Abstract
Under long-term hypoxia, noradrenaline (NA) content in the carotid body (CB) increases, suggesting that NA plays an important role in CB chemotransduction. However, it is unknown whether short-term hypoxia upregulates NA biosynthesis in CB. Therefore, we examined dopamine β-hydroxylase (DBH) expression in the CB of rats exposed to hypoxia (10% O2) for 0 to 24 hr with immunoblotting and immunohistochemistry. Using immunoblotting, the signal intensity for DBH appeared to be the most intense in rats exposed to hypoxia for 12 hr. Using immunohistochemistry, DBH immunoreactivity was observed in the cytoplasm of some glomus cells and varicosities in controls and rats exposed to hypoxia for 6 hr. In rats exposed to hypoxia for 12 hr, DBH immunoreactive intensities in DBH-positive glomus cells were significantly higher compared with controls (p<0.05). In the CB of rats exposed to hypoxia for 18 and 24 hr, DBH immunoreactive intensities in DBH-positive glomus cells were significantly lower than that of rats exposed to hypoxia for 12 hr (p<0.05). These results demonstrate that DBH immunoreactivity is transiently increased in glomus cells by short-term hypoxia, suggesting that NA biosynthesis is transiently facilitated in glomus cells at an early stage of hypoxia.
Keywords: hypoxia, carotid body, glomus cell, dopamine β-hydroxylase, noradrenaline
The carotid body (CB) is located bilaterally at the bifurcation of the common carotid artery and is the peripheral chemoreceptor responsible for monitoring changes in PO2, PCO2, and pH in arterial blood (Nurse 2005; Lahiri et al. 2006; Prabhakar 2006). Decreases in arterial PO2 are detected by glomus cells (type I cells) within the CB, which are synaptically connected with the carotid sinus nerve, leading to an increase in afferent sensory discharge to the nucleus of the solitary tract (Gonzalez et al. 1994; Lahiri et al. 2006). As a result, appropriate autonomic changes, including stimulation of breathing and a rise in blood pressure, are caused under environmental hypoxia (Prabhakar 2006).
It is known that glomus cells contain many neurotransmitters and neuromodulators (Lahiri et al. 2006). In an electrochemical study, acetylcholine and ATP are excitatory neurotransmitters and mediate hypoxic chemotransduction between glomus cells and nerve terminals of the carotid sinus nerve (Zhang et al. 2000). On the other hand, dopamine is contained in glomus cells (Gonzalez et al. 1994) and inhibits L-type Ca2+ channels via dopaminergic D2 autoreceptors in these cells (Benot and López-Barneo 1990). It was reported that dopamine content in the CB was increased under long-term hypoxia, that is, 2 days to 4 weeks (Hanbauer et al. 1981; Olson et al. 1983; Pequignot et al. 1987; Hui et al. 2003). It has been suggested that dopamine inhibits excitatory neurotransmitter release from glomus cells during hypoxia (Nurse 2005).
In addition to dopamine, noradrenaline (NA) is one of the other catecholamines that exist in the glomus cells of the CB (Gonzalez et al. 1994). There are a few reports concerning the role of NA in CB chemotransduction compared with dopamine, but it was reported that α2-adrenoceptors were present within cat CBs by a receptor binding assay, and intracarotid infusion of guanabenz, an α2-adrenoceptor agonist, depressed the magnitude of carotid sinus nerve activity to isocapnic hypoxia (Kou et al. 1991). It was also reported in an electrophysiological study that NA inhibited the Ca2+ current in glomus cells of rabbit CBs via α2-adrenoceptors (Almaraz et al. 1997; Overholt and Prabhakar 1999). The authors suggested that NA in glomus cells has an inhibitory role for the secretion of excitatory neurotransmitters from these cells. In addition, it was reported that NA content in the CB was increased in rats exposed to hypoxia for 2 weeks (Hui et al. 2003) and 4 weeks (Pequignot et al. 1987). From these reports, it could be considered that NA contributes to the inhibition of excessive excitation of the CB under long-term hypoxia together with dopamine.
In contrast to long-term hypoxia, there are only a few reports for the effect of short-term hypoxia (within 1 day) on the expression of catecholamines in the CB. However, we previously reported that mRNA for tyrosine hydroxylase (EC1.14.16.2), the rate-limiting enzyme in catecholamine biosynthesis, was increased in the CB of rats exposed to hypoxia for 4 hr or more (Wakai et al. 2010), and immunoreactivity for this enzyme was enhanced in glomus cells after rats were exposed to hypoxia for 12 hr (Kato et al. 2010). These findings suggest that biosynthesis of catecholamines is upregulated in glomus cells of the CB by short-term hypoxia. Thus, in addition to dopamine, NA is likely to contribute to inhibitory regulation of excessive CB excitation at an early stage of hypoxia. Therefore, it is expected that dopamine β-hydroxylase (DBH; EC1.14.17.1), the biosynthetic enzyme for NA, is also enhanced in the CB by short-term hypoxia as in the case of tyrosine hydroxylase.
In the present study, we confirmed expression of mRNA for DBH in the CB by RT-PCR. To investigate the effect of short-term hypoxia on NA biosynthesis, we examined DBH protein expression and immunoreactivity in the CB of rats exposed to hypoxia for 0 to 24 hr.
Materials and Methods
Animals
Male Wistar rats (8–9 weeks old) from Japan SLC, Inc. (Slc: Wistar, Japan SLC, Hamamatsu, Japan) were used in the present study. The total number of rats provided for this study was 45. All procedures for animal handling were performed in accordance with the guidelines of the Local Animal Ethics Committee of Iwate University (accession number: 201047).
RT-PCR
RT-PCR analysis was performed to confirm expression of mRNA for DBH in the CB. For RT-PCR analysis, rats were anesthetized with diethyl ether and euthanized by exsanguination from the abdominal aorta. The CB was dissected out and frozen with liquid N2. The adrenal gland and superior cervical ganglion were also dissected out as control organs. Total RNA of the three organs was collected using a magnetic beads method (MELT total nucleic acid isolation system; Ambion, Austin, TX). Details of the primers used in the present study are shown in Table 1. RT-PCR was performed with a Qiagen OneStep RT-PCR kit (Qiagen, Hilden, Germany) with specific primers for DBH. Primers for 18S rRNA were also used to detect mRNA expression of housekeeping genes. Reverse transcription was performed for 30 min at 50C, and initial PCR activation was incubated for 15 min at 95C. After reverse transcription, PCR amplifications were performed 40 times as follows: 30 sec at 94C for denaturation, 30 sec at 60C for annealing, and 1 min at 72C for extension. After PCR amplification, final extension was performed for 10 min at 72C. PCR end products were visualized on 2% agarose gels using ethidium bromide. For negative control experiments, mRNA templates were omitted.
Table 1.
Primers Used in RT-PCR
| mRNA (Accession Number) | Primer Sequences | Position | Product Length (bp) |
|---|---|---|---|
| DBH (NM_013158) | 5′-TGGAATCTTGGