyte gate was established based on the linear forward scatter and side scatter.
Quantification of mouse TCRα excision circles
Thymocytes were precisely counted and centrifuged for 2 min at 12000 rpm. Cell pellets were immediately resuspended in a 0.8 mg/ml solution of proteinase K (Boehringer Mannheim, Germany) digestion buffer (1 × 105 cells/10 μl of buffer). Samples were incubated for 1 h at 56°C followed by 10 min at 95°C to inactivate the proteinase K. Real-time quantitative PCR was performed on 5 μl of cell lysate (equivalent to 50 000 cells) using the mδRec primer 5′-CAAAAGAGGAAAGGAAGGCAGTC, the ψJα primer 5′-AAGGCATAAAGCGACACGAAGA and the mδRec-ψJα probe 5′-FAM-CTGCTGTGTGCCCTACCCTGCCC-TAMRA-3′. Primers and fluorescent probes were synthesized by Invitrogen (Carlsbad, CA, USA). PCR reactions contained 0.5 μM each of primer, 0.3 μM fluorescent probe, 1x Platinum Quantitative PCR Supermix-UDG (Invitrogen), and 1x Blue-636 reference dye (MegaBases, Evanston, IL, USA). Amplifications were performed in duplicate on a STRATAGENE MX3500P sequence Detection System (Agilent Technologies, Inc. Santa Clara, CA, USA) and were analyzed using the associated software as previously described [15]. PCR conditions were as follows: 95°C for 5 min, then 40 cycles of 95°C for 30 s, 59°C for 30 s and 72°C for 30 s. The number of molecules of murine TRECs was doubled to normalize the values per 100 000 cells.
Standard curves for murine TRECs were generated by cloning a 257-bp fragment of the δRec-ψJα PCR product into the pGEM-T Easy vector (Invitrogen). The plasmid was transformed into E. coli and was isolated by using IPTG (Merck & Co.). Stock dilutions of 107, 106, 105, 104, 103 and 102 plasmid copies per 5 μl were generated and were stored at –80°C until needed. One vial of each standard dilution was thawed immediately before use and was run in duplicate to generate a standard curve for each real-time PCR run. All acceptable runs had r2 values of at least 0.995 and y-intercepts of 45 ± 2.
T cell proliferation assay
T cell proliferation was assessed by using the Cell Counting Kit-8 (Dojindo, Kumomoto, Japan) according to the manufacturer's instructions. Briefly, the splenic mononuclear cells (MNCs) were collected and were suspended in RPMI 1640 medium supplemented with 10% FCS to a final concentration of 1 × 106 cells/ml. One hundred microliters of each cell suspension was added to a 96-well plate and was stimulated with 5 μg/ml ConA. After 48 h, 10 µl of CCK-8 reagent was added to each well. The plates were incubated at 37°C for 1.5 h, and then the absorbance (A) was measured at 450 nm using a microplate reader (Bio-TeK).
Statistical analysis
The results are expressed as the mean ± SD. P-values were calculated by using Student's two-tailed t-test. P-values less than 0.05 were considered significant for all tests.
RESULTS
Effects of G-CSF on thymocyte proliferation and T cell subset distribution
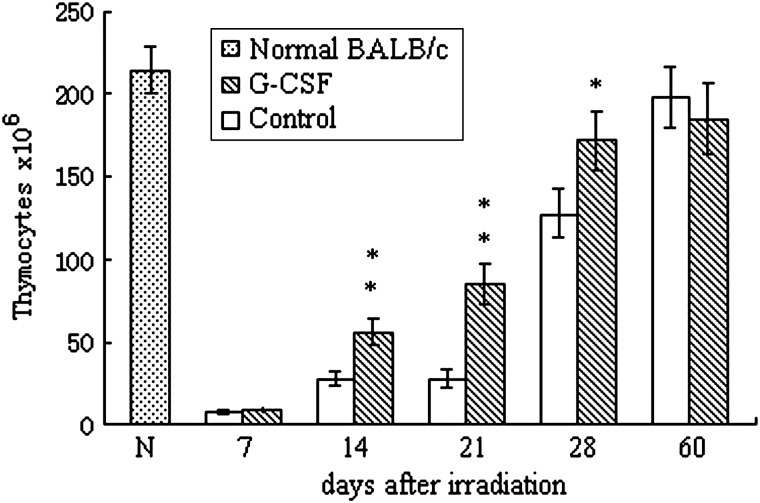
The total number of thymocytes decreased sharply after irradiation and reached approximately 5% of the basal level by Day 7. The thymocyte count began to increase by Day 14 and returned to the basal level at Day 60. Although G-CSF treatment had a minimal effect on the nadir level of the thymocytes post-irradiation, G-CSF significantly accelerated thymocyte reconstitution. The number of thymocytes in the G-CSF-treated group was significantly higher than the time-matched control-treated samples at Days 14, 21 and 28 post-irradiation (Fig. 1).
Fig. 1.
Effects of G-CSF administration on the number of total thymocytes
BALB/c mice were exposed to 6 Gy total body irradiation and were treated with 100 μg/kg G-CSF or a similar volume of PBS (control) once daily for 14 days. Thymocytes were collected, and the cell numbers were carefully counted. Values represent the mean ± SD of six independent animals per group. *P < 0.05, **P < 0.01 compared with PBS-treated control.
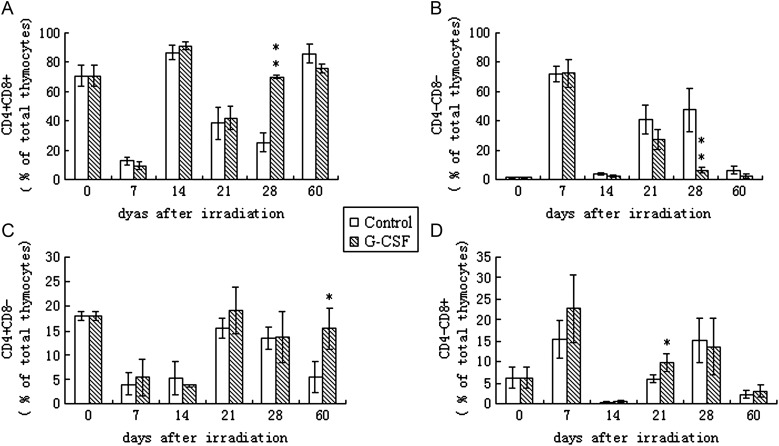
During thymopoiesis, bone-marrow-derived T progenitors (CD4–CD8– double-negative (DN) cells) migrate to the thymus, expand and mature. Thymopoiesis is the process of thymocyte differentiation and maturation resulting in CD4 or CD8 expression and the export of the mature T cells into the periphery. During thymopoiesis, T cells begin as CD4–CD8– double negative (DN) cells and then gain the expression of both CD4 and CD8 to become CD4 + CD8+ double positive (DP) cells. During the last step of maturation, the DP T cells down-regulate the expression of either CD4 or CD8 and become CD4+ or CD8+ single positive (SP) cells [16]. During the steady-state of the adult mouse thymus, phenotypic studies using flow cytometry analysis have shown that approximately 70–80% of thymocytes are CD4 + CD8+ DP cells, 10–20% are CD4+ SP cells, 5–6% are CD8+ SP cells and less than 3% of thymocytes are CD4–CD8– DN cells. Furthermore, the CD4:CD8 ratio in the thymus appears to be similar to the peripheral blood. Among the four subsets of thymocytes, CD4 + CD8+ DP cells showed the most radio-sensitivity, and the reconstitution of DP cells appears to follow a biphasic pattern. As shown in Fig. 2, CD4 + CD8+ DP cells were strongly affected by irradiation and decreased to less than 10% of the thymocytes by Day 7. Simultaneously, the relatively radio-resistant CD4–CD8– thymocytes showed a relative increase in numbers to nearly 90%. Upon the maturation and differentiation of the DN cells, the percentage of CD4 + CD8+ DP cells in both G-CSF- and PBS-treated groups returned to their basal levels by Day 14. However, this recovery was apparently temporary because a second decrease in the CD4 + CD8+ DP cell population was detected in both groups at Day 21 post-irradiation, which led to different recovery rates between the two groups. In G-CSF-treated mice, CD4 + CD8+ DP cells regenerated rapidly and permanently to the basal level by Day 28, whereas the PBS-treated control mice did not recover from the second decrease in the CD4 + CD8+ DP cell population until Day 60 post-irradiation. CD4+ SP and CD8+ SP cells were also significantly affected by irradiation and reached to their nadir at Day 7 and Day 14, respectively. After this decline, both CD4+ SP cells and CD8+ SP cells returned to their respective basal levels by Day 28. G-CSF appeared to increase the preference of DP cells to differentiate into CD8+ SP cells because by Day 21, the percentage of CD8+ SP cells was significantly higher than that of the control group (P < 0.05).
