Abstract
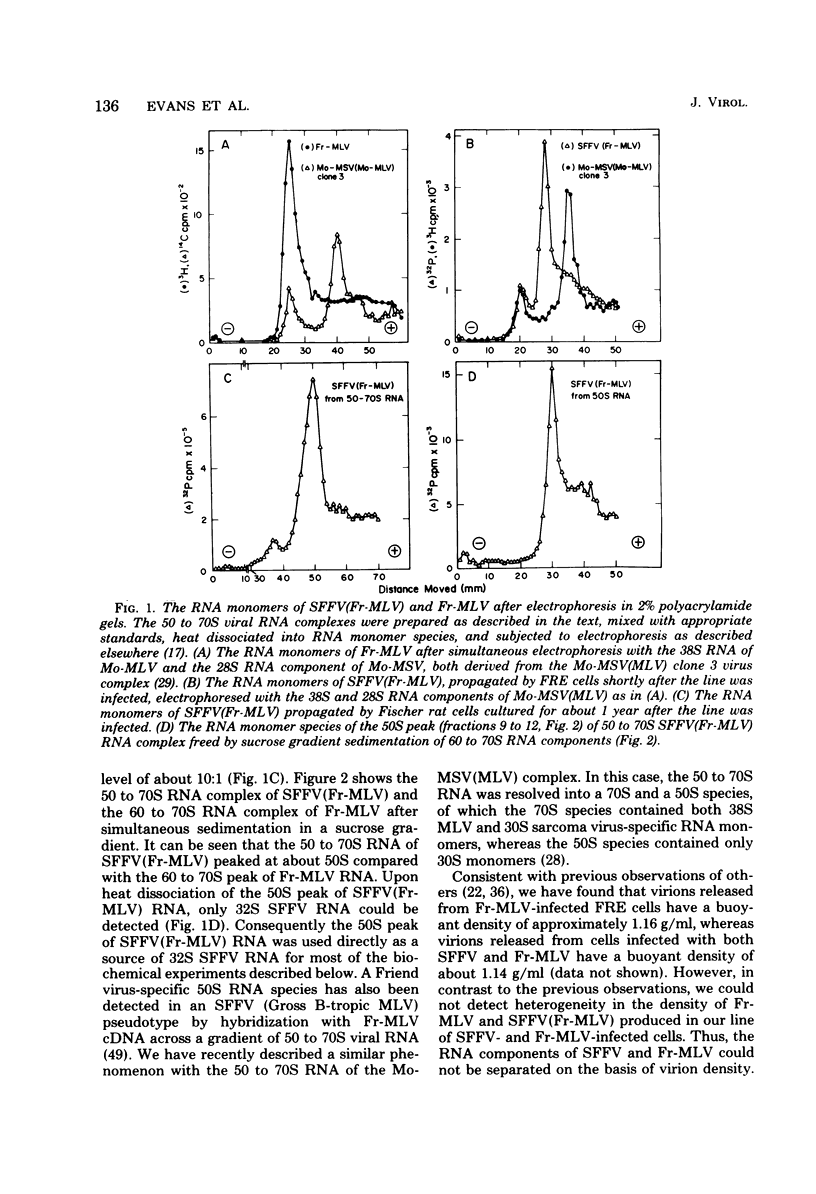
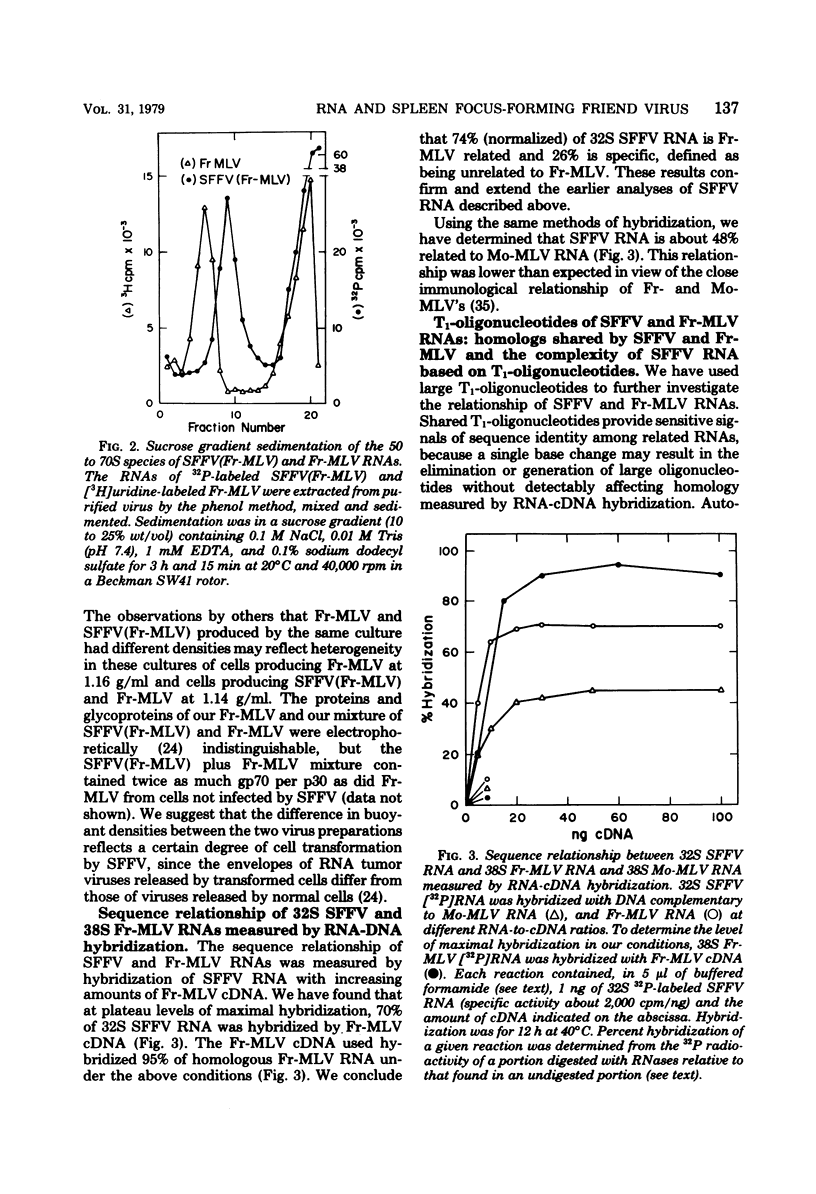
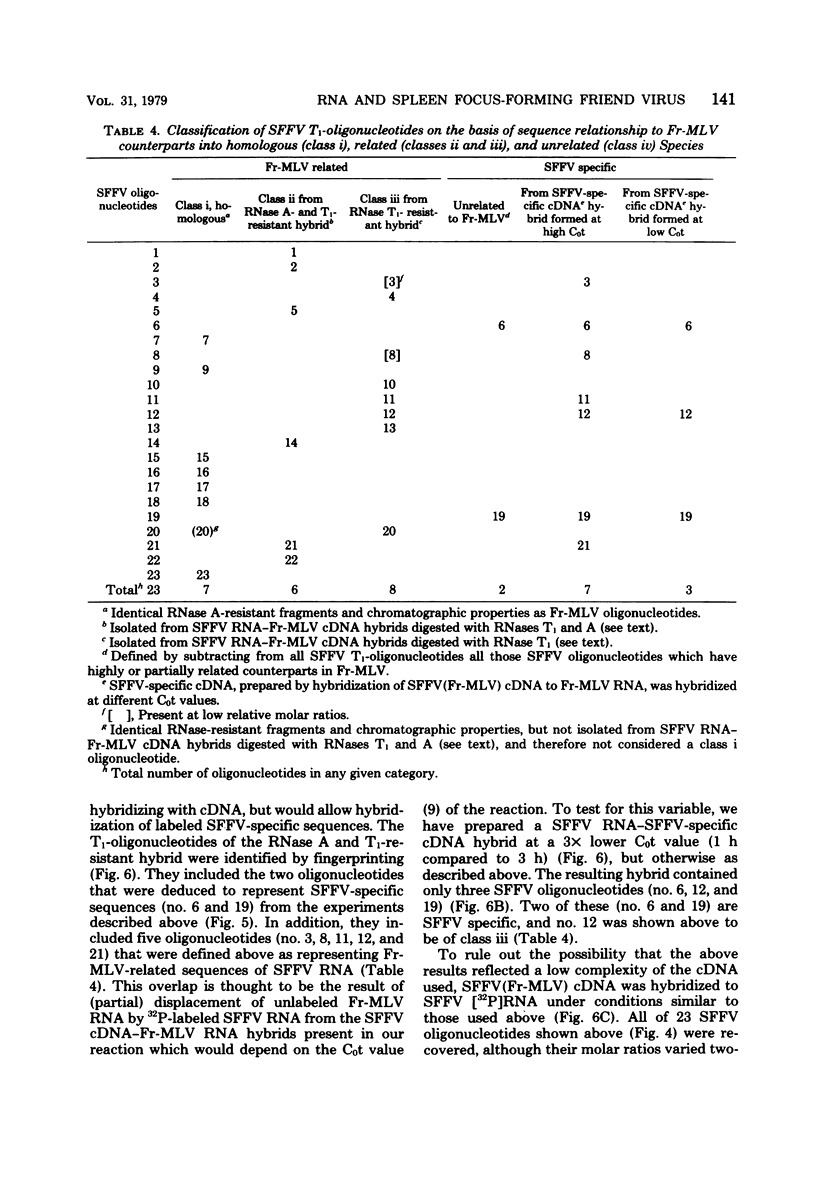
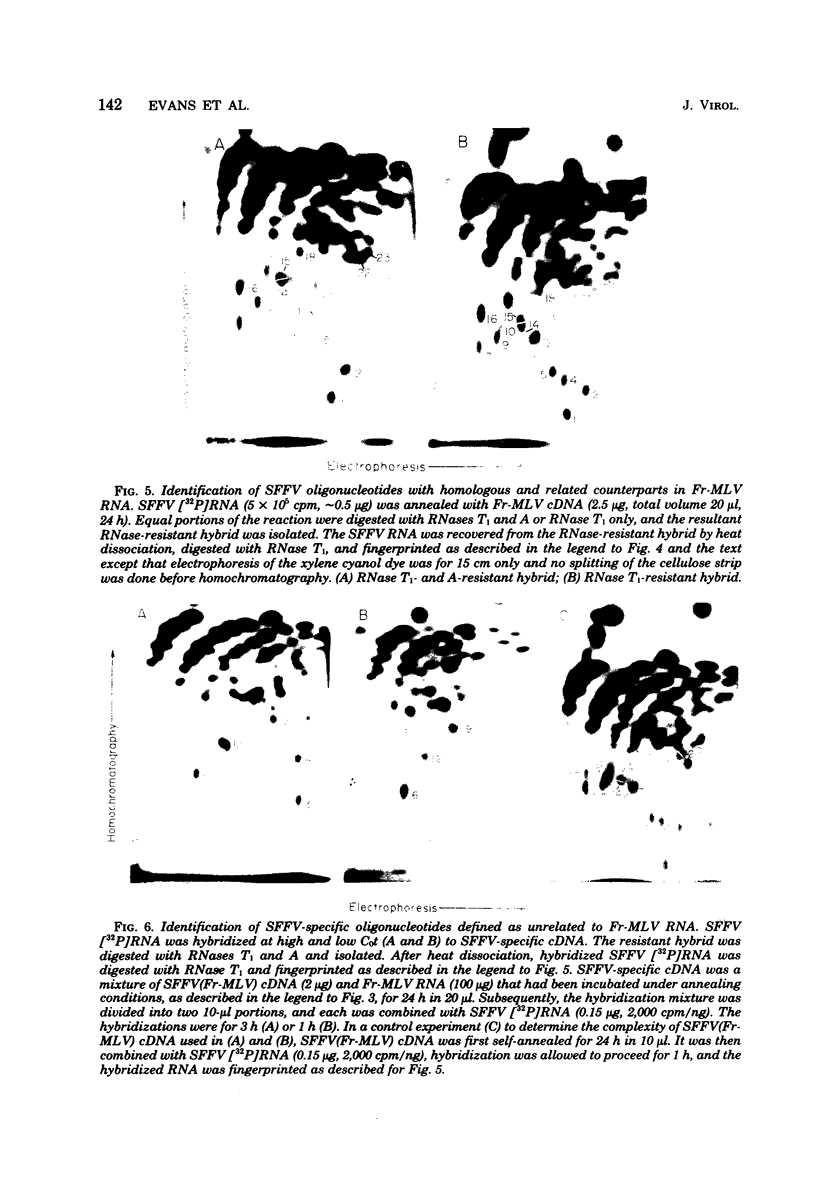
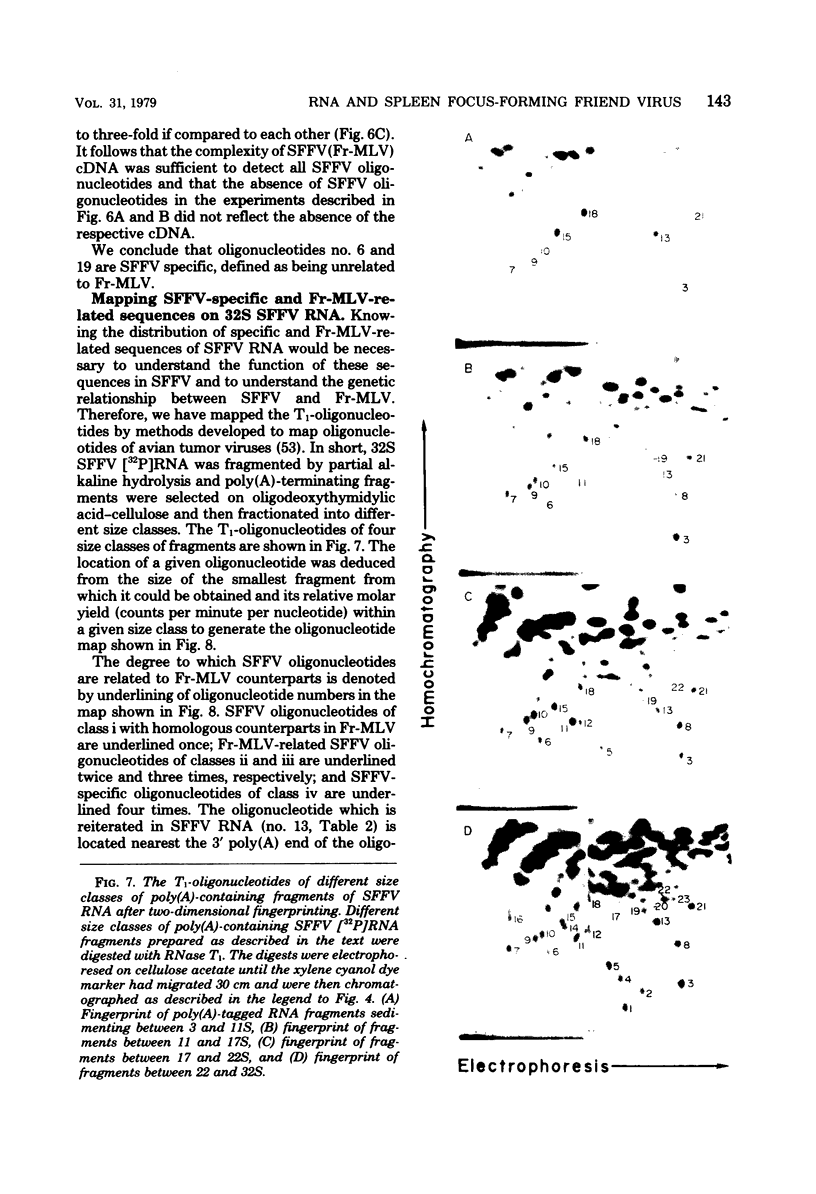
The genome of the defective, murine spleen focus-forming Friend virus (SFFV) was identified as a 50S RNA complex consisting of 32S RNA monomers. Electrophoretic mobility and the molecular weights of unique RNase T1-resistant oligonucleotides (T1-oligonucleotides) indicated that the 32S RNA had a complexity of about 7.4 kilobases. Hybridization with DNA complementary to Friend murine leukemia virus (Fr-MLV) has distinguished two sets of nucleotide sequences in 32S SFFV RNA, 74% which were Fr-MLV related and 26% which were SFFV specific. By the same method, SFFV RNA was 48% related to Moloney MLV. We have resolved 23 large T1-oligonucleotides of SFFV RNA and 43 of Fr-MLV RNA. On the basis of the relationship between SFFV and Fr-MLV RNAs, the 23 SFFV oligonucleotides fell into four classes: (i) seven which had homologous equivalents in Fr-MLV RNA; (ii) six more which could be isolated from SFFV RNA-Fr-MLV cDNA hybrids treated with RNases A and T1; (iii) eight more which were isolated from hybrids treated with RNases A and T1; and (iv) two which did not have Fr-MLV-related counterparts. Surprisingly, the