Abstract
Purpose
Tonometry, or measurement of intraocular pressure (IOP), is one of the most important examination procedures in ophthalmic clinics, and IOP is an important parameter in the diagnosis of glaucoma. Because there are numerous types of tonometer available, it is important to evaluate the differences in readings between different tonometers. Goldmann applanation tonometers (GATs) and noncontact air-puff tonometers (APTs) are largely available in ophthalmic clinics. The purpose of this study was to evaluate the role of AP tonometer by comparing the measurements of IOP made using this device with those made using a GAT.
Patients and methods
This study involved 196 eyes from 98 study participants, all of whom were patients attending an ophthalmic outpatient clinic. Each patient’s IOP was measured using both Goldmann applanation tonometry and AP tonometry, and the difference in readings between the two methods was calculated.
Results
The mean IOP as measured by GAT was 13.06 ± 4.774 mmHg, while that as measured by AP tonometer was 15.91 ± 6.955 mmHg. The mean difference between the two methods of measurement was 2.72 ± 2.34 mmHg. The readings obtained by AP tonometer were higher than those obtained by GAT in 74% of patients, and this difference was most obvious when the GAT measurement of IOP exceeded 24 mmHg. No statistically significant variation in IOP was noted between the devices when the patients’ age, sex, and laterality (right and left eyes) were considered.
Conclusion
There is a significant difference in the measurement of IOP between GATs and AP tonometers. Goldmann applanation tonometry remains the most suitable and reliable method for measuring IOP. Because measurements of IOP by AP tonometer are usually higher than those obtained by GAT regardless of the patient’s age, sex, or laterality of eyes, AP tonometry is a suitable method for community or mass screenings of IOP.
Keywords: tonometry, comparison, glaucoma, noncontact tonometry, goldmann applanation tonometer
Introduction
Tonometry, or the measurement of intraocular pressure (IOP), the pressure of the fluid inside the eye, is one of the most important examination procedures in ophthalmic clinics, and IOP is an important parameter in the diagnosis of glaucoma. IOP varies among individuals,1 with normal IOP essentially maintained by the dynamic equilibrium between aqueous humor formation and outflow, and by episcleral venous pressure.2 Aqueous humor helps to maintain appropriate IOP.3 The circulating aqueous humor nourishes the cornea and lens (both structures that must be transparent and therefore devoid of blood vessels), as well as the trabecular meshwork.4 IOP helps to maintain the proper shape of the eyeball.5 Aqueous humor provides a transparent and colorless medium between the cornea and the lens and constitutes an important component of the eye’s optical system.6 The aqueous humor is secreted by the nonpigmented ciliary epithelium at a flow rate of 2–3 μL per minute.3 Anterior chamber volume in humans is estimated to be ~250–300 μL. Aqueous humor turnover is ~1% of anterior chamber volume (~2.5 μL per minute).7
Pooled data from large epidemiologic studies indicate that the mean IOP is approximately 16 mmHg; however, these pooled data have a non-Gaussian distribution with a skew toward higher pressures, especially in individuals over the age of 40. The value 22 mmHg has been used in the past to both separate normal and abnormal pressures and define which patients required ocular hypotensive therapy. This division was based largely on the erroneous assumptions that glaucomatous damage is caused exclusively by pressures that are higher than normal and that normal pressures do not cause damage.8
Screening for glaucoma based solely on an IOP > 21 mmHg may miss up to half of the people with glaucoma in the screened population. It is now generally agreed that, for the population as a whole, no clear line exists between safe and unsafe IOP: some eyes undergo damage at an IOP of 18 mmHg or less, whereas other eyes tolerate IOPs in the 30s. However, IOP is still seen as a very important risk factor for the development of glaucomatous damage. Although other risk factors affect an individual’s susceptibility to glaucomatous damage, IOP is the only risk factor that can be altered at this time.8
In normal individuals, IOP varies by 2–6 mmHg over the course of a 24-hour period as aqueous humor production changes. Higher IOP is associated with greater fluctuation and a diurnal fluctuation > 10 mmHg is suggestive of glaucoma. Many people reach their peak IOP in the morning hours, but others do so in the afternoon, in the evening, or during sleep; still others follow no reproducible pattern.8
IOP is an important risk factor for the development of glaucoma as well as for the progression of an already established glaucoma.9 Reduction of IOP is the best, and only evidence-based, treatment modality; pharmacologic as well as surgical interventions aimed at reducing IOP may successfully slow the progression of structural damage and visual field loss in patients with glaucoma.10 Therefore, IOP measurement by tonometry is essential in ophthalmological assessment. However, glaucoma may continue to progress despite IOP reduction to targeted levels; this indicates that factors other than IOP may play an important role in the pathogenesis of glaucoma.11
