Abstract
The present study aimed to evaluate the expression of neuro-oncological ventral antigen 1 (Nova1) in cerebral ischemia/reperfusion (I/R) insults by immunohistochemistry. The focal cerebral I/R model was induced by right middle cerebral artery occlusion (MCAO) for 120 min followed by 1 day, 7 days, and 14 days of reperfusion in Sprague-Dawley (SD) rats. The results showed that Nova1 was expressed in nearly the whole brain, although with higher density in hippocampus, hypothalamus, cingulate cortex, and medial habenular nucleus. The immunoreactivity of Nova1 neurons was increased dramatically, especially on both sides of the hippocampal CA1 region, after 1 day of reperfusion. A strong response occurred at the ipsilateral CA1 region between 1 day and 7 days of reperfusion. Likewise, strong compensatory responses of Nova1 expression were observed on the contralateral side of the striate cortex, dentate gyrus, and hypothalamus. Interestingly, more Nova1 neurons were observed to translocate to the dendrites and growth cones of the axons in the hypothalamus on the ischemic side after 7 days of reperfusion. In conclusion, our data suggest that Nova1 might mediate neuronal responsiveness, and its expression might positively correlate with neural repair after I/R insults in the rat brain.
Keywords: ischemia/reperfusion insults, middle cerebral artery occlusion, neuron splicing factor Nova 1, nuclear-cytoplasmic-neuritic translocation
Alternative splicing (AS) plays a major role in the regulation of gene expression and the generation of proteomic and functional diversity in eukaryotic genes (Black 2000; Blencowe 2006). AS is abundantly used in the brain in comparison to other tissues (Yeo et al. 2004; Blencowe 2006). In the brain, AS can influence neurophysiology through spatial and/or temporal alterations in proteins that comprise ion channels and membrane-bound receptors, which play a role in neurotransmitter storage and release (Grabowski and Black 2001; Lipscombe 2005; Stamm et al. 2005). Although numerous reports have described links between AS and neurologic disease, in many cases, the role of AS in disease is not well understood.
Cerebral ischemia/reperfusion (I/R) insults result in neuronal cell death, brain tissue loss, and severe neurological deficits. I/R insults usually result in severe functional losses in the central nervous system (CNS). Neuroprotection for ischemic brain injury has emerged only recently as a topic of serious biomedical inquiry. A growing body of evidence suggests that some pathological incidents in I/R are associated with gene expression and posttranscriptional regulation (Keyvani et al. 2002). The expression of more than 1000 genes is modulated during I/R, and many of these may be directly involved in propagation of the injury according to gene expression analyses (Prass et al. 2000). The integrated pattern of genomic dysregulation during the I/R insult might be further complicated by mis-splicing and alternative splicing, although this has not been confirmed. Recently, we reported that transcription and splicing were regulated in human umbilical vein endothelial cells under hypoxic stress (Hang et al. 2009). Daoud et al. (2002) also report that ischemia causes the cytoplasmic accumulation and hyperphosphylation of the splicing factor Tra2-β1, which changes alternative splicing patterns in the brain. We, similarly, showed that hnRNP A2/B1 participated in the posttranscriptional regulation of neurons in the cerebral cortex and hippocampus after suffering from I/R insults (Liu et al. 2010). These reports strongly suggest that the transcriptional and posttranscriptional regulation of neurons is helpful for better understanding the mechanism of cerebral I/R insults. Therefore, we asked whether the neuron-specific splicing factor, Nova1, is also altered under such pathological conditions and applied an established ischemia paradigm to answer this question.
Nova is a brain-specific splicing factor that was first identified as an antigen in a neurologic disorder termed paraneoplastic opsoclonus-myoclonus ataxia (POMA) (Darnell and Posner 2003). Nova proteins are sequence-specific RNA-binding proteins that harbor three KH-type RNA-binding domains. Extensive genetic, biochemical, and crystallographic studies have demonstrated the ability of Nova to bind RNA-containing repeats of the sequence YCAY in vivo and in vitro (Buckanovich and Darnell 1997; Yang et al. 1998; Jensen et al. 2000; Lewis et al. 2000). Nova regulates the AS of the GABAAγ2 and GlyRα2 inhibitory neurotransmitter receptor subunit pre-mRNAs via binding to the intronic YCAY-repeat elements (Dredge and Darnell 2003). Recently, analyses using a combination of techniques, including biochemical (Jensen et al. 2000; Dredge et al. 2005), cross-linking and immunoprecipitation (CLIP) (Ule et al. 2003; Licatalosi et al. 2008), microarray (Ule et al. 2005), and bioinformatic (Ule et al. 2006) assays, have led to the conclusion that Nova regulates the AS of neuronal transcripts encoding synaptic proteins that are important for the balance of neuronal excitation and inhibition. Such analyses have identified Nova as a regulatory protein at the top of a hierarchical network.
Two members of the Nova family have been found, termed Nova1 and Nova2. The activity of Nova1 has been shown to be necessary for the development and survival of motor neurons (Jensen et al. 2000). Moreover, the identification of Nova targets has begun to predict specific defects in the synaptic physiology of Nova knockout (KO) mice (Huang et al. 2005; Ule et al. 2005, 2006). Recently, Zhang et al. (2010) used Bayesian networks to identify Nova mRNA targets in the mouse brain and found that Nova directly affects the in vivo phosphorylation patterns of brain proteins via AS regulation. However, there is no knowledge about the role of Nova in the brain after I/R insults. To further understand the function of Nova, including its potential role in neuronal posttranscriptional regulation, the present study examined the expression pattern of Nova1 in the brain after transient forebrain I/R insults in the rat. The expression of Nova1 was assessed in the rat cerebral cortex, hippocampus, medial habenular nucleus, and hypothalamus after I/R insults by immunohistochemistry using a panel of polyclonal antibodies against the Nova1 protein.
Materials and Methods
Surgical Procedure
Cerebral focal ischemia or sham surgery was performed in male Sprague Dawley (SD) rats, weighing 260 to 280 g, by right middle cerebral artery occlusion (MCAO). All procedures involving animals were performed in accord with the Principles of Laboratory Animal Care (NIH publication No. 85–23, revised 1985) and were reviewed and approved by the Institutional Animal Care and Use Committee. The animals were allowed free access to food and water and housed in a climate-controlled environment (25C).
The animals were randomly divided into six groups: three groups of rats suffered 120 min ischemia followed by 1 day, 7 days, or 14 days of reperfusion (n=5 in each group) before being sacrificed; the three remaining rat groups did not suffer ischemia but were given a sham operation as controls for the three I/R groups (n=5 in each group). In the control groups, the external carotid artery (ECA) and the internal carotid artery (ICA) were exposed during the sham operation. The control rats were killed concurrently with the MCAO experimental groups (at 1 day, 7 days, and 14 days). The rats were subjected to right MCAO operation for 120 min using the intraluminal filament technique. Briefly, the rats were anesthetized with chloral hydrate (350 mg/kg, intraperitoneally [IP]). The right common carotid artery was exposed at the level of the ECA and ICA bifurcation. A 4–0 monofilament nylon suture was inserted into the ECA from the common carotid artery bifurcation and pushed into the ICA for 17 to 20 mm until a slight resistance was felt, which blocked the origin of the middle cerebral artery. The suture was slowly and carefully withdrawn at 120 min after MCAO. Afterward, the skin incision was sutured, and the animals were maintained in an incubator at 37C until they regained consciousness. The animals were then returned to their cages and closely monitored; their body temperature was maintained at 37 ± 0.5C.
Neurological Deficits
Twenty-four hours after I/R, the rats were tested for neurological sensorimotor deficits and scored by a blinded observer, as described by Brisman et al. (1999), with the following minor modification: 0 = no observable deficit (normal), 1 = failure to extend the right forepaw (mild), 2 = circling to the contralateral side (moderate), and 3 = loss of walking or righting reflex (severe). Animals were excluded from further studies if they exhibited either no neurological deficit after MCAO or evidence of subarachnoid hemorrhage.
Preparation of Histological Samples
At various time points after surgery (1 day, 7 days, and 14 days), the two groups of animals (MCAO treatment and sham operated) were reanesthetized. Their thoraxes were exposed, and blood was removed by transcardial perfusion with normal saline, followed by cold 4% phosphate-buffered saline (PBS)–buffered paraformaldehyde. Whole brains were removed and postfixed in 4% PBS-buffered paraformaldehyde for 48 hr. A standard block, at a level of 1.0 to 1.8 mm posterior to the bregma, was obtained and embedded in paraffin. A series of consecutive sections (5 µm in thickness) were prepared from the paraffin-embedded tissues for conventional histological examination and immunohistochemistry, according to our previously published methods (Shang et al. 2006; Xu et al. 2009).
Immunohistochemistry
After de-waxing and rehydration, the sections were washed three times with 0.01M PBS. Endogenous peroxidase activity was inactivated by incubation in 0.3% H2O2 in methanol for 15 min. Then, the sections were heated in a microwave for 20 min for antigen retrieval. After washing in PBS, the sections were incubated in 10% normal goat serum for 30 min at room temperature to prevent nonspecific immunoreactions. The sections were then incubated with an anti-Nova1 polyclonal antibody (1:1000; cat no. HPA004155; Sigma, St. Louis, MO) containing 0.3% Triton X-100 at 4C overnight. The sections were subsequently washed in PBS and incubated with biotinylated goat anti-rabbit IgG secondary antibody (1:500; Zymed, South San Francisco, CA) for 1 hr at room temperature. Finally, the sections were incubated with avidin-biotin-peroxidase reagents for 1 hr, and peroxidase activity was revealed by incubating the slides with 0.5 mg/mL of 3,3′-diaminobenzidine solution and 0.03% H2O2 for 3 to 5 min. The stained sections were then washed in distilled water, dehydrated in a graded series of alcohols, cleared in xylenes, and coverslipped after the final treatment. Immunoreacted sections of each brain region (cerebral cortex, hypothalamus, and hippocampus) were observed under a microscope (Olympus, Tokyo, Japan). Incubation with PBS served as a negative control for immunohistochemistry, which replaced incubation with the anti-Nova1 primary antibody. To ensure the specificity of the anti-Nova1 antibody used in this study as well as the immunohistochemical staining results, we also used another anti-Nova1 antibody from the LifeSpan Biosciences (Seattle, WA; cat no. LS-B3085). This antibody was raised against a synthetic peptide consisting of amino acids 21 to 37 of the Nova1 sequence [DPPDSRKRPLEAPPEAG], which is 100% conserved across the human, rat, and mouse (see Suppl. Information S1—CLUSTAL 2.0.12 multiple sequence alignment). As the immunohistochemistry results obtained by the LifeSpan antibody were similar to that of the Sigma antibody, we provide the immunohistochemistry results only by the Sigma antibody below. The immunohistochemistry results by the LifeSpan anti-Nova1 antibody as well as the identification of this antibody by using Western blot can be seen in the supplemental figures (Suppl. Figs. S1–S4).
Morphometric Analysis
Images of the stained sections (five sections per animal) were captured with a microscope using a 20× objective connected to a three-color (red-green-blue) CCD camera and a PC computer. Four representative brain regions (striatum cortex, hippocampus, medial habenular nucleus, and hypothalamus) of the hemisphere ipsilateral to the surgery and the corresponding regions on the contralateral side were analyzed. The number of immunoreactive neurons per visual field (2.5 × 1.8 mm2) was calculated using the Image-Pro Plus software by stereological methods (version 5.1; Media Cybernetics, Bethesda, MD). In detail, each image was captured with 2560 × 1920–pixel resolution. The image was first optimized by clicking the “Enhance/Equalize/Best Fit” tool in the Image-Pro Plus software. Next, the optical density of the image was calibrated using the menu item “Measure/Calibration/Intensity” (black level = 0 and incident level = 255). Immunoreactive neurons were manually circumscribed using the “area of interest (AOI)” tool. The command “Select colors” in the “Count/Size” dialog box was then applied to manually set the color threshold representing the positive immunostained signals. To define the mean optical density (MOD) and total per area (TPA) immunoreactivity, “Select Measurements” was selected from the “Measure” menu in the “Count/Size” dialog box. Finally, the data were displayed in the “Ranges Statistics” window. The parameter “MOD × TPA” was regarded as the density of Nova1 expression in rat brain, where TPA equals the area of [positive signals]/[area of AOI].
Data Analysis
All data were expressed as the mean ± SD. Statistical analyses of the morphometric quantification of Nova1-immunoreactive cells and the expression of Nova1 were performed with the one-way ANOVA test. Student’s t-test was used to compare two means from different groups with significance at p<0.05(*).
Results
Mortality and Neurological Scores
Prior to the completion of the experimental protocol, rat mortality was recorded as follows: one of five rats from the MCAO group (7 days) and two of five in the MCAO group (14 days) succumbed to focal hemorrhages in the infarct. No animals died during or immediately after the induction of cerebral ischemia. Thus, the number of rats that successfully completed the experimental protocol and were included in the final analysis was the following: MCAO (1 day), n=5; controls (1 day), n=5; MCAO (7 days), n=4; controls (7 days), n=5; MCAO (14 days), n=3; and controls (14 days), n=5. In general, the average neurological score recorded at 24 hr after I/R was 2 points, indicating that the MCAO model in this study was successful.
Normal Expression of Nova1 in the Rat Brain
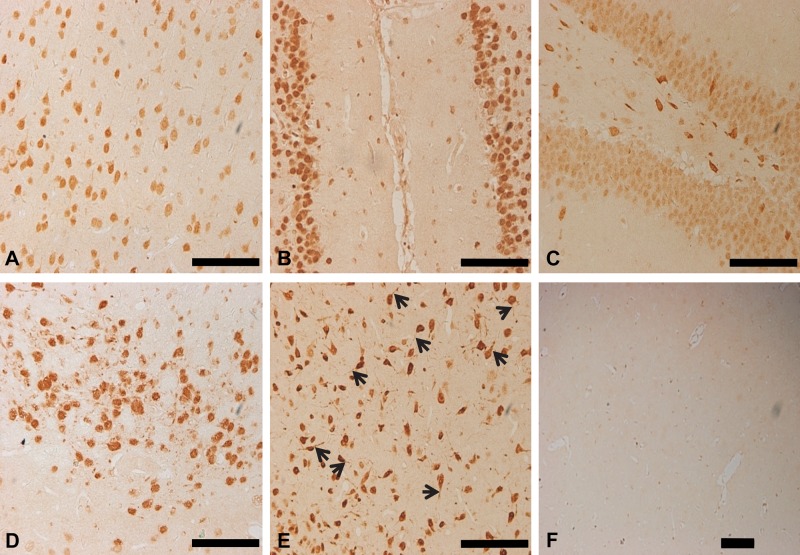
In agreement with previous studies (Buckanovich et al. 1993, 1996; Yang et al. 1998), Nova1-immunoreactive (Nova1-IR) cells were widely expressed in the rat brain, primarily in the midbrain and brainstem, as observed in sham-operated SD rats. In the cerebral cortex, Nova1-IR cells were scattered evenly throughout layer II to layer VI (Fig. 1). As expected, Nova1 was mainly expressed in the nuclei and cytoplasm of neurons. Strong signals were observed in the nucleus and cytoplasm of neurons in the cingulum cortex, dentate gyrus, medial habenular nucleus, hypothalamus, and especially the proximal dendrites of hypothalamic neurons (Fig. 1B–E). No typical immunoreactive signals were observed in the negative control (Fig. 1F).