two class iv oligonucleotides had homologous counterparts in the RNA of six amphotropic MLV's including mink cell focus-forming and HIX-MLVs analyzed previously. The map locations of the 23 SFFV T1-oligonucleotides relative to the 3' polyadenylic acid coordinate of SFFV RNA were deduced from the size of the smallest polyadenylic acid-tagged RNA fragment from which a given oligonucleotide was isolated. The resulting oligonucleotide map could be divided roughly into three segments: two terminal segments which are mosaics of oligonucleotides of classes i, ii, and iii and an internal segment between 2 and 2.5 kilobases from the 3' end containing the two oligonucleotides shared with amphotropic MLVs. Since SFFV RNA consists predominantly of sequence elements related to ecotropic and amphotropic helper-independent MLVs, it would appear that the transforming gene of SFFV is not a major specific sequence unrelated to genes of helper viruses, as is the case with Rous sarcoma and probably withe other defective sarcoma and acute leukemia viruses.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Beemon K. L., Faras A. J., Hasse A. T., Duesberg P. H., Maisel J. E. Genomic complexities of murine leukemia and sarcoma, reticuloendotheliosis, and visna viruses. J Virol. 1976 Feb;17(2):525–537. doi: 10.1128/jvi.17.2.525-537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A., Mak T. W., Stephenson J. R. The Friend virus genome: evidence for the stable association of MuLV sequences and sequences involved in erythroleukemic transformation. Cell. 1977 Sep;12(1):287–294. doi: 10.1016/0092-8674(77)90206-9. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Duesberg P. H. Structure of Rauscher mouse leukaemia virus RNA. Nature. 1968 Oct 26;220(5165):396–399. doi: 10.1038/220396a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Dawson P. J., Tacke R. B., Fieldsteel A. H. Relationship between Friend virus and an associated lymphatic leukaemia virus. Br J Cancer. 1968 Sep;22(3):569–576. doi: 10.1038/bjc.1968.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina D., Beemon K., Duesberg P. The 30S Moloney sarcoma virus RNA contains leukemia virus nucleotide sequences. Cell. 1976 Oct;9(2):299–309. doi: 10.1016/0092-8674(76)90120-3. [DOI] [PubMed] [Google Scholar]
- Dube S., Kung H. J., Bender W., Davidson N., Ostertag W. Size, subunit composition, and secondary structure of the Friend virus genome. J Virol. 1976 Oct;20(1):264–272. doi: 10.1128/jvi.20.1.264-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Eckner R. J., Hettrick K. L. Defective Friend spleen focus-forming virus: interfering properties and isolation free from standard leukemia-inducing helper virus. J Virol. 