Fig. 2.
Effects of G-CSF administration on the thymocyte subset distribution.
BALB/c mice were exposed to 6 Gy of total body irradiation and were treated with 100 μg/kg G-CSF or an identical volume of PBS (control) once daily for 14 days. Thymocyte subsets CD4 + CD8+ (Fig. 2A), CD4–CD8– (Fig. 2B), CD4 + CD8– (Fig. 2C) and CD4–CD8+ (Fig. 2D) were detected once a week post-irradiation by flow cytometry. Values represent the mean ± SD of six independent animals per group. *P < 0.05, **P < 0.01 compared with PBS-treated control.
Effects of G-CSF on recent thymic emigrant cells
Signal-joint T cell receptor excision circles (sjTRECs) are stable circular DNA fragments that are excised in developing T lymphocyte at the double-negative thymocyte stage when the TCRα chain undergoes rearrangement [17]. These circular episomes are detected in T cells that are recent thymic emigrants or long-lived naive T cells. Because sjTRECs are not replicated during T cell proliferation, the number of sjTRECs per mg of thymus tissue or per 100 000 lysed cells can be used as a molecular marker of thymopoiesis. The sjTREC assay in mice was established by Sempowski et al. [18] and was used in the present study to assess thymocyte recovery in the control-treated and G-CSF-treated mice. In female BALB/c mice, the mean sjTREC frequency was 13 800 ± 873, which is similar to values that have been previously reported [19]. Table 1 shows that mice treated with G-CSF had a significantly higher sjTREC frequency when compared with the control mice at Day 30 after irradiation (P < 0.01). However, by Day 60 post-irradiation, the sjTREC frequency was no longer statistically significantly different between the two groups.
Table 1:
Effects of G-CSF on thymocyte sjTREC frequency
| sjTRECs/100 000 thymocytes |
|||
|---|---|---|---|
| Groups | n | 30 days post-irradiation | 60 days post-irradiation |
| Control | 5 | 3630 ± 595 | 3870 ± 1585 |
| G-CSF | 5 | 6450 ± 783a | 4950 ± 896 |
aP < 0.01 compared with PBS-treated control.
G-CSF accelerates peripheral T cell proliferation and T cell subset reconstitution
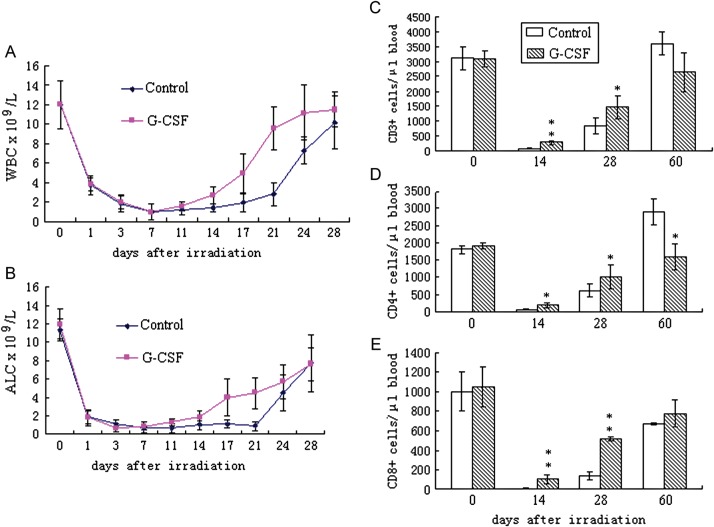
To determine whether G-CSF may have some influence on peripheral lymphocyte proliferation and subset distribution, an automated hematologic analyzer and flow cytometry were used to detect the absolute lymphocyte count (ALC) and the lymphocyte composition. As shown in Fig. 3, profound lymphopenia developed in both treatment groups following 6 Gy of total body irradiation. ALC in the PBS-treated controls reached a nadir at approximately one-tenth of the basal level at Day 7, remained substantially reduced for a period of 13 to 15 days and then increased to approximately 50% of the basal level by Day 28. Similar to the effects on WBCs such as neutrophils (Fig. 3A), G-CSF markedly accelerated the recovery of lymphocytes (Fig. 3B) from similar nadir levels. In mice treated with G-CSF, lymphocytes began to increase approximately 11 days post-irradiation and a significantly higher ALC was observed at Day 14 (P < 0.05), Day 17 (P < 0.01) and Day 21 (P < 0.01). From Day 28 to Day 60, the ALC in the two groups showed no significant differences (data not shown).