Applanation tonometry is based on the Imbert–Fick principle, which states that a perfect sphere has its internal pressure equally distributed and that the external force needed to flatten a known area of that sphere is directly proportional to the internal pressure of the sphere.12
The Goldmann applanation tonometer (GAT) is currently the most popular tonometer available. It consists of a double prism mounted on a standard slit lamp.2 The GAT represents the gold standard for IOP measurement and is used in all major randomized glaucoma clinical trials. With the GAT, the force required to flatten, or applanate, a constant area of the cornea is measured and related to the IOP using the Imbert–Fick principle. The GAT uses an applanation diameter of 3.06 mm and is performed with the patient seated at the slit lamp.13
Air-puff tonometry is an applanation method using a standardized puff of air to flatten the cornea. This method has the advantage that no topical anesthetic or risk of corneal abrasion is involved.14 The system consists of a central air plenum flanked either side by a light emitter and a light detector. As the pressure of the air pulse directed to the cornea increases to deform the cornea, the corneal surface behaves like a plane mirror, reflecting light to the detector.15 Corneal applanation is measured by collecting light reflected from the central cornea. A parallel beam of light is directed onto the central cornea at an angle of 30° and the reflected light is measured by a photo detector at an angle of reflection of 30°. The reflected beam of light will be strongest at this angle when the cornea is flat and acting as a plane mirror, rather than as a curved mirror. The instrument records the force of air required to flatten the cornea and displays the IOP that corresponds to that force. The AP tonometer must be used at a set distance from the cornea, and the instrument incorporates an optical alignment system to facilitate this.16
The puff of air can startle the patient, both with its apparent force and with its noise. An AP tonometer may be nonportable or portable,17 and because the AP tonometer is a noncontact tonometer, the risk of transmitting infectious agents from one eye to another via the tonometer tip is eliminated. However, the force of the air puff can aerosolize the tear film and may theoretically transmit viruses by an airborne route.13
The purpose of this study was to evaluate the difference, if any, between IOP measurements taken by a GAT and those taken by an AP tonometer, in view of what the author considers to be an increasing dependency in the medical community on the AP method of IOP measurement.
Material and methods
Subjects
This was a comparative study using a convenience sample. The study population comprised 196 eyes from 98 patients who were attending an ophthalmic outpatient clinic for various ophthalmic complaints and diseases. The patients (51 males, 47 females) had an age range of 15–84 years, and mean age was 55.32 ± 14.72 years. All subjects had a negative history of corneal diseases. Exclusion criteria were as follows: patients who are uncooperative in the measurement of IOP by either method, those with severe visual loss who are unable to maintain fixation for both methods, history of intraocular surgery, history of refractive surgery, a known case of glaucoma, or history of antiglaucoma medications. Prior to the assessment of IOP, to minimize the effect of astigmatism on the accurate determination of IOP, subjects with astigmatism of 3 diopters or more (as detected by autorefraction) were excluded from the study.18 The research follows the tenets of the Declaration of Helsinki, and each patient gave his or her informed consent to participate in the study.
Technique
IOP was measured in all patients using both a GAT and an AP tonometer, and the difference in readings between the two methods was calculated. The AP tonometer used was a Topcon CT-80 model (Topcon Corporation, Tokyo, Japan). For the assessment of IOP, three readings were averaged to get the IOP values for an eye; this procedure was adopted to suit the principle of IOP measurement used by the Topcon CT-80 noncontact tonometer. The IOP assessment with the GAT was always subsequent to that with the Topcon CT-80 noncontact tonometer; this was done to prevent bias due to a reduction of measured IOP caused by applanation. For the measurement by GAT, the eyes were anesthetized using Alcaine® (proparacaine hydrochloride ophthalmic solution) 0.5% eye drops (Alcon Laboratories, Inc, Fort Worth, TX) and a fluorescein strip was applied to the inferior conjunctival fornix for a few seconds. The period of contact with the applanation probe was kept under 5 seconds to minimize the IOP-reducing effect of aqueous massage on repeated applanation readings. All readings of IOP were taken between 8 am and 1 pm, and the same examiner took both measurements.
Classification of data
The data collected were classified into three groups according to the IOP measurements by GAT and AP tonometer: Group 1, IOP < 12 mmHg; Group 2, IOP 12–24 mmHg; and Group 3, IOP > 24 mmHg. The difference in readings between the two devices was calculated for each patient in each group. The data were also classified according to the patients’ age, sex, and laterality (right and left eyes).
Statistical analysis
The data were analyzed using statistical software (SPSS, v 17; IBM Corporation, Armonk, NY). The paired t-test was used and a P-value < 0.05 was considered statistically significant.
Results
The data obtained from the 196 eyes in this study gave the following results. The mean IOP value for all patients as measured by GAT was 13.06 ± 4.774 mmHg, with a range of 6–30 mmHg. The mean IOP value for all patients as measured by AP tonometer was 15.91 ± 6.955 mmHg, with a range of 7–30 mmHg. The mean difference of IOP values between GAT and AP tonometer measurements was 2.72 ± 2.345 mmHg, with a range of 6–10 mmHg. The difference in IOP values between the two devices was statistically significant (P = 0.001) (Table 1). In 74% of patients, the IOP measurement by AP tonometer was higher than that measured by GAT (Table 2).
Table 1.
Mean intraocular pressure (IOP) values for study participants, as measured by Goldmann applanation tonometer (GAT) and air-puff tonometer
Note:
Data presented as mean plus or minus standard deviation.
Table 2.
Intraocular pressure (IOP) values measured by air-puff tonometer as related to those measured by Goldmann applanation tonometer (GAT)
| IOP measurement by air-puff tonometer | Patients (%) | Right eyes | Left eyes |
|---|---|---|---|
| Below GAT measurement | 5% | 6% | 7% |
| Equal to GAT measurement | 21% | 19% | 20% |
| Above GAT measurement | 74% | 75% | 73% |
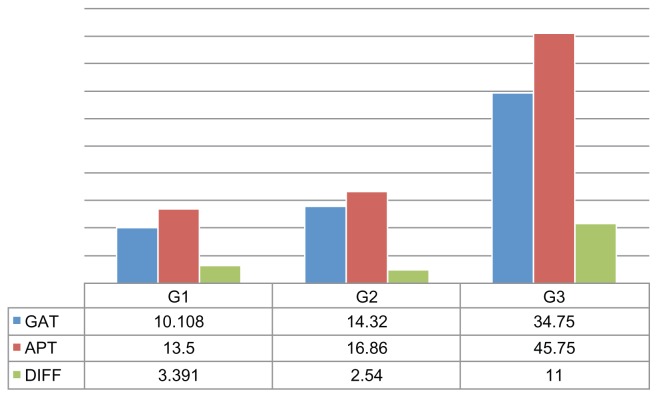
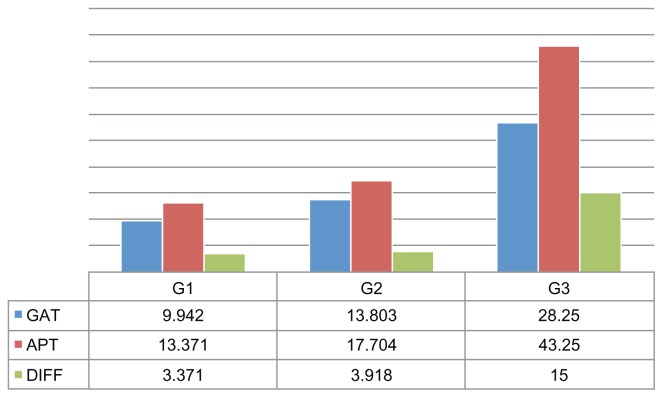
This study showed that IOP values measured with an AP tonometer were higher than those measured with a GAT (Figures 1 and 2), especially when the GAT measurement of IOP exceeded 24 mmHg, and that this difference was statistically significant (P < 0.0001).
Figure 1.
Right eyes in study: mean intraocular pressure (IOP) as measured by Goldmann applanation tonometer (GAT), mean IOP as measured by air-puff tonometer (APT), and the difference (DIFF) between the two readings according to three groups of IOP values (Group 1 [G1], <12 mmHg; Group 2 [G2], 12–24 mmHg; and Group 3 [G3], >24 mmHg).
Figure 2.
