Abstract
Glioblastoma (GB) is one of the most lethal forms of cancer, with an invasive growth pattern that requires the use of adjuvant therapies, including chemotherapy and radiation, to prolong survival. Temozolomide (TMZ) is an oral chemotherapy with a limited side effect profile that has become the standard of care in GB treatment. While TMZ has made an impact on survival, tumor recurrence and TMZ resistance remain major challenges. Molecular markers, such as O6-methylguanine-DNA methyltransferase methylation status, can be helpful in predicting tumor response to TMZ, and therefore guides clinical decision making. This review will discuss the epidemiology and possible genetic underpinnings of GB, how TMZ became the standard of care for GB patients, the pharmacology of TMZ, the practical aspects of using TMZ in clinic, and how molecular diagnostics – particularly the use of O6-methylguanine-DNA methyltransferase status – affect clinical management.
Keywords: glioblastoma, temozolomide, PredictMDx™, MGMT
Introduction to glioblastoma (GB) and its management
GB is the most common primary brain cancer and among the most lethal, accounting for approximately 1% of all cancer diagnoses and 2% of cancer deaths in the US.1 GB is defined histologically by hypercellularity, necrosis, pleomorphism, vascular proliferation, and pseudopalisading necrosis.2 Its characteristically invasive growth pattern makes a complete surgical resection nearly impossible, therefore requiring the use of adjuvant therapies to prolong survival.
When using anticancer therapies such as radiation and chemotherapy in the central nervous system, clinicians must balance the need to kill malignant cells with the need to preserve normal, functional tissue. Additionally, chemotherapy agents must be able to reach cancer cells, which may reside behind the blood–brain barrier or be impenetrable due to high interstitial pressures. These challenges all contribute to the relative resistance of GB to treatment. Despite many advances, the 5-year survival rate of GB patients is less than 5%.3
Since chemotherapy first became available in the 1950s, nearly every new class of drug to come to market has been tested in GB patients. The nitrosoureas, a class of small, lipophilic alkylating agents that includes 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU), were the first drugs to demonstrate activity against GB.4,5 These agents add an alkyl group to the guanine residues in DNA, causing crosslinking and preventing replication. Other drugs in this class cause base pair mismatches, which also disrupts replication. Alkylating agents quickly became the backbone of medical glioma therapy, but broad use was limited by relatively severe side effects such as myelosuppression and, in the case of BCNU, pulmonary fibrosis. When compared with radiation, neither added a significant survival advantage.6,7 Therefore, many patients received first-line radiotherapy. Chemotherapy was considered an alternate option, frequently reserved for disease progression.
In the late 1970s, a team of scientists in England synthesized a new class of alkylating drugs, the imidazotetrazinones, which included the compound that would ultimately go on to be called temozolomide (TMZ). The imidazotetrazinones are related to dacarbazine (DTIC®), a drug that was widely used for metastatic melanoma in the late 1970s and early 1980s, and also had some success in glioma.8 DTIC is a prodrug that requires enzymatic conversion to its active form, 5-(3-methyltriazen-1yl)-imidazole-4-carboxamide (MTIC). The conversion occurs rapidly in mice, but minimally in humans,8 which probably contributed to its limited clinical efficacy. Because TMZ does not rely on an enzymatic reaction and is converted to MTIC at physiologic pH, it was an attractive alternative to DTIC. TMZ also had the advantage of uniform tissue distribution (including brain) in mouse models, so the compound was advanced to human trials.9 Although it was initially intended for use in melanoma patients, TMZ has become the drug of choice for treatment of GB. This paper will briefly review what is known about the causes of GB, how TMZ is used to treat GB, and how molecular diagnostics impact how TMZ is used to treat GB patients.
Epidemiology and etiology of GB
The incidence of GB is 3.19/100,000/year in the US.3 Highly developed, industrialized countries appear to have an overall higher incidence than developing nations, but this may be a result of better access to health care and diagnostics. There is a slight gender predilection for men over women (3.99 versus 2.53 per 100,000 person-years),3 though the underlying reason remains unclear. GB is generally a disease of older patients: average age at diagnosis in the US is 64 years.10 Although poor outcome continues to be the rule in GB, age has historically been the most significant prognostic factor. For each additional decade of life at the time of diagnosis there is a statistically significant decrease in survival.11
Multiple epidemiologic studies have failed to identify major risk factors for developing GB. Small, occupation-specific studies have identified some environmental and lifestyle factors such as exposure to industrial chemicals, eg, formaldehyde, that may be linked to a higher incidence of central nervous system cancers. However, none have been clearly borne out in larger population-based studies.12 Personal habits such as diet, alcohol use, caffeine intake, and nicotine use have been studied, but no clear association with GB has been identified. Multiple studies have also failed to correlate cell phone usage with tumor incidence.13,14 In the past 5 years, the role of cytomegalovirus genes in malignant gliomas has been explored. Although infection with cytomegalovirus is not specifically linked to tumor formation, it has been proposed that cytomegalovirus infection may drive the oncogenic process by modulating growth factor and receptor expression in tumors.15 Nevertheless, like many other purported causes, the GB–cytomegalovirus link remains highly speculative. To date, the only exposure that confers an undeniable increased risk of GB is prior high-dose ionizing radiation. Children treated with radiation therapy for acute lymphoblastic leukemia have a significantly increased risk of gliomas, with up to a 22% excess of central nervous system neoplasms in patients followed for more than 15 years after initial treatment.16
In addition to environmental exposures, several genetic syndromes, eg, Li–Fraumeni and the neurofibromatoses, are associated with a higher risk of low-grade gliomas and GB. Patients with Li–Fraumeni syndrome carry mutations in TP53, the gene that encodes cell cycle modulatory protein p53. Somatic TP53 mutations can be found in the tumors of patients who initially develop low-grade gliomas that eventually transform into GB (known as secondary GB) over the course of years, presumably as the cells accumulate more progrowth mutations. Secondary GBs make up around 5% of all GBs; therefore, TP53 mutations are neither necessary nor sufficient for GB formation. Primary GBs, or de novo GBs, are frequently but not always associated with mutations in the cell cycle regulatory gene, PTEN.11,17 Numerous additional mutations, eg, epidermal growth factor receptor variant III, have been found with high frequency in GB, but no single, causative genetic mutation has been identified.
Cancer epigenetics, which explores genome-wide expression, silencing, and gene–gene interplay, may ultimately provide more information about the etiology of cancers. One of the first and most important epigenetic markers identified was methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter. The MGMT gene encodes a DNA repair enzyme that may reverse the damage done by alkylating agents, and is associated with better response to the TMZ–radiation combination.18 Genome-wide methylation patterns (as opposed to gene-specific methylation) have been associated with cancer phenotypes in a number of tumors, such as colon, breast, and lung. The GB genome is largely hypomethylated with specific areas of hypermethylation, leading to increased genetic instability, silencing of tumor suppressors (eg, p53 and PTEN), and activation of oncogenes. In 2010, Noushmehr et al identified a glioma –cytosine–phosphate–guanine– island methylator phenotype after correlating overall methylation patterns with patient characteristics. Patients with such tumors were younger, had a high frequency of IDH1 mutations, and had longer survival.19 This technique, and others used for identifying genome-wide changes, are becoming more affordable, and may soon be used routinely.
Although being able to identify both specific epigenetic and genetic changes in GB has improved the ability to discuss outcomes or predict response to therapy, it has not had a significant impact on treatment for patients. To date, no chemotherapeutic agent has been more successful or efficacious against GB than TMZ.
Pharmacology of TMZ
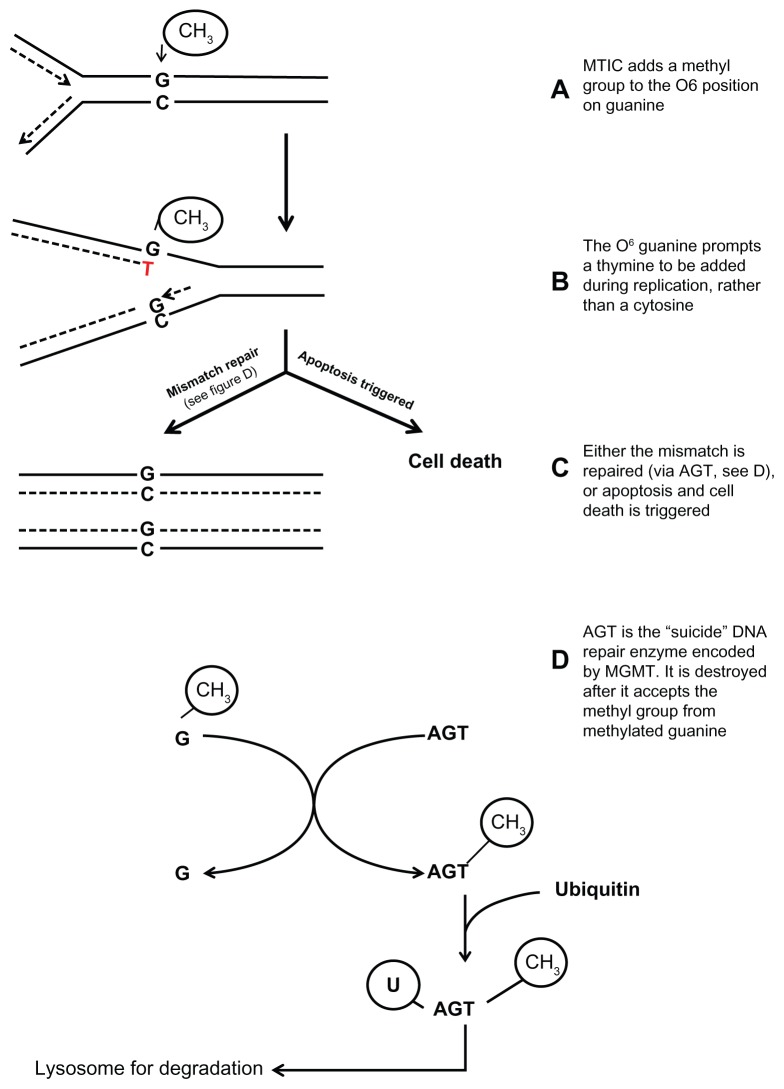
TMZ is a small (194 Da), lipophilic prodrug that is rapidly metabolized at physiologic pH to its active metabolite, MTIC. MTIC prevents cell division by disrupting normal DNA replication. Addition of a methyl group to the O6 position of guanine in genomic DNA results in the incorporation of a thymine residue opposite O6-methylguanine instead of the normal cytosine residue. The resulting abnormal guanine–thymine pair leads to a pause in the DNA replication fork and triggers the DNA mismatch repair pathway cell cycle arrest. However, if this repair mechanism is incapable of keeping up with the rate and extent of DNA damage, apoptosis results (Figure 1).20,21
Figure 1.
MTIC, the active metabolite of TMZ, adds a methyl group to guanine which causes a base pair mismatch.
