Abstract
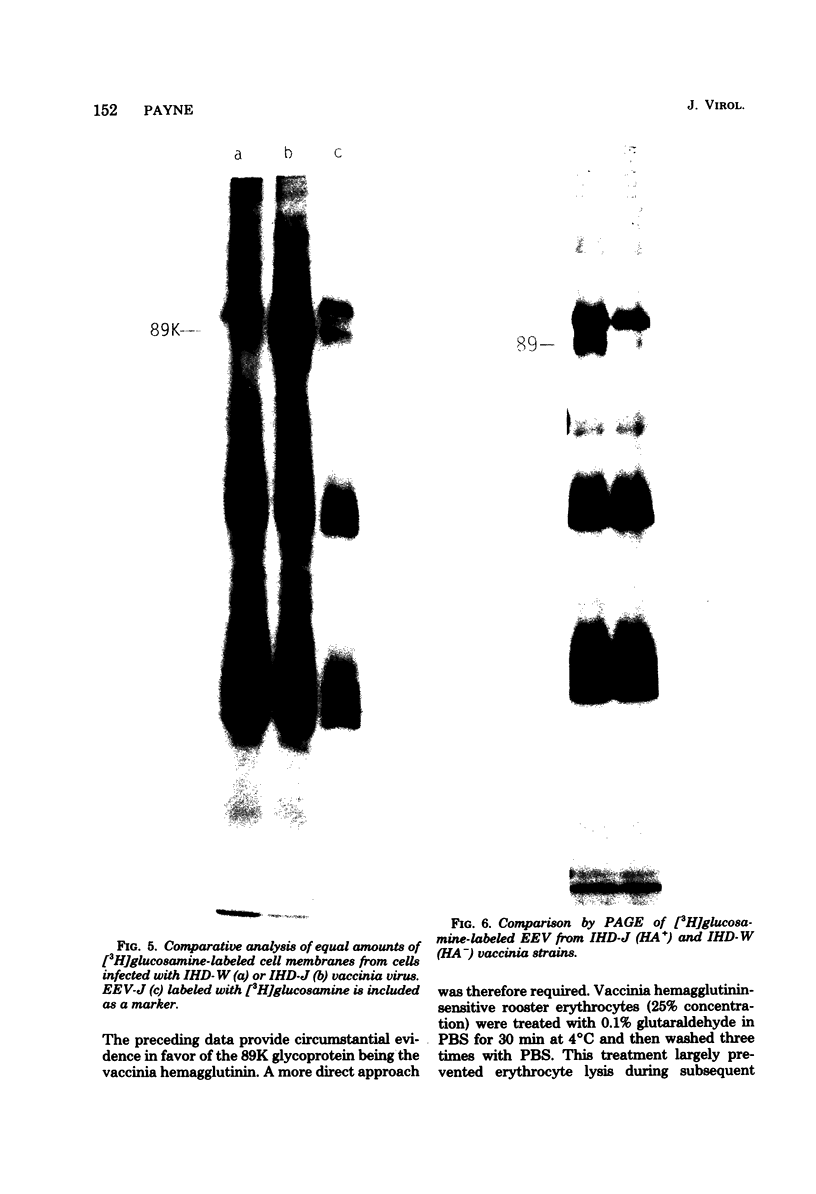
HeLa, SIRC, and RK-13 cells were compared as to their production of intracellular naked vaccinia virus (INV) and extracellular enveloped vaccinia virus (EEV) after infection with vaccinia strains WR and IHD-J. IHD-J produced more EEV from all three cell lines than did WR, although both strains produced approximately the same quantity of INV. The most efficient EEV release was from RK-13 cells infected with IHD-J, which was 200 times more than from WR-infected SIRC cells. This permitted for the first time the purification of milligram quantities of EEV that contained much fewer cell protein contaminants than could be obtained from HeLa or SIRC cells. The INV surface proteins 200K, 95K, 65K, and 13K were present in both HeLa and RK-13 cell-derived INV but were absent in SIRC cell INV. These proteins were absent in EEV from all three cell lines. Four glycoproteins of molecular weights 210 x 10(3) (210K), 110K, 89K, and 42K and five glycoproteins in the 23K to 20K range plus a nonglycosylated protein of 37K were detected in EEV from the hemagglutinin-positive IHD-J vaccinia strain. The 89K glycoprotein was not present in EEV or membranes from cells infected with the hemagglutinin-negative vaccinia strain IHD-W. Antisera to IHD-W lacking hemagglutinin-inhibiting antibodies did not precipitate the 89K glycoprotein of IHD-J. The only glycoprotein that specifically attached to rooster erythrocytes was the 89K glycoprotein. This evidence indicates that the 89K glycoprotein is the vaccinia hemagglutinin.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Hapel A. J., Boulter E. A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971 Oct;13(1):9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H. HeLa cell plasma membranes. Methods Cell Biol. 1973;7:157–188. doi: 10.1016/s0091-679x(08)61776-8. [DOI] [PubMed] [Google Scholar]
- Boulter E. A., Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y. Vaccinia-specific hemagglutinin. Virology. 1977 Feb;76(2):527–538. doi: 10.1016/0042-6822(77)90235-5. [DOI] [PubMed] [Google Scholar]
- KAKU H., KAMAHORA J. GIANT CELL FORMATION IN L CELLS INFECTED WITH ACTIVE VACCINIA VIRUS. Biken J. 1964 Jan;6:299–315. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Ghetie V., Sjöquist J. A human lymphoid cell line with an IgG-like membrane component. Eur J Immunol. 1975 Aug;5(8):518–526. doi: 10.1002/eji.1830050803. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 1978 Jul;27(1):19–27. doi: 10.1128/jvi.27.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash V. J., Norrby E., Payne L. Single radial immunodiffusion test for detecting antibodies against surface antigens of intracellular and extracellular vaccinia virus. J Gen Virol. 1977 Jun;35(3):465–472. doi: 10.1099/0022-1317-35-3-463. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Turner G. S., Squires E. J. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J Gen Virol. 1971 Oct;13(1):19–25. doi: 10.1099/0022-1317-13-1-19. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Dales S. Biogenesis of poxviruses: genetically controlled modifications of structural and functional components of the plasma membrane. Virology. 1974 Jul;60(1):96–127. doi: 10.1016/0042-6822(74)90369-9. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Stern W., Dales S. Biogenesis of vaccinia. Effects of inhibitors of glycosylation on virus-mediated activities. Virology. 1977 May 1;78(1):315–322. doi: 10.1016/0042-6822(77)90102-7. [DOI] [PubMed] [Google Scholar]