Left eyes in study: mean intraocular pressure (IOP) as measured by Goldmann applanation tonometer (GAT), mean IOP as measured by air-puff tonometer (APT), and the difference (DIFF) between the two readings according to three groups of IOP values (Group 1 [G1], <12 mmHg; Group 2 [G2], 12–24 mmHg; and Group 3 [G3], >24 mmHg).
Discussion
GATs and AP (ie, noncontact) tonometers are the most common devices for measuring IOP in daily practice. AP tonometers are easier to use and are more convenient, for both the patient and the examiner, than GATs. In view of this, the author considers there is an increasing dependency in the medical community, especially in outpatient clinics, on the AP method of IOP measurement, despite there being some doubt regarding the acceptance of all AP tonometer readings.
This study shows there is a significant difference in measurements of IOP between GATs and AP tonometers. The readings obtained by AP tonometer were higher than those obtained by GAT in 74% of the patients in this study. The difference in readings between the two instruments increased when the GAT measurement of IOP exceeded 24 mmHg.
Several other studies have compared IOP measurements obtained with GAT and those obtained by noncontact tonometers.19–23 Firat et al’s19 study concluded that noncontact tonometer measurements were higher than those obtained by GATs and that this difference was statistically significant. Martinez-de-la-Casa et al20 compared IOP measurements obtained with GATs and with noncontact tonometers and found that the mean GAT measurement was lower than the mean noncontact tonometer measurement. Tonnu et al21 showed that the mean difference in IOP between GAT measurements and AP tonometer measurements was 0.7 mmHg. The present study showed the mean difference was 2.72 ± 2.345 mmHg between the two devices. Rao22 showed that noncontact tonometer readings were more accurate when the IOP was <20 mmHg. Lagerlöf23 showed that measurements by a noncontact tonometer were found to be unreliable between 20 and 30 mmHg. Our present study shows that the lower the IOP as measured by GAT, the more reliable the corresponding readings made with AP tonometer.
There was no statistically significant difference found in IOP measurements between GATs and AP tonometers according to patient, sex, or laterality of the eyes. Some previous studies have shown that IOP is equal between the sexes,24,25 while some have found sex-specific differences (typically, higher IOP in females and the magnitude of the difference increasing after 40 years of age).26
The main advantages of noncontact tonometers are that they are easier than GATs to use (they can even operate automatically, as the readings are largely operator independent); they are noninvasive, so there is no requirement for topical anesthesia or fluorescein and there is minimum risk of infection; there is no risk of corneal abrasion, so the method is more comfortable than applanation tonometry for the patient, and repeated measurements do not reduce IOP (unlike the ocular massage effect that occurs with applanation tonometry); and IOP screening with a noncontact tonometer may be performed by an ophthalmic assistant without the direct supervision of an ophthalmologist. A disadvantage is that where readings by AP tonometer are abnormally high, the readings should be checked – the measurements should be repeated with another tonometric device before giving an opinion or a final diagnosis.
Conclusion
The GAT remains the most suitable, reliable device and is the international gold standard for measuring IOP. Measurements of IOP by AP tonometer are usually higher than those obtained by GAT, regardless of the patient’s age, sex, or laterality of eyes (particularly for higher IOP values), and therefore the AP tonometer is suitable for community or mass screenings of IOP. Considering this difference in IOP measurements between AP tonometers and GATs, the increasing use in the medical community, especially in outpatient clinics, of the seemingly more user-friendly AP tonometry devices is worrying. It should be kept in mind that AP tonometers can produce significantly higher results. It is necessary when supplying the primary health care centers with AP tonometers an emphasis is placed on training the users of APTs. It may be reasonable to find correcting values (nomogram) for AP tonometer readings.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Parker JN, Parker PM, editors. Ophthalmology: A Medical Dictionary, Bibliography and Annotated Research Guide to Internet References. San Diego, CA: ICON Health Publications; 2004. p. 210. [Google Scholar]