Notes: This leads either to repair and continued DNA replication, or programed cell death. AGT, the enzyme that repairs damage caused by TMZ, is a “suicide” enzyme (it can be used only once per mismatch repair) and therefore can be overwhelmed.
Abbreviations: AGT, O6-alkylguanine-DNA alkyltransferase; MGMT, O6-methylguanine-DNA methyltransferase; MTIC, 5-(3-methyltriazen-1yl)-imidazole-4-carboxamide; TMZ, temozolomide.
TMZ is available in an oral and intravenous formulation, with nearly equivalent bioavailability (276 ng/mL intravenous versus 282 ng/mL oral for a 150 mg/m2 dose). Therefore, the intravenous formulation is rarely used in clinical practice. Bioavailability of the oral formulation is nearly 100%, with rapid and complete absorption (<1% fecal elimination), and peak serum concentrations reached within 1–2 hours.22 The kinetics appears the same in patients who have had a gastric bypass.23 Once absorbed, TMZ is only 15% protein bound, limiting its interactions with other common medications used in brain tumor patients, eg, phenytoin, warfarin, and birth control pills.24 As a small, lipophilic molecule, TMZ is known to enter the cerebrospinal fluid with an area under the curve cerebrospinal fluid/plasma ratio of 20%.25 Cerebrospinal fluid penetrance of a drug does not always correlate with its activity in brain parenchyma, but TMZ’s efficacy in the clinical setting provides additional evidence that it is accumulating within central nervous system tumors.
Once in plasma, TMZ is converted to MTIC, its active metabolite, by a nonenzymatic chemical degradation process, entirely bypassing the liver, and thereby avoiding interactions with other medications. MTIC is then irreversibly degraded to 4-amino-5-imidazole-carboxamide and a methyldiazonium cation. The methyldiazonium cation transfers its methyl group to DNA, leaving a dinitrogen to be eliminated by the lungs.9 4-amino-5-imidazole-carboxamide, a natural intermediate in purine synthesis found in normal serum, is excreted by the kidney.26 Because the kidney does not directly excrete more than 5%–6% of TMZ, renal dosing is not routinely used. There is no data available that specifically addresses safety in patients receiving renal dialysis.
Clinical safety, efficacy, and tolerability of TMZ
TMZ was first tested in humans in 1992 after animal studies demonstrated even tissue distribution, predictable kinetics, and tolerability. The first Phase I trial included 51 patients, nearly all of who had melanoma.27 The trial confirmed the linear pharmacokinetics seen in animal studies and proposed the 150 mg/m2 5-day on/23-day off dosing schedule that was used in later glioma trials. Myelotoxicity was the dose-limiting side effect, but compared to mitozolomide, an earlier drug of the same class, it was less severe and less frequent. Mild to moderate nausea and vomiting was also dose related, but easy to control with antiemetic medications. This study included only two patients with recurrent high-grade glioma, but both had partial responses by computed tomography scan along with “dramatic clinical improvements.”27 These results quickly generated excitement in the neurooncology community, as did the small follow-up study run by the same authors, in which nine of 17 patients with astrocytomas had improvement by computed tomography scan.28
The Cancer Research Campaign, which funded the Phase I TMZ study above (as well as some preclinical work), went on to support two multicenter Phase II trials using TMZ in recurrent glioma patients. Both studies confirmed TMZ’s tolerability profile.29,30 Adverse events were generally not severe, were reversible, and related to those identified in the Phase I trials: thrombocytopenia, leukopenia, fatigue, nausea, and vomiting. There were no deaths attributed to TMZ. Of the 103 patients enrolled in the European study, 47% had disease stability and 11% had an objective radiographic response.29 The US study enrolled 225 patients and compared TMZ with procarbazine, another oral agent that was being used frequently in patients with recurrent GB multiforme.30 Progression free survival was 12.4 weeks in the TMZ group compared with 8.32 weeks in the procarbazine group, with a 6-month overall survival rate for TMZ patients of 60% compared with 44% in the procarbazine group. This study also concluded that freedom from brain tumor progression was associated with better health-related quality of life scores. While an improvement of more than 4 weeks in median progression free survival seems lackluster, these results, in combination with supportive evidence from smaller Phase II trials, were enough to make TMZ the first new drug in 20 years to gain approval for use in glioma patients by both the Food and Drug Administration and European Medicines Evaluation Agency.
Most Phase II trials in the late 1990s used a 5-day on/23-day off dose schedule. Brock et al designed a Phase II study to investigate a prolonged, daily dosing schedule based on the results of their Phase I study. A subset of the patients in the initial Phase I study that progressed through the 5-day on/23-day off dose schedule were transitioned to a daily dose schedule, and had disease stabilization. The primary goal of the study was to establish a dose that could be given concurrently with radiation therapy. At 75 mg/m2/day, patients were able to tolerate up to 7 weeks of therapy without grade III or IV hematologic toxicity, thereby allowing the potential combination with radiation therapy for gliomas.31
In 2002, Stupp et al published the results of their pilot Phase II protocol designed to test the safety and tolerability of concomitant radiation plus TMZ therapy at 75 mg/m2/day followed by adjuvant TMZ, dosed 150–200 mg/m2 5 days on/23 days off. During the concomitant phase, there was a modest increase in myelosuppression (6%), but the combination was otherwise well tolerated. The median survival was 16 months, with 58% and 31% survival at 1 year and 2 years, respectively.32 This study was quickly followed by a European Organization for Research and Treatment of Cancer Phase III trial (EORTC 26981), which compared surgery followed by radiotherapy plus concomitant TMZ to surgery followed by radiotherapy alone in GB patients. There was a statistically significant increase in median survival of approximately 10 weeks (12.1 versus 14.6 months).33 In the neurooncology community, there was also excitement about the apparent increase in 2-year survivors in patients receiving concomitant chemoradiation when compared to those receiving radiation alone (26.5% versus 10.4%). Interestingly, in 1998, a Radiation Therapy Oncology Group (RTOG) trial comparing radiation alone to radiation with BCNU or CCNU demonstrated a 23% 2-year survival rate in 40–60 year olds receiving combined therapy.34 However, a significant number of these patients (about 20% in both the CCNU and BCNU groups) had dose-limiting leukopenia, which limited the number of patients who could safely be offered the regimen. Patients receiving BCNU or CCNU also had significant fatigue and nausea. Conversely, adding TMZ to radiotherapy caused no decrease in health-related quality of life,35 which may explain why there was no move to test the Stupp protocol directly against an analogous regimen containing CCNU or BCNU.
Radiation with concomitant TMZ quickly became accepted as the standard of care for newly diagnosed GB patients in Europe and the US. No new cytotoxic agent has been more effective. Since its initial approval, there have been an enormous number of nonrandomized trials testing different dose regimens, including prolonged administration, 7 days on/7 days off, and a “dose-dense” 21-day on/7-day off regimen.36 Some alternate regimens have shown promise, but only one has been tested against the standard regimen in an RTOG Phase III trial. RTOG 0525 compared a dose-dense adjuvant regimen to the standard 5-day regimen and demonstrated no benefit to the alternate dose strategy.37 It is suspected that this will hold true for most alternate dosing regimens.
In general, TMZ is a well-tolerated drug. Fatigue was the most commonly reported adverse event in both the Phase II recurrent anaplastic glioma and Phase III GB trials, with more than 50% of patients reporting this side effect.38 In the Phase III trial, it was severe (grade III–IV) in 13% of patients. Nausea and vomiting were frequent in both trials, but usually easily controlled. However, 16% of patients in the Phase II trial experienced severe and more difficult to control nausea and vomiting. Constipation was also quite common (roughly 20%), but rarely severe. The dose-limiting side effect for TMZ, as for most chemotherapy, is myelosuppression, which usually occurs early, after the first few cycles. In the Phase III trial, grade III–IV thrombocytopenia occurred in 12% of patients and grade III–IV neutropenia and leukopenia occurred in 7% of patients.33 However, it is important to remember that even patients with grade I thrombocytopenia (<lower limit of normal to 75,000 mm3) may experience dose delays in order to recover to a minimum platelet count of 100,000 prior to the next cycle. Rare but life-threatening side effects have been reported since TMZ became more widely used, including anaphylaxis, Stevens–Johnson’s reaction, and aplastic anemia.38–40
Though the Stupp regimen is more effective and tolerable than prior therapy options, it is not ideal for many GB patients. This is particularly true for patients over 65 years, who have significantly shorter survival than their younger counterparts, even when treated with radiation and chemotherapy.41 Elderly patients are more vulnerable to chemotherapy side effects, such as myelosuppression, mucositis, and neurotoxicity after radiation.42–44 Elderly patients are also less likely to participate in clinical trials, which makes translating clinical trial results to the general GB population more challenging. The average patient in the Stupp et al trial was 56 years old, while the average GB patient in the US is 64 years old.10 Therefore, clinicians need to consider alternate treatment plans for elderly patients with comorbidities or low performance status. Two recent randomized studies demonstrated that TMZ alone is a reasonable, noninferior option for patients older than 65 years who may not tolerate the combined regimen.45,46 Patients with MGMT promoter methylation had a significant survival benefit when treated on the TMZ arms, but no survival benefit when receiving radiotherapy alone. This suggests MGMT-positive patients who cannot tolerate the combined regimen should receive TMZ rather than radiation.45,46
Given that the goal of GB therapy is primarily survival prolongation rather than cure, close attention is paid to the impact of therapy on quality of life. As a result, the authors have modified their prescribing practices in order to maximize treatment and minimize side effects in patients. For example, the use of oral ondansetron 1 hour prior to every dose of TMZ is advised as prophylaxis against nausea. Constipation is nearly ubiquitous, so all patients are advised to begin a stool softener, eg, docusate, the same evening they begin TMZ. Concurrent use of ondansetron may aggravate the constipation, and in these cases, prochlorperazine or metoclopramide is used instead. Although liver failure has been seen in association with the conventional dosing strategy, it remains a rare complication. All patients are routinely tested for hepatitis B and C prior to therapy with TMZ because disease reactivation has been seen in patients, which can lead to liver failure. For patients who have had hepatitis B, but are not immune, Epivir® is prescribed to minimize the risk of reactivation. Pneumocystis pneumonia (PCP) prophylaxis during the concomitant TMZ and radiotherapy period is recommended on the package insert. However, retrospective studies exploring the incidence of PCP in GB patients during TMZ therapy found PCP occurred in patients using prolonged, high-dose corticosteroids and those who developed significant lymphopenia (<500 cells/μL).47,48 Sulfamethoxazole/trimethoprim, the most commonly used drug for PCP prophylaxis, is generally well tolerated but may cause gastrointestinal upset, nausea, and headache in 6%–8% of patients.49 More serious events can occur in 3%–5% of patients receiving the drug, including bone marrow suppression and life-threatening rashes.50 Therefore, for most GB patients, the additional risks of PCP prophylaxis may outweigh the benefits. PCP prophylaxis should be reserved for high-risk patients, ie, those receiving prolonged courses of high-dose steroids or who have lymphocyte counts <500 cells/μL.