Figure 1.
The expression pattern of Nova1 in the normal rat brain. Nova1 was mainly expressed in the nuclei and cytoplasm of neurons. (A) Strong immunohistochemical staining signals were observed in the nucleus and cytoplasm of neurons in the cingulum cortex (B), dentate gyrus (C), medial habenular nucleus (D), hypothalamus (E), and especially the proximal dendrites of neurons in the hypothalamus (E, arrowheads). The negative control shows no immunoreactive signals typical of Nova1 (F). Scale bar = 100 µm.
Changes of Nova1 Expression in Rat Brain upon I/R Insults
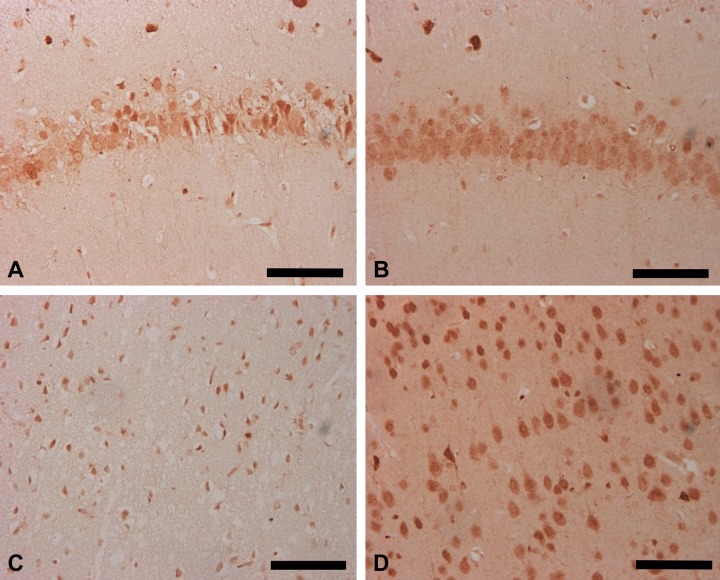
To investigate the expression and localization pattern of Nova1 under I/R pathological conditions, the MCAO model of the rat brain was examined. Sham-operated animals did not exhibit any brain damage, and their cortex regions appeared undamaged at various time points. However, apparent damage was observed in the ipsilateral neurons of the cerebral cortex and hippocampal CA1 region in the I/R group after 1 day of reperfusion (Fig. 2A, C); the contralateral side appeared normal (Fig. 2B, D).
Figure 2.
Expression pattern of Nova1 in neuronal cells of the cortex and CA1 region of the hippocampus in the rat brain following middle cerebral artery occlusion (MCAO). The expression of Nova1 was downregulated in ischemia/reperfusion groups after 1 day of reperfusion on the ipsilateral side following MCAO treatment (A and C), whereas the contralateral side appeared normal (B and D). Scale bar = 100 µm.
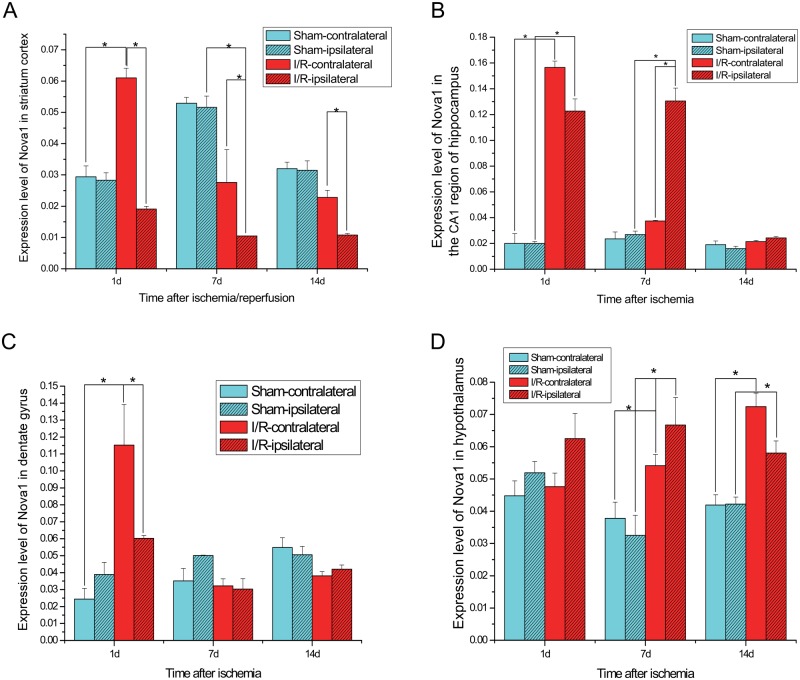
Most of the Nova1-IR cells on the ipsilateral side were morphologically damaged, whereas the residual neuronal cells showed intense immunoreactive signals to the anti-Nova1 antibodies. After 1 day of reperfusion, death of the neuronal cells extended throughout the entire cerebral cortex, and the observed damage was nearly identical at each time point examined: 1 day, 7 days, and 14 days following reperfusion. The immunoreactivity of Nova1 neurons in the ipsilateral striate cortex was notably decreased compared with that of the contralateral side, regardless of the time of reperfusion (1 day, 7 days, or 14 days; Fig. 3A). However, we noted that the immunoreactivity of Nova1 neurons in the striate cortex of the contralateral side was significantly increased compared with either the ipsilateral side or the sham group at 1 day of reperfusion, suggesting that a compensatory response may have occurred. The loss of Nova1 neurons on the ipsilateral side was accompanied by an increase in Nova1 expression on the contralateral side. These data suggest that strong splicing activity of Nova1 might occur within 1 day of reperfusion.
Figure 3.
Dynamic expression pattern of Nova1 on both sides of the striate cortex (A), CA1 region of the hippocampus (B), dentate gyrus (C), and hypothalamus (D) of the rat brain subjected to ischemia-reperfusion insults. I/R, ischemia/reperfusion. *p<0.05. Student’s t-test was used to compare two means from different groups with significance at p<0.05.
In the hippocampus, the Nova1 neurons were distributed mainly in the pyramidal cell layer. We observed an intense immunoreactivity in the neuronal nucleoplasm from the CA1 to CA4 hippocampal subfields. Compared with the sham group, the immunoreactivity of Nova1 in the CA1 subfield was clearly increased in both hemispheres after 1 day of reperfusion, although the most obvious damage at this time point was observed in the CA1 region of the ipsilateral hemisphere (Fig. 3B). The expression of Nova1 in the ipsilateral hippocampal CA1 subfield was also significantly upregulated after 7 days of reperfusion when compared with the sham group or the contralateral side. However, at 14 days after reperfusion, the expression of Nova1 was slightly higher than that in the sham group in both hemispheres of the CA1 region, suggesting that the strong splicing regulation of Nova1 in the CA1 region occurred between 1 day and 7 days of reperfusion. In the dentate gyrus, the immunoreactivity of Nova1 was increased on both sides after 1 day of reperfusion compared with the sham group, especially on the contralateral side (Fig. 3C). At the same time, the number of Nova1-IR neurons in both hemispheres of the dentate gyrus was gradually reduced to normal levels between 7 and 14 days of reperfusion. Therefore, the strong splicing activity of Nova1 probably occurred in the dentate gyrus after 1 day of reperfusion.
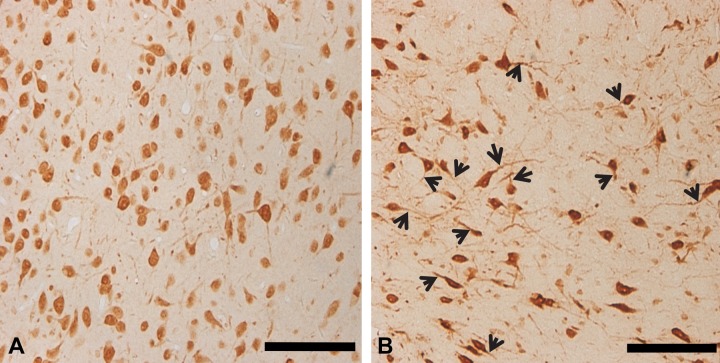
Between 1 and 14 days after the I/R insult, the immunoreactivity of Nova1 neurons was gradually increased in the hypothalamus. Specifically, the neurons of the ipsilateral side were obviously increased at the 7-day time point compared with those of the sham group (Fig. 3D). Interestingly, no obvious neuronal damage could be observed in the hypothalamus, although dendrites and axons were longer than those of the control group (Fig. 4). This may suggest that neurite outgrowth occurred when more Nova1 proteins translocated from the nucleus to the cytoplasm. The average length of the dendrites was gradually increased between 1 day and 7 days after reperfusion. At 7 days after reperfusion, it was obvious that the length of each dendrite was much longer on the ipsilateral side compared with the sham group. Therefore, it seemed that even after a long time of reperfusion, the splicing activity of Nova1 in the hypothalamus remained increased. These results also demonstrate that focal cerebral ischemia leads to complex pathogenic events in the hypothalamus long after the initial ischemic insult.
Figure 4.
A large number of the Nova1-immunoreactive signals were observed to translocate from the nucleus to the cytoplasm, and its expression extended to the neuronal dendrites and growth cones of the axons in the hypothalamus of the rat brain. The average length of the dendrites was largest after 7 days of reperfusion (B, arrowheads) on the ipsilateral side compared with the other time points of reperfusion or the sham group (A). Scale bar = 100 µm.