Fig. 3.
Effects of G-CSF administration on peripheral WBC and lymphocyte reconstitution.
BALB/c mice were exposed to 6 Gy of total body irradiation and were treated with 100 μg/kg G-CSF or an identical volume of PBS (control) once daily for 14 days. Peripheral blood samples were collected, and the white blood count (WBC) (Fig. 3A), absolute lymphocyte count (ALC) (Fig. 3B), and the absolute number of CD3+ (Fig. 3C), CD4+ (Fig. 3D) and CD8+ T cells (Fig. 3E) were determined. Values represent the mean ± SD of 10 independent animals per group. *P < 0.05, **P < 0.01 compared with PBS-treated control mice.
To determine to what extent the improved lymphocyte recovery in the G-CSF-treated group was due to T cell reconstitution, the absolute numbers of peripheral CD3 + , CD4 + and CD8+ cells were quantified at Days 14, 28 and 60 after irradiation. As shown in Fig. 3, G-CSF significantly accelerated the recovery of both CD4+ and CD8+ cells, which was particularly evident by Day 14 and Day 28 underscored by a prominent increase in CD3+ cells. Both the percentage and the absolute number of CD8+ T cells showed an increase in the G-CSF-treated mice. The G-CSF-treated mice harbored five-fold more CD8+ T cells per microliter of blood by Day 14 and four-fold more CD8+ T cells per microliter of blood by Day 28 when compared with the control-treated mice. Although the relative number of CD4+ T cells was similar between the two groups, the absolute number of CD4+ T cells per microliter of blood was significantly higher in the G-CSF-treated mice due to an overall increase in the CD3+ lymphocyte population. In addition, mice treated with G-CSF tended to maintain the CD4/CD8 ratio within a relatively normal range better than the control-treated mice.
In vitro proliferative responses to ConA of lymphocytes from G-CSF-treated mice
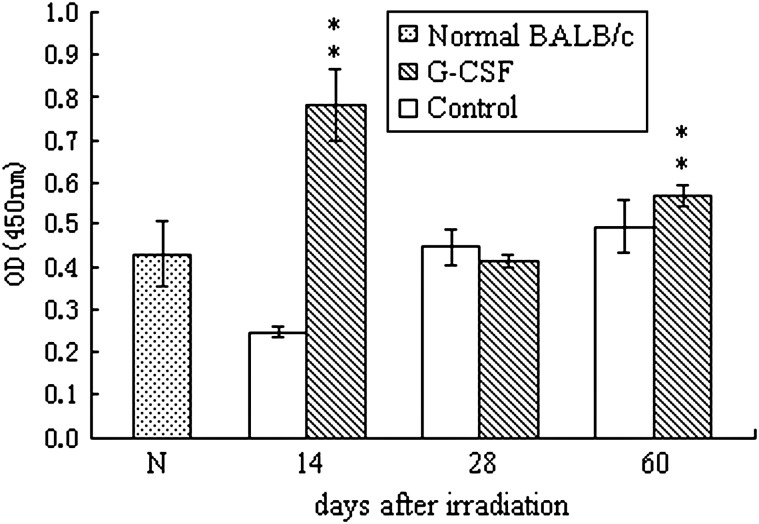
To assess the effects of G-CSF administration on the proliferative capacity of mouse T cells after irradiation, the CCK-8 kit was used following ConA stimulation of T cells. As shown in Fig. 4, G-CSF significantly promoted T cell proliferation. The OD value of splenic mononuclear cells from G-CSF-treated mice was almost two-fold higher than the control-treated mice on Day 14 after irradiation. By Day 60, the number of T cells in both groups returned to the basal level, but slight albeit significantly higher proliferation was observed in cells derived from G-CSF-treated mice. These results suggest that G-CSF may have a profound influence on T cell proliferation even several days after irradiation.
Fig. 4.
Proliferation of splenic T cells upon stimulation with ConA as determined by the CCK-8 assay.
The results are presented as the mean ± SD (n = 5). Double asterisks (**) represent statistically significant differences where P < 0.01 compared with the time-matched control-treated mice.