- 2.Khurana AJ, Khurana I. Anatomy and Physiology of the Eye. 2nd ed. New Delhi, India: CBS; 2006. pp. 71–80. [Google Scholar]
- 3.American Academy of Ophthalmology. 2007–2008 Basic and Clinical Science Course Section 2: Fundamentals and Principles of Ophthalmology. San Francisco, CA: American Academy of Ophthalmology; 2007. pp. 315–322. [Google Scholar]
- 4.Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye: Clinical Application. 10th ed. St Louis, MO: Mosby; 2009. p. 237. [Google Scholar]
- 5.Northrop RB. Noninvasive Instrumentation and Measurement in Medical Diagnosis. Boca Raton, FL: CRC Press; 2002. pp. 271–272. [Google Scholar]
- 6.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra AK, editor. Ophthalmic Drug Delivery Systems. 2nd ed. New York, NY: Marcel Dekker; 2003. pp. 224–225. [Google Scholar]
- 8.American Academy of Ophthalmology. Practicing Ophthalmologists Curriculum: Glaucoma, basic and clinical science course; 2007–2008. San Francisco, CA: American Academy of Ophthalmology; 2007. pp. 22–25. [Google Scholar]
- 9.Nucci C, Osborne NN, Bagetta G, Cerulli L, editors. Progress in Brain Research. Vol. 173. Philadelphia: Elsevier; 2008. Glaucoma: An Open Window to Neurodegeneration and Neuroprotection; p. 25. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Ishikawa S, Nakamura Y, Sakai H, Henzan I, Sawaguchi S. 24-hour intraocular pressure in glaucoma patients randomized to receive dorzolamide or brinzolamide in combination with latanoprost. Clin Ophthalmol. 2009;3:395–400. [PMC free article] [PubMed] [Google Scholar]
- 11.Topouzis F, Founti P. Weighing in ocular perfusion pressure in managing glaucoma. Open Ophthalmol J. 2009;3:43–45. doi: 10.2174/1874364100903010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin WJ, editor. Borish’s Clinical Refraction. 2nd ed. Butterworth-Heinemann; 2006. pp. 501–503. [Google Scholar]
- 13.Morrison JC, Pollack IP, editors. Glaucoma Science and Practice. New York, NY: Thieme Medical Publishers; 2003. pp. 60–64. [Google Scholar]
- 14.Crick RP, Khaw PT. A Textbook of Clinical Ophthalmology: A Practical Guide to Disorders of the Eyes and Their Management. 3rd ed. Singapore: World Scientific; 2003. p. 557. [Google Scholar]
- 15.Weinreb RN, Brandt JD, Garway-Heath DF, Medeiros FA, editors. World Glaucoma Association: Intraocular Pressure; Consensus Series 4. The Hague, The Netherlands: Kugler Publications; 2007. pp. 22–23. [Google Scholar]
- 16.Elkington AR, Frank HJ, Greaney MJ. Clinical Optics. 3rd ed. Oxford: Blackwell Science; 1999. pp. 205–207. [Google Scholar]
- 17.Kanski JJ, Bowling B. Clinical Ophthalmology: A Systematic Approach. 7th ed. Philadelphia: Elsevier; 2011. pp. 315–644. [Google Scholar]
- 18.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38(1):1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 19.Firat PG, Cankaya C, Doganay S, et al. The influence of soft contact lenses on the intraocular pressure measurement. Eye (Lond) 2012;26(2):278–282. doi: 10.1038/eye.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-de-la-Casa JM, Jimenez-Santos M, Saenz-Frances F, et al. Performance of the rebound, noncontact and Goldmann applanation tonometers in routine clinical practice. Acta Ophthalmol. 2011;89(7):676–680. doi: 10.1111/j.1755-3768.2009.01774.x. [DOI] [PubMed] [Google Scholar]
- 21.Tonnu PA, Ho T, Sharma K, White E, Bunce C, Garway-Heath D. A comparison of four methods of tonometry: method agreement and interobserver variability. Br J Ophthalmol. 2005;89(7):847–850. doi: 10.1136/bjo.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao BS. Clinical evaluation of the non-contact tonometer and comparison with Goldmann applanation tonometer. Indian J Ophthalmol. 1984;32(5):432–434. [PubMed] [Google Scholar]
- 23.Lagerlöf O. Airpuff tonometry versus applanation tonometry. Acta Ophthalmol (Copenh) 1990;68(2):221–224. doi: 10.1111/j.1755-3768.1990.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 24.Krieger N, Ketcher G, Fulk GW. Physiological variables affecting intraocular pressure in a population study. Am J Optom Physiol Opt. 1988;65(9):739–744. doi: 10.1097/00006324-198809000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Shields MB, editor. Intraocular Pressure and Tonometry: Textbook of Glaucoma. 3rd ed. Baltimore, MD: Williams and Wilkins; 1992. pp. 53–83. [Google Scholar]
- 26.Qureshi IA. Intraocular pressure: a comparative analysis in two sexes. Clin Physiol. 1997;17(3):247–255. doi: 10.1111/j.1365-2281.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]