Discussion
Here, we examined the brain-specific splicing factor, Nova1, by immunohistochemistry and identified dynamic changes in its expression level in the cerebral cortex, hippocampus, and hypothalamus of the rat brain after an I/R insult. Strong immunostaining signals were observed in the hippocampal CA1 region and dentate gyrus in both brain hemispheres at 1 day following I/R. There was also a strong compensatory response in the cortex, CA1 region, dentate gyrus, and hypothalamus on the contralateral side. Furthermore, in the hypothalamus of the ischemic side after 7 days of reperfusion, we found that more Nova1 proteins translocated to the proximal dendrites and growth cones of the neuronal axons, indicating that strong splicing activity is present in the brain during I/R insults. Strong histochemical staining signals were also found in the medial habenular nucleus, but there was no significant difference between the ipsilateral and contralateral sides after analysis (data not shown), suggesting that different areas responded differently to the I/R insult. As a neuronal splicing factor, Nova1 appears to play an important role in the posttranscriptional regulation of neurons from the cerebral cortex, CA1 region, dentate gyrus, and hypothalamus following I/R insults. Further investigation of these changes in gene expression may reveal that the regulatory mechanisms found in different brain regions are unique. As such, our data indicate that posttranscriptional regulation of neuronal genes may provide a new avenue of investigation for the development of novel therapeutic approaches for stroke.
Our results show that Nova1 responds to I/R insults with a strong compensatory increase in expression in the cortex, CA1 region, dentate gyrus, and hypothalamus, specifically on the contralateral side. A number of studies have reported such contralateral gene induction before. Several members of the immediate early gene and transcription factor family were bilaterally upregulated after ischemic insults (Kokaia et al. 1995; Keyvani et al. 2000). Schneider et al. (2004) detected several genes that showed bilateral upregulation in the cortex of mice after ischemia. Possible mechanisms for the induction of these genes during ischemia could include seizures, transhemispherical communication, and/or electrical spreading depression (Koistinaho and Chan 2000). Following ischemia, there is a rapid electrophysiological response in the contralateral cortex (Reinecke et al. 1999). Here, the expression of Nova1 was significantly upregulated on the contralateral side, which revealed that Nova1 responded strongly to I/R insults and, thus, orchestrated a coordinated change in alternative splice-site selection of potential target genes for neuroprotection after ischemia.
A striking example of the biological importance of this response is given by the observed changes in alternative splicing patterns of genes, as well as the alterations in splicing factor expression, under different pathological conditions (Pajares et al. 2007; Skotheim and Nees 2007). For example, human Tra2-β1 is a splicing factor specifically induced in breast cancer that regulates the alternative splicing of the CD44 gene (Daoud et al. 2002; Watermann et al. 2006). This study shows that ischemia also induces the translocation of Nova1 and changes alternative splicing patterns in the brain. Several other cellular or viral proteins are known to shuttle between the nucleus and the cytoplasm (Borer et al. 1989; Meier and Blobel 1992). It has been reported that an inhibition of the oxygen supply can cause the translocation of the splicing factor SRPK1 (SR-protein specific kinase) from the nucleus to the cytosol. This translocation blocks its nuclear function and later leads to complete downregulation in the kernel infarcted area (Erdo et al. 2004). In line with our observations, these data suggest that important splicing factors are activated during ischemia. Recently, our group has also demonstrated that hnRNP A2/B1 translocated from the nucleus to the cytoplasm and dendrites in the cerebral cortex from 3 to 24 hr after I/R (Liu et al. 2010). Darnel et al. found that Nova shuttles between the nucleus and cytoplasm and colocalizes with target RNAs in the dendrites (GIRK2 and GlyRα2) (Racca et al. 2010). In our current study, we observed the translocation of Nova1 in the dendrites of the hypothalamus after 7 days of reperfusion, thereby further confirming the suggestion that the regulation of alternative splicing is coupled to the expression of the same RNA in neuronal dendrites in vivo. Nova1 might play a role in the regulation of mRNAs within dendrites and may be able to act to coordinate nuclear RNA processing with local mRNA expression. There is now considerable evidence that some mRNAs enable the local synthesis of the encoded proteins (Steward et al. 1996; Steward 1997). Many of the mRNAs that are present in dendrites have been identified. Polyribosomes are very abundant in the dendrites of developing neurons and appear to be preferentially localized beneath postsynaptic sites. Nova1 has well-documented splicing activity in the normal brain and may also be involved in the local translation of target mRNAs in dendrites under pathological circumstances, such as in the I/R insult described herein (Racca et al. 2010). Our results, together with previous reports, suggest that Nova1 might enhance local translation in dendrites and promote neural recovery after I/R damage.
Nova1 is evolutionarily conserved based on sequence comparisons between human, rat, and mouse, which exhibit high Nova1 homology (almost 99% at the amino acid level; data not shown). Such strong conservation indicates the functional importance of Nova1. Future studies will focus on the target genes of Nova1 during neuritogenesis and axonogenesis, as well as its expression pattern in the rat brain after I/R insults, which may permit a greater understanding of mammalian RNA regulation and translation at the systems level. Inferring RNA target networks regulated by Nova1 may provide general insights into the mechanisms of regulation and their role in a number of neurological diseases.