Testing of MGMT methylation status
One of the strongest predictive biomarkers for response to alkylating agents is MGMT methylation status. The MGMT gene encodes O6-alkylguanine-DNA alkyltransferase (AGT), the DNA repair enzyme that reverses damage inflicted by TMZ. AGT is a “suicide enzyme” – one molecule of AGT removes only one alkyl molecule, inactivating itself in a stoichiometric fashion.51 In principle, hypermethylation of – cytosine–phosphate–guanine – islands in the MGMT promoter region leads to lower MGMT expression,20 and should enhance the response to alkylating agents by inhibiting DNA repair. Indeed, MGMT promoter methylation is associated with a significantly higher median survival after therapy with TMZ (21.7 months versus 15.3 months in the 2005 Stupp et al trial).18,51
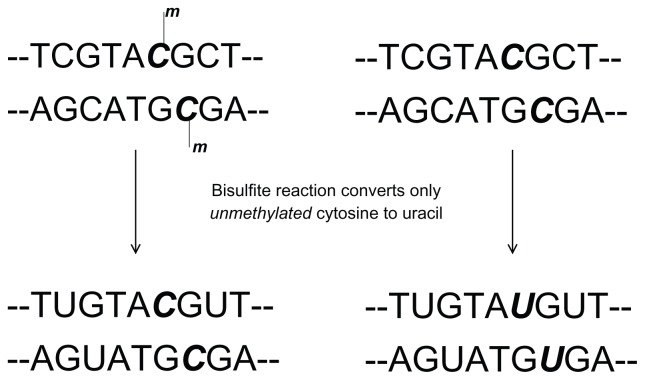
There are multiple methods being used to test for MGMT promoter methylation status, each with potential benefits and pitfalls. There is no consensus regarding which method is most accurate or most feasible across varying clinical settings (academic hospital versus small community practice). There is general agreement, however, that immunohistochemical staining for MGMT protein expression does not correlate with polymerase chain reaction (PCR)-based methods of detection or overall survival.52,53 Early reports using enzyme activity assays appear to correlate level of activity with level of methylation detected by PCR, but they have not been widely adopted at this time.54,55 Nested, gel-based PCR, real-time PCR (also called quantitative PCR), and DNA pyrosequencing are the most commonly cited techniques. All three sequencing tests utilize the bisulfite reaction as the initial step, where unmethylated cytosine bases are deaminated to uracil, while methylated cytosine bases remain unchanged (Figure 2). Gel-based PCR techniques yield bands of differing intensity on an agarose gel. Results are determined by the human eye: a qualitative, rather than quantitative, method. Gel-based PCR was used and validated by Hegi et al, in conjunction with the 2005 Phase III trial that established TMZ in combination with radiation as the standard of care.18
Figure 2.
The bisulfite reaction is the first step in detection of MGMT methylation status for both gel-based and real-time polymerase chain reaction.
Abbreviation: MGMT, O6-methylguanine-DNA methyltransferase.
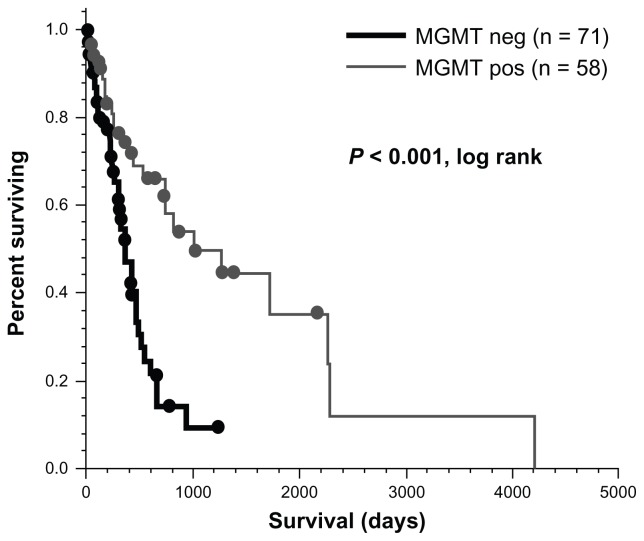
The authors’ institution uses the gel-based technique outlined above. All GB cases from 2006–2011 consecutively were reviewed, regardless of their treatment course (patients who went directly to hospice were also included), under an Institutional Review Board-approved protocol. Patients with both survival and MGMT data available were identified (n = 129). Forty-five percent were positive for promoter methylation. Figure 3 illustrates that MGMT-positive patients in this population had significantly longer overall survival when compared to MGMT- negative patients (32.8 months versus 12.4 months; P < 0.001, log-rank). These results mirror what Hegi et al and others have reported.56,57 Compared to other biomarkers (such as epidermal growth factor receptor overexpression), MGMT promoter methylation status has such a pronounced correlation with survival that it has now become routine at the authors’ institution.
Figure 3.
MGMT status has a significant impact on overall survival in glioblastoma patients.
Abbreviation: MGMT, O6-methylguanine-DNA methyltransferase.