DISCUSSION
ARS following an accidental or intentional exposure to radiation leads to multi-organ failure. Depending on the energy, dose and geometric distribution of the exposure, bone marrow aplasia could arise concomitantly with gastrointestinal syndrome, skin burns, central nervous system failure and failure of several other organs. Until recently, only hematopoietic disorders could be counteracted with the appropriate therapies [20]. With the development of hematopoietic stem cell transplantation (HCT) and intense supportive therapy, bone marrow aplasia may not be a fatal outcome of radiation sickness, but injury to the immune system remains a hurdle in the effective management of ARS.
Of the multiple cell lineages affected by ARS, this study focused on the reconstitution of the T cell population of the immune system. Two pathways of T cell regeneration were identified: the thymic-dependent maturation of new T cells from bone marrow progenitors and thymic-independent expansion of mature peripheral T cells [5]. In irradiated lymphopenic hosts, both processes appear to contribute to the reconstitution of subsets of CD4+ and CD8+ cells.
G-CSF has been the golden standard therapy for patients suffering from 2–6 Gy of total body irradiation [2, 3]. Previous studies have shown that G-CSF can enhance hematological recovery and improve the survival of irradiated mice, which is most likely due to the effect of G-CSF on the differentiation and proliferation of the myeloid progenitor cells [21]. However, this study shows that G-CSF can support the recovery of both thymic-dependent and thymic-independent pathways of T cell regeneration, which promotes a more rapid and successful T cell immune reconstitution.
The thymus is the primary site for T-cell development, and the thymic-dependent pathway becomes severely compromised after irradiation due to the high radiation sensitivity of thymocytes. Sublethal radiation damage is transient as the regeneration of the irradiated thymus is remarkably rapid; the percentage of CD4 + CD8+ T cells returned to basal levels by Day 14 after irradiation. However, the process of CD4 + CD8+ double positive cell reconstitution was biphasic showing two waves of regeneration between Days 7 and 14 and after Day 21. Between Days 14 and 21 a severe relapse was observed similarly to other studies [22]. The thymic regeneration process depends not only on the proliferation of intrathymic radio-resistant precursors but also on the mobilization of stem cells from the BM to the thymus and differentiation to mature T lymphocytes. Radio-resistant thymocytes, mainly of the CD4–CD8– phenotype, appeared to be responsible for the first wave of reconstitution, which started relatively rapidly and gave rise to all thymocyte subsets. The second wave of regeneration was mainly driven by the early T cell progenitors as they migrated from the bone marrow and matured in the thymus. The results from previous experiments have shown that when irradiation was immediately followed by an intrathymic injection of bone marrow cells, the relapse in thymus reconstitution was no longer observed [22]. These results suggest that increased availability of functional early T cell progenitors may dramatically enhance CD4 + CD8+ cell reconstitution [23, 24]. As one of the most important hematopoietic cytokines, G-CSF mobilizes bone marrow stem cells into the peripheral blood, which eventually reach the thymus. However, as previously described [25], very few stem cells can give rise to the optimal T cell development in a sublethally irradiated thymus if exogenous growth factors are provided. In our study, although G-CSF did not prevent the interruption in CD4 + CD8+ cell regeneration, G-CSF significantly shortened the period of reduced T cells and quickly promoted the second phase of regeneration. Our data support a model whereby G-CSF potentiates stem cell migration to the thymus and possibly induces stem cell proliferation and differentiation within the thymus.
In addition to the effects of G-CSF on the migration of bone marrow precursor cells to the thymus, hematopoietic cytokine expression (mRNA and protein) by thymic epithelial cells (TECs) or whole thymus tissue has suggested additional possible regulatory mechanisms of thymopoiesis and thymic involution. In previous studies [26], the transcription of G-CSF mRNA was detected in the TECs and functional G-CSF was found in the supernatant of TEC culture systems in vitro. Thymic epithelial cells also produce a variety of other colony stimulating factors and hematopoietic cytokines such as IL-1, IL-3 and IL-6 [27], which can influence the complex process of thymopoiesis. Although G-CSF seemed unlikely to play a direct role in thymocyte proliferation, G-CSF can synergize with these cytokines to enhance intrathymic T cell development.