In conclusion, the presence of Nova1 indicates ongoing transcription, and the amount of Nova1 reflects splicing activity after I/R insults. It is likely that improved splicing activity is an important factor for neurofunctional recovery in the brain. Our observations emphasize the potential role of alternative splicing as a strong posttranscriptional regulator of gene expression in the brain. To our knowledge, this is the first report on the expression of the splicing factor Nova1 in a rat model of cerebral I/R injury. The dynamic expression of Nova1 in neurons suggests distinct roles of Nova1 at different time points in the postischemic brain. We have shown here that increased expression of Nova1 correlates with neuronal survival after I/R. Thus, our data suggest that Nova1 participates in the posttranscriptional regulation of neurons in the CA1 region, striatum cortex, dentate gyrus, and hypothalamus following I/R insults. Molecular evidence for the involvement of Nova1 in cerebral I/R injuries will be required for a better understanding of their differential biological actions.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by The General Program (grants 30973107, 81070741, 81100862, and 81100979) of the National Natural Science Foundation of China; the National Key Technologies R&D Program for New Drugs (grant 2012ZX09102301–016); the General Program of the State Key Laboratory of Medical Neurobiology (grants 09–03 and 10–06); and The National Basic Research Project (973 program) (2012CB518200).
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Black DL. 2000. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell. 103:367–370 [DOI] [PubMed] [Google Scholar]
- Blencowe BJ. 2006. Alternative splicing: new insights from global analyses. Cell. 126:37–47 [DOI] [PubMed] [Google Scholar]
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. 1989. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 56:379–390 [DOI] [PubMed] [Google Scholar]
- Brisman MH, Tuhrim S, Jenkins A, Bederson JB. 1999. Thyrocervical to vertebral artery transposition and ipsilateral carotid endarterectomy. Surg Neurol. 51:327–330; discussion 330–321. [DOI] [PubMed] [Google Scholar]
- Buckanovich RJ, Darnell RB. 1997. The neuronal RNA binding protein Nova-1 recognizes specific RNA targets in vitro and in vivo. Mol Cell Biol. 17:3194–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckanovich RJ, Posner JB, Darnell RB. 1993. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron. 11:657–672 [DOI] [PubMed] [Google Scholar]
- Buckanovich RJ, Yang YY, Darnell RB. 1996. The onconeural antigen Nova-1 is a neuron-specific RNA-binding protein, the activity of which is inhibited by paraneoplastic antibodies. J Neurosci. 16:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud R, Mies G, Smialowska A, Olah L, Hossmann KA, Stamm S. 2002. Ischemia induces a translocation of the splicing factor tra2-beta 1 and changes alternative splicing patterns in the brain. J Neurosci. 22:5889–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell RB, Posner JB. 2003. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 349:1543–1554 [DOI] [PubMed] [Google Scholar]
- Dredge BK, Darnell RB. 2003. Nova regulates GABA(A) receptor gamma2 alternative splicing via a distal downstream UCAU-rich intronic splicing enhancer. Mol Cell Biol. 23:4687–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dredge BK, Stefani G, Engelhard CC, Darnell RB. 2005. Nova autoregulation reveals dual functions in neuronal splicing. Embo J. 24:1608–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdo F, Trapp T, Mies G, Hossmann KA. 2004. Immunohistochemical analysis of protein expression after middle cerebral artery occlusion in mice. Acta Neuropathol. 107:127–136 [DOI] [PubMed] [Google Scholar]
- Grabowski PJ, Black DL. 2001. Alternative RNA splicing in the nervous system. Prog Neurobiol. 65:289–308 [DOI] [PubMed] [Google Scholar]
- Hang X, Li P, Li Z, Qu W, Yu Y, Li H, Shen Z, Zheng H, Gao Y, Wu Y, et al. 2009. Transcription and splicing regulation in human umbilical vein endothelial cells under hypoxic stress conditions by exon array. BMC Genomics. 10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Shi SH, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY. 2005. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell. 123:105–118 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Dredge BK, Stefani G, Zhong R, Buckanovich RJ, Okano HJ, Yang YY, Darnell RB. 2000. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron. 25:359–371 [DOI] [PubMed] [Google Scholar]
- Keyvani K, Reinecke S, Abts HF, Paulus W, Witte OW. 2000. Suppression of proteasome C2 contralateral to ischemic lesions in rat brain. Brain Res. 858:386–392 [DOI] [PubMed] [Google Scholar]