While it is clear that MGMT methylation status correlates with longer survival, the frequency of methylation status reported in the literature varies, probably because each laboratory uses a slightly different technique. Primer choice and band intensity guidelines can vary from laboratory to laboratory, and both can affect the results of gel-based PCR. Not surprisingly, there is now a movement towards quantitative methods of measuring MGMT methylation. Real-time PCR detects the number of copies of double-stranded DNA being made from a target sequence in relation to a standard dilution. This technique requires that a numeric cutoff value for MGMT methylation status be established, by choosing a point on the bimodal result curve that delineates a positive or negative test.58 This method was used in the Phase III RTOG 0525 trial comparing standard dose adjuvant TMZ with a dose-dense schedule. Though the trial was negative, it confirmed the predictive significance of MGMT and demonstrated the utility of a single commercial laboratory (PredictMDx™; MDxHealth, Inc, Liege, Belgium) for MGMT testing in clinical trials.37
Pyrosequencing relies on detecting specific wavelengths of light emitted as each DNA nucleotide is added during PCR. This method is quantitative and also correlates with outcomes.59 Interestingly, Havik et al compared MGMT detection by pyrosequencing and quantitative PCR in a cohort of glioma patients and found that those with discordant results (positive by one, negative by another) had intermediate-length survival, inferring that methylation status is not best described in a bimodal fashion.59 When comparing all three techniques, pyrosequencing may be the most efficient and accurate in high throughput settings, although accessibility may limit its widespread use.60
At the time of this paper’s writing – because no better alternative first-line therapy to concurrent radiation and TMZ is available to patients with MGMT-negative GB multiforme – MGMT remains largely a predictive factor, rather than a test on which therapy decisions are based. In certain situations, however, MGMT status can alter therapy. When evaluating magnetic resonance imaging changes in patients who have completed chemoradiation for example, patients with MGMT-positive tumors are more likely to demonstrate pseudoprogression (treatment injury that appears like tumor progression) than patients who are MGMT-negative.61 Therefore, MGMT status, along with positron emission tomography and magnetic resonance perfusion data, plays a role in decision making. In patients with MGMT-positive tumors, changes in the immediate field of radiation and reassuring perfusion imaging are generally treated as pseudoprogression. Alternatively, patients with MGMT-negative tumors with similar appearance in magnetic resonance imaging may be moved more quickly to a second-line therapy. MGMT status also influences clinical decision making in elderly MGMT-positive patients who are less likely to tolerate 6 weeks of concurrent chemoradiation. These patients may benefit from TMZ alone, while MGMT-negative patients may benefit from a 3-week course of radiation therapy rather than standard chemoradiation.45,46
Future directions
Although there is good evidence that GB patients respond to repeated challenges with TMZ,62 cure occurs rarely, if ever. Not surprisingly, several lines of investigation have sought to enhance TMZ’s impact in GB multiforme, including alternate delivery methods and amplifying responses through modulating MGMT.
One strategy to improve the efficacy of TMZ is to increase the effective dose of TMZ the tumor received via alternate delivery mechanisms. Local delivery via wafer or convection-enhanced delivery could theoretically circumvent an intact blood–brain barrier. A polymer wafer (analogous to Gliadel®) that combines TMZ with BCNU has been tested in rat models and may be more effective than either agent alone.63 Slow-release microspheres containing TMZ have also been used in animals, but not yet in humans.64 Both of these options would be available to patients with surgical disease and near total resections. PEGylation and targeted nanovesicles are two potential systemic approaches that could increase TMZ delivery to tumor cells while minimizing effects on normal cells. PEGylation is the process of attaching polyethylene glycol to another molecule, which can shield it from degradation or recognition by the immune system, reduce its clearance, and increase the length of time the drug is available in the body. Some investigators are using PEGylation in combination with nanoparticle engineering, to create nanoparticles that avoid the immune system, but can also target tumor cells for drug delivery via a combination of monoclonal antibodies. This strategy has yielded promising results in vitro and in basic animal models, but currently no clinical evidence exists to support this approach.65–67
Preventing tumors from developing alkylator resistance is another alternate mechanism of enhancing TMZ’s efficacy. O6-benzylguanine is a purine analog thought to potentiate the action of alkylating agents, including TMZ, by blocking the activity of DNA repair enzyme AGT (the product of the MGMT gene). Although it appears safe, it is not clear that this strategy is effective.68,69 Data from the Phase I trial suggests O6-benzylguanine’s activity is transient, so future directions may include TMZ in combination with a sustained release O6-benzylguanine.
More recently, some investigators have proposed a strategy that is “opposite” to the O6-benzylguanine strategy: to intensify treatment by rendering normal, vulnerable tissue more resistant to TMZ’s effects. In a pilot study, patients received infused hematopoietic stem and progenitor cells with a mutant MGMT gene that confers resistance to O6-benzylguanine. This resulted in highly resistant hematopoietic cells that allowed dose intensification, producing promising preliminary results in three patients.70
Conclusion
In summary, TMZ is a well-tolerated oral chemotherapy that has become the standard of care for GB patients following initial surgery. Alone or in combination with radiation, it increases survival and health-related quality of life. MGMT status can predict response to TMZ, and therefore can help guide treatment decisions, particularly in patients who appear to have early recurrence on magnetic resonance imaging, and in elderly patients who are unlikely to tolerate chemoradiation. Alternate dosing schedules using “more” TMZ do not appear to be beneficial. The addition of drugs that potentiate TMZ’s effect or overcome DNA repair mechanisms may improve outcomes in the future.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Howlader NA, Noone AM, Krapcho M, et al. National Cancer Institute. SEER cancer statistics review, 1975–2009 (vintage 2009 populations) 2011. [Accessed November 19, 2012]. [Updated August 20, 2012]. Available from: http://seer.cancer.gov/csr/1975_2009_pops09/index.html.
- 2.Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer. 1985;56(5):1106–1111. doi: 10.1002/1097-0142(19850901)56:5<1106::aid-cncr2820560525>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Central Brain Tumor Registry of the United States. CBTRUS Statistical Report: Primary Brain and Central Nervous System Diagnosed in the United States in 2004–2007. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2011. [Accessed November 19, 2012]. Available from: http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf. [Google Scholar]
- 4.Fewer D, Wilson CB, Boldrey EB, Enot KJ, Powell MR. The chemotherapy of brain tumors. Clinical experience with carmustine (BCNU) and vincristine. JAMA. 1972;222(5):549–552. [PubMed] [Google Scholar]
- 5.Wilson CB, Gutin P, Boldrey EB, Drafts D, Levin VA, Enot KJ. Single-agent chemotherapy of brain tumors. A five-year review. Arch Neurol. 1976;33(11):739–744. doi: 10.1001/archneur.1976.00500110007002. [DOI] [PubMed] [Google Scholar]
- 6.Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52(6):997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Solero CL, Monfardini S, Brambilla C, et al. Controlled study with BCNU vs CCNU as adjuvant chemotherapy following surgery plus radiotherapy for glioblastoma multiforme. Cancer Clin Trials. 1979;2(1):43–48. [PubMed] [Google Scholar]
- 8.Stevens MF, Newlands ES. From triazines and triazenes to temozolomide. Eur J Cancer. 1993;29A(7):1045–1047. doi: 10.1016/s0959-8049(05)80221-7. [DOI] [PubMed] [Google Scholar]
- 9.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23(1):35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 11.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 12.National Toxicology Program. Final report on carcinogens background document for formaldehyde. Rep Carcinog Backgr Doc. 2010;10-5981:i-512. [PubMed] [Google Scholar]
- 13.Deltour I, Auvinen A, Feychting M, et al. Mobile phone use and incidence of glioma in the Nordic countries1979–2008: consistency check. Epidemiology. 2012;23(2):301–307. doi: 10.1097/EDE.0b013e3182448295. [DOI] [PubMed] [Google Scholar]
- 14.Little MP, Rajaraman P, Curtis RE, et al. Mobile phone use and glioma risk: comparison of epidemiological study results with incidence trends in the United States. BMJ. 2012;344:e1147. doi: 10.1136/bmj.e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson JH, Mitchell DA. Is cytomegalovirus a therapeutic target in glioblastoma? Clin Cancer Res. 2011;17(14):4619–4621. doi: 10.1158/1078-0432.CCR-11-0992. [DOI] [PubMed] [Google Scholar]
- 16.Neglia JP, Meadows AT, Robison LL, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;325(19):1330–1336. doi: 10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, Sato K, Biernat W, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res. 1997;3(4):523–530. [PubMed] [Google Scholar]
- 18.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 19.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tentori L, Orlando L, Lacal PM, et al. Inhibition of O6-alkylguanine DNA-alkyltransferase or poly(ADP-ribose) polymerase increases susceptibility of leukemic cells to apoptosis induced by temozolomide. Mol Pharmacol. 1997;52(2):249–258. doi: 10.1124/mol.52.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26(2):186–197. doi: 10.1038/sj.onc.1209785. [DOI] [PubMed] [Google Scholar]
- 22.Hammond LA, Eckardt JR, Baker SD, et al. Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol. 1999;17(8):2604–2613. doi: 10.1200/JCO.1999.17.8.2604. [DOI] [PubMed] [Google Scholar]
- 23.Park DM, Shah DD, Egorin MJ, Beumer JH. Disposition of temozolomide in a patient with glioblastoma multiforme after gastric bypass surgery. J Neurooncol. 2009;93(2):279–283. doi: 10.1007/s11060-008-9773-4. [DOI] [PubMed] [Google Scholar]
- 24.Gander M, Leyvraz S, Decosterd L, et al. Sequential administration of temozolomide and fotemustine: depletion of O6-alkyl guanine-DNA transferase in blood lymphocytes and in tumours. Ann Oncol. 1999;10(7):831–838. doi: 10.1023/a:1008304032421. [DOI] [PubMed] [Google Scholar]
- 25.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 26.Baker SD, Wirth M, Statkevich P, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5(2):309–317. [PubMed] [Google Scholar]
- 27.Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Br J Cancer. 1992;65(2):287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Reilly SM, Newlands ES, Glaser MG, et al. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29A(7):940–942. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- 29.Bower M, Newlands ES, Bleehen NM, et al. Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol. 1997;40(6):484–488. doi: 10.1007/s002800050691. [DOI] [PubMed] [Google Scholar]
- 30.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brock CS, Newlands ES, Wedge SR, et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58(19):4363–4367. [PubMed] [Google Scholar]
- 32.Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20(5):1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 34.Nelson DF, Diener-West M, Horton J, Chang CH, Schoenfeld D, Nelson JS. Combined modality approach to treatment of malignant gliomas – re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988;(6):279–284. [PubMed] [Google Scholar]
- 35.Taphoorn MJ, Stupp R, Coens C, et al. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(12):937–944. doi: 10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- 36.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11(1):69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert MR, Wang M, Aldape KD, et al. RTOG 0525: a randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (dd) schedule in newly diagnosed glioblastoma (GBM) [abstract] J Clin Oncol. 2011;29:2006. [Google Scholar]
- 38.Temodar® [prescribing information] Whitehouse Station, NJ: Merck and Co, Inc; 2012. [Google Scholar]