Renewed thymopoiesis may be assessed by using several indicators including thymic imaging, naive T cell frequency, TCR excision circle quantity and spectrotype patterns [28–30]. Measurement of TCR rearrangement excision circles (TRECs) provides a means of quantifying thymic productivity [31]. Robust thymopoiesis is reflected by a high frequency of TREC-bearing naive cells, termed ‘recent thymic emigrants’ (RTEs). RTEs are proportional to the level of BM precursors that migrated to the thymus as well as the availability of the thymic stroma [32, 33]. The frequency of sjTRECs in G-CSF-treated mice was significantly higher than that of the control-treated mice at Day 30 after irradiation. However, the effects of G-CSF decreased with time and by Day 60 after irradiation, the two groups had similar sjTREC frequencies, which were below the basal level. A possible explanation for these findings is that the dosage and/or duration of G-CSF administration used in our experiments were insufficient to sustain a long-term effect on thymic RTE levels. Future studies should determine whether increasing the G-CSF dosage or prolonging the treatment duration would correlate with an increased effect on thymic RTEs.
Although the thymic-dependent pathway can provide stable, diverse T cell reconstitution, the contribution of this pathway to immune system reconstitution following a radiological insult is delayed by the period of thymic reconstruction. An alternative pathway for T cell development is through the rapid cell division of mature T cell clones termed homeostatic peripheral expansion ‘HPE’ [34, 35]. In this study, 6 Gy of TBI depleted most of the lymphocytes causing severe lymphopenia that persisted for approximately 18 days. Following the period of lymphopenia, residual T cells began rapid regeneration consistent with a predominant HPE mechanism. Treatment with G-CSF had a significant effect on the expansion of residual T lymphocytes and shortened the period of lymphopenia. The T cell-promoting effects of G-CSF have also been observed when G-CSF was used to mobilize hematopoietic progenitors into the peripheral blood of a healthy donor. Previous studies have demonstrated that in G-CSF–treated animals, the yield of T cells per milliliter of blood increases three-fold to four-fold higher without changing the CD4/CD8 ratio [36]. Our study shows that G-CSF has similar effects on T cells under conditions of myelosuppression.
As a hematopoietic growth factor, G-CSF also possesses immunomodulatory effects. In previous studies, Joshi and colleagues [37] found that T cell proliferation in response to standard mitogens was significantly impaired after G-CSF-mediated mobilization. Studies have also shown that G-CSF mobilization skews the cytokines secreted by T cells towards a type 2 profile upon alloantigen stimulation [38]. In our study, we found that G-CSF enhances T lymphocyte proliferation upon mitogen stimulation. This discrepancy may be due to differences in the circumstances (steady state or myelosuppression) in which G-CSF was used.
Although the precise mechanism remains unknown, our results demonstrate that G-CSF promotes thymocyte regeneration and peripheral T cell expansion after sublethal irradiation thus contributing to a more rapid and efficient T cell immune reconstitution.
REFERENCES
- 1.Capo G, Waltzman R. Managing hematologic toxicities. J Support Oncol. 2004;2:65–79. [PubMed] [Google Scholar]
- 2.Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Friesecke I, Beyrer K, Fliedner TM, et al. How to cope with radiation accidents: the medical management. Br J Radiol. 2001;74:121–2. doi: 10.1259/bjr.74.878.740121. [DOI] [PubMed] [Google Scholar]
- 4.Petersen SL, Ryder LP, Bjork P, et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplantation. 2003;32:65–72. doi: 10.1038/sj.bmt.1704084. [DOI] [PubMed] [Google Scholar]
- 5.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 6.Storek J, Joseph A, Dawson MA, et al. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002;73:1154–8. doi: 10.1097/00007890-200204150-00026. [DOI] [PubMed] [Google Scholar]
- 7.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–56. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 8.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JS, Prosper F, McCullar V. Natural killer (NK) cells are functionally abnormal and NK cells are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood. 1997;90:3098–105. [PubMed] [Google Scholar]
- 10.Verma UN, Yankelevich B, Hodgson J, et al. Effect of the in vivo priming regimen for peripheral blood stem cells (PBSC) mobilization on in vitro generation of cytotoxic effectors by IL-2 activation of PBSC in a murine model. Bone Marrow Transplant. 1997;19:265–73. doi: 10.1038/sj.bmt.1700632. [DOI] [PubMed] [Google Scholar]
- 11.Rondelli D, Raspodiri D, Anasetti C, et al. Alloanti-gen presenting capacity, T cell alloreactivity and NK function of G-CSF-mobilized peripheral blood cells. Bone Marrow Transplant. 1998;22:631–7. doi: 10.1038/sj.bmt.1701413. [DOI] [PubMed] [Google Scholar]
- 12.Marmier-Savet C, Larosa F, Legrand F, et al. Persistence of lymphocyte function perturbations after granulocyte–colony-stimulating factor mobilization and cytapheresis in normal peripheral blood stem cell donors. Transfusion. 2010;12:2676–85. doi: 10.1111/j.1537-2995.2010.02781.x. [DOI] [PubMed] [Google Scholar]
- 13.Patchen M, MacVittie T, Solberg B, et al. Survival enhancement and hemopoietic regeneration following radiation exposure: Therapeutic approach using glucan and granulocyte colony-stimulating factor. Exp Hematol. 1990;18:1042–8. [PubMed] [Google Scholar]
- 14.Suzuki I, Martin S, Boursalian TE, et al. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J Immunol. 2000;165:5537–43. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland JS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunology. 2005;175:2741–53. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 16.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita S, Toda M, Yamagishi H. Excision products of the T cell receptor gene support a progressive rearrangement model of the alpha/delta locus. EMBO J. 1989;8:3261–70. doi: 10.1002/j.1460-2075.1989.tb08486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sempowski G.D., Gooding ME, Liao HX, et al. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–8. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 19.Broers AE, Meijerink JP, van Dongen JJ, et al. Quantification of newly developed T cells in mice by real-time quantitative PCR of T-cell receptor rearrangement excision circles. Exp Hematol. 2002;30:745–50. doi: 10.1016/s0301-472x(02)00825-1. [DOI] [PubMed] [Google Scholar]
- 20.MacVittie TJ, Farese AM. Cytokine-based treatment for acute radiation-induced myelosuppression: preclinical and clinical perspective. In: Ricks RC, Berger ME, O'Hara FM Jr., editors. The Medical Basis for Radiation Accident Preparedness. London: The Partenon Publishing Group; 2002. pp. 53–72. [Google Scholar]
- 21.Tanikawa S, Nose M, Aoki Y, et al. Effects of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:445–9. [PubMed] [Google Scholar]
- 22.Penit C, Ezine S. Cell proliferation and thymocyte subset reconstitution in sublethally irradiated mice: compared kinetics of endogenous and intrathymically transferred progenitors. Proc Nadl Acad Sci. 1989;86:5547–51. doi: 10.1073/pnas.86.14.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen BJ, Cui X, Sempowski GD, et al. Hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation. Blood. 2004;103:4344–52. doi: 10.1182/blood-2003-07-2534. [DOI] [PubMed] [Google Scholar]
- 24.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nature Medicine. 2006;12:1039–47. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 25.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. J Immunol. 1990;145:3661–8. Kinetics and phenotype of progeny. [PubMed] [Google Scholar]
- 26.Le PT, Kurtzberg J, Brandt SJ, et al. Human thymic epithelial cells produce granulocyte and macrophage colony-stimulating factors. J Immunol. 1988;141:1211–17. [PubMed] [Google Scholar]
- 27.Le PT, Lazorick S, Whichard LP, et al. Human thymic epitheli cells produce IL-6, granulocyte-monocyte-CSF, and leukemia inhibitor factor. J Immunol. 1990;145:3310–15. [PubMed] [Google Scholar]
- 28.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. New Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 29.Mackall CL, Granger L, Sheard MA, et al. T-cell regeneration after bone marrow transplantation:differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood. 1993;82:2585–94. [PubMed] [Google Scholar]
- 30.Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. Journal Clin Invest. 2005;115:930–9. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–81. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 32.Berzins SP, Boyd RL, Miller JFAP. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–48. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berzins SP, Godfrey DI, Miller JFAP, et al. A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci USA. 1999;96:9787–91. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackall CL, Granger L, Sheard MA, et al. T-cell regeneration after bone marrow transplantation:differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood. 1993;82:2585–94. [PubMed] [Google Scholar]
- 35.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald KA, Rowe V, Filippich C, et al. Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood. 2003;101:2033–42. doi: 10.1182/blood-2002-05-1529. [DOI] [PubMed] [Google Scholar]
- 37.Joshi SS, Lynch JC, Pavletic SZ, et al. Decreased immune functions of blood cells following mobilization with granulocyte colony-stimulating factor: association with donor characteristics. Blood. 2001;98:1963–70. doi: 10.1182/blood.v98.6.1963. [DOI] [PubMed] [Google Scholar]
- 38.Pan L, Delmonte J, Jalonen CK, et al. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–9. [PubMed] [Google Scholar]