- Keyvani K, Witte OW, Paulus W. 2002. Gene expression profiling in perilesional and contralateral areas after ischemia in rat brain. J Cereb Blood Flow Metab. 22:153–160 [DOI] [PubMed] [Google Scholar]
- Koistinaho J, Chan PH. 2000. Spreading depression-induced cyclooxygenase-2 expression in the cortex. Neurochem Res. 25:645–651 [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, Siesjo BK, Lindvall O. 1995. Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol. 136:73–88 [DOI] [PubMed] [Google Scholar]
- Lewis HA, Musunuru K, Jensen KB, Edo C, Chen H, Darnell RB, Burley SK. 2000. Sequence-specific RNA binding by a Nova KH domain: implications for paraneoplastic disease and the fragile X syndrome. Cell. 100:323–332 [DOI] [PubMed] [Google Scholar]
- Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, et al. 2008. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 456:464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D. 2005. Neuronal proteins custom designed by alternative splicing. Curr Opin Neurobiol. 15:358–363 [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao Y, Wu Y, Wu Y, Wang H, Zhang C. 2010. Histochemical mapping of hnRNP A2/B1 in rat brain after ischemia-reperfusion insults. J Histochem Cytochem. 58:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier UT, Blobel G. 1992. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 70:127–138 [DOI] [PubMed] [Google Scholar]
- Pajares MJ, Ezponda T, Catena R, Calvo A, Pio R, Montuenga LM. 2007. Alternative splicing: an emerging topic in molecular and clinical oncology. Lancet Oncol. 8:349–357 [DOI] [PubMed] [Google Scholar]
- Prass K, Wiegand F, Schumann P, Ahrens M, Kapinya K, Harms C, Liao W, Trendelenburg G, Gertz K, Moskowitz MA, et al. 2000. Hyperbaric oxygenation induced tolerance against focal cerebral ischemia in mice is strain dependent. Brain Res. 871:146–150 [DOI] [PubMed] [Google Scholar]
- Racca C, Gardiol A, Eom T, Ule J, Triller A, Darnell RB. 2010. The neuronal splicing factor Nova co-localizes with target RNAs in the dendrite. Front Neural Circuits. 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke S, Lutzenburg M, Hagemann G, Bruehl C, Neumann-Haefelin T, Witte OW. 1999. Electrophysiological transcortical diaschisis after middle cerebral artery occlusion (MCAO) in rats. Neurosci Lett. 261:85–88 [DOI] [PubMed] [Google Scholar]
- Schneider A, Fischer A, Kruger C, Aronowski J. 2004. Identification of regulated genes during transient cortical ischemia in mice by restriction-mediated differential display (RMDD). Brain Res Mol Brain Res. 124:20–28 [DOI] [PubMed] [Google Scholar]
- Shang A, Zhou D, Wang L, Gao Y, Fan M, Wang X, Zhou R, Zhang C. 2006. Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Res. 1078:219–226 [DOI] [PubMed] [Google Scholar]
- Skotheim RI, Nees M. 2007. Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol. 39:1432–1449 [DOI] [PubMed] [Google Scholar]
- Stamm S, Ben-Ari S, Rafalska I, Tang Y, Zhang Z, Toiber D, Thanaraj TA, Soreq H. 2005. Function of alternative splicing. Gene. 344:1–20 [DOI] [PubMed] [Google Scholar]
- Steward O. 1997. mRNA localization in neurons: a multipurpose mechanism? Neuron. 18:9–12 [DOI] [PubMed] [Google Scholar]
- Steward O, Falk PM, Torre ER. 1996. Ultrastructural basis for gene expression at the synapse: synapse-associated polyribosome complexes. J Neurocytol. 25:717–734 [DOI] [PubMed] [Google Scholar]
- Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. 2003. CLIP identifies Nova-regulated RNA networks in the brain. Science. 302:1212–1215 [DOI] [PubMed] [Google Scholar]
- Ule J, Stefani G, Mele A, Ruggiu M, Wang X, Taneri B, Gaasterland T, Blencowe BJ, Darnell RB. 2006. An RNA map predicting Nova-dependent splicing regulation. Nature. 444:580–586 [DOI] [PubMed] [Google Scholar]
- Ule J, Ule A, Spencer J, Williams A, Hu JS, Cline M, Wang H, Clark T, Fraser C, Ruggiu M, et al. 2005. Nova regulates brain-specific splicing to shape the synapse. Nat Genet. 37:844–852 [DOI] [PubMed] [Google Scholar]
- Watermann DO, Tang Y, Zur Hausen A, Jager M, Stamm S, Stickeler E. 2006. Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer Res. 66:4774–4780 [DOI] [PubMed] [Google Scholar]
- Xu Z, Hou B, Zhang Y, Gao Y, Wu Y, Zhao S, Zhang C. 2009. Antidepressive behaviors induced by enriched environment might be modulated by glucocorticoid levels. Eur Neuropsychopharmacol. 19:868–875 [DOI] [PubMed] [Google Scholar]
- Yang YY, Yin GL, Darnell RB. 1998. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc Natl Acad Sci U S A. 95:13254–13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo G, Holste D, Kreiman G, Burge CB. 2004. Variation in alternative splicing across human tissues. Genome Biol. 5:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Frias MA, Mele A, Ruggiu M, Eom T, Marney CB, Wang H, Licatalosi DD, Fak JJ, Darnell RB. 2010. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science. 329:439–443 [DOI] [PMC free article] [PubMed] [Google Scholar]