1977 Oct;24(1):383–396. doi: 10.1128/jvi.24.1.383-396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotide maps of Moloney and HIX murine leukemia viruses. Virology. 1978 Oct 15;90(2):265–273. doi: 10.1016/0042-6822(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Galehouse D. M., Duesberg P. H. Glycoproteins of avian tumor virus recombinants: evidence for intragenic crossing-over. J Virol. 1978 Jan;25(1):86–96. doi: 10.1128/jvi.25.1.86-96.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howk R. S., Troxler D. H., Lowy D., Duesberg P. H., Scolnick E. M. Identification of a 30S RNA with properties of a defective type C virus in murine cells. J Virol. 1978 Jan;25(1):115–123. doi: 10.1128/jvi.25.1.115-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H., Horst J., Vogt P. K. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Bender W., Hu S., Duesberg P. H., Davidson N. Structure of 50 to 70S RNA from Moloney sarcoma viruses. J Virol. 1978 Jan;25(1):384–394. doi: 10.1128/jvi.25.1.384-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Dina D., Duesberg P. Murine sarcoma viruses: the helper-independence reported for a Moloney variant is unconfirmed; distinct strains differ in the size of their RNAs. Virology. 1977 Jan;76(1):295–312. doi: 10.1016/0042-6822(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A., STOCKERT E. TYPING OF MOUSE LEUKAEMIAS BY SEROLOGICAL METHODS. Nature. 1964 Feb 22;201:777–779. doi: 10.1038/201777a0. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Pragnell I. B. Changes in genome composition of the Friend virus complex in erythroleukemia cells during the course of differentiation induced by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3278–3282. doi: 10.1073/pnas.75.7.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell I. B., McNab A., Harrison P. R., Osterag W. Are spleen focus-forming virus sequences related to xenotropic viruses and expressed specifically in normal erythroid cells? Nature. 1978 Mar 30;272(5652):456–458. doi: 10.1038/272456a0. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson K. E., Parr I. B. A new virus of minimal pathogenicity associated with Friend virus. I. Isolation by end-point dilution. Int J Cancer. 1970 Jan 15;5(1):96–102. doi: 10.1002/ijc.2910050113. [DOI] [PubMed] [Google Scholar]
- Scolnic E. M., Goldberg R. J., Parks W. P. A biochemical and genetic analysis of mammalian RNA-containing sarcoma viruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):885–895. doi: 10.1101/sqb.1974.039.01.103. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Vass W. C., Howk R. S., Duesberg P. H. Defective retrovirus-like 30S RNA species of rat and mouse cells are infectious if packaged by type C helper virus. J Virol. 1979 Mar;29(3):964–972. doi: 10.1128/jvi.29.3.964-972.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin S. A., Rapp U. R., Benveniste R. E., Sen A., Todaro G. J. Rescue of endogenous 30S retroviral sequences from mouse cells by baboon type C virus. J Virol. 1978 May;26(2):257–264. doi: 10.1128/jvi.26.2.257-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Troxler D. H., Coffin J. M., Scolnick E. M. Mapping host range-specific oligonucleotides within genomes of the ecotropic and mink cell focus-inducing strains of Moloney murine leukemia virus. J Virol. 1978 Apr;26(1):71–83. doi: 10.1128/jvi.26.1.71-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Williams D. R., Weeks M. O., Maryak J. M., Vass W. C., Scolnick E. M. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. doi: 10.1128/jvi.27.1.45-55.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Parks W. P., Vass W. C., Scolnick E. M. Isolation of a fibroblast nonproducer cell line containing the Friend strain of the spleen focus-forming virus. Virology. 1977 Feb;76(2):602–615. doi: 10.1016/0042-6822(77)90242-2. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]