- 39.Oh J, Kutas GJ, Davey P, Morrison M, Perry JR. Aplastic anemia with concurrent temozolomide treatment in a patient with glioblastoma multiforme. Curr Oncol. 2010;17(4):124–126. doi: 10.3747/co.v17i4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarma N. Stevens–Johnson syndrome and toxic epidermal necrolysis overlap due to oral temozolomide and cranial radiotherapy. Am J Clin Dermatol. 2009;10(4):264–267. doi: 10.2165/00128071-200910040-00007. [DOI] [PubMed] [Google Scholar]
- 41.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 42.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol. 2003;1(4 Suppl 2):18–24. [PubMed] [Google Scholar]
- 43.Hottinger AF, DeAngelis LM, Yahalom J, Abrey LE. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69(11):1178–1182. doi: 10.1212/01.wnl.0000276986.19602.c1. [DOI] [PubMed] [Google Scholar]
- 44.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24(28):4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 45.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 46.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 47.De Vos FY, Gijtenbeek JM, Bleeker-Rovers CP, van Herpen CM. Pneumocystis jirovecii pneumonia prophylaxis during temozolomide treatment for high-grade gliomas. Crit Rev Oncol Hematol. 2012 Aug 25; doi: 10.1016/j.critrevonc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi H, Saito Y, Kokuho N, et al. Fatal pneumonia associated with temozolomide therapy in patients with malignant glioma. Jpn J Clin Oncol. 2012;42(7):632–636. doi: 10.1093/jjco/hys058. [DOI] [PubMed] [Google Scholar]
- 49.Jick H. Adverse reactions to trimethoprim-sulfamethoxazole in hospitalized patients. Rev Infect Dis. 1982;4(2):426–428. doi: 10.1093/clinids/4.2.426. [DOI] [PubMed] [Google Scholar]
- 50.Smilack JD. Trimethoprim-sulfamethoxazole. Mayo Clin Proc. 1999;74(7):730–734. doi: 10.4065/74.7.730. [DOI] [PubMed] [Google Scholar]
- 51.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 52.Uno M, Oba-Shinjo SM, Camargo AA, et al. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clinics (Sao Paulo) 2011;66(10):1747–1755. doi: 10.1590/S1807-59322011001000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez FJ, Thibodeau SN, Jenkins RB, et al. MGMT immunohistochemical expression and promoter methylation in human glioblastoma. Appl Immunohistochem Mol Morphol. 2008;16(1):59–65. doi: 10.1097/PAI.0b013e31802fac2f. [DOI] [PubMed] [Google Scholar]
- 54.Kishida Y, Natsume A, Toda H, et al. Correlation between quantified promoter methylation and enzymatic activity of O6-methylguanine-DNA methyltransferase in glioblastomas. Tumour Biol. 2012;33(2):373–381. doi: 10.1007/s13277-012-0319-1. [DOI] [PubMed] [Google Scholar]
- 55.Georgiadis P, Polychronaki N, Kyrtopoulos SA. Progress in high-throughput assays of MGMT and APE1 activities in cell extracts. Mutat Res. 2012;736(1–2):25–32. doi: 10.1016/j.mrfmmm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Kim YS, Kim SH, Cho J, et al. MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys. 2012;84(3):661–667. doi: 10.1016/j.ijrobp.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 57.Minniti G, Salvati M, Arcella A, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomide. J Neurooncol. 2011;102(2):311–316. doi: 10.1007/s11060-010-0324-4. [DOI] [PubMed] [Google Scholar]
- 58.Vlassenbroeck I, Califice S, Diserens AC, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008;10(4):332–337. doi: 10.2353/jmoldx.2008.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Havik AB, Brandal P, Honne H, et al. MGMT promoter methylation in gliomas-assessment by pyrosequencing and quantitative methylation-specific PCR. J Transl Med. 2012;10:36. doi: 10.1186/1479-5876-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christians A, Hartmann C, Benner A, et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS One. 2012;7(3):e33449. doi: 10.1371/journal.pone.0033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 62.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 63.Recinos VR, Tyler BM, Bekelis K, et al. Combination of intracranial temozolomide with intracranial carmustine improves survival when compared with either treatment alone in a rodent glioma model. Neurosurgery. 2010;66(3):530–537. doi: 10.1227/01.NEU.0000365263.14725.39. [DOI] [PubMed] [Google Scholar]
- 64.Dong J, Zhou G, Tang D, et al. Local delivery of slow-releasing temozolomide microspheres inhibits intracranial xenograft glioma growth. J Cancer Res Clin Oncol. 2012;138(12):2079–2084. doi: 10.1007/s00432-012-1290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patil R, Portilla-Arias J, Ding H, et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid) Pharm Res. 2010;27(11):2317–2329. doi: 10.1007/s11095-010-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujita M, Lee BS, Khazenzon NM, et al. Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(β-L-malic acid) J Control Release. 2007;122(3):356–363. doi: 10.1016/j.jconrel.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain A, Chasoo G, Singh SK, Saxena AK, Jain SK. Transferrin-appended PEGylated nanoparticles for temozolomide delivery to brain: in vitro characterisation. J Microencapsul. 2011;28(1):21–28. doi: 10.3109/02652048.2010.522257. [DOI] [PubMed] [Google Scholar]
- 68.Friedman HS, Kokkinakis DM, Pluda J, et al. Phase I trial of O6-benzylguanine for patients undergoing surgery for malignant glioma. J Clin Oncol. 1998;16(11):3570–3575. doi: 10.1200/JCO.1998.16.11.3570. [DOI] [PubMed] [Google Scholar]
- 69.Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide plus O6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27(8):1262–1267. doi: 10.1200/JCO.2008.18.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adair JE, Beard BC, Trobridge GD, et al. Extended survival of glioblastoma patients after chemoprotective HSC gene therapy. Sci Transl Med. 2012;4(133):133ra57. doi: 10.1126/scitranslmed.3003425. [DOI] [PMC free article] [PubMed] [Google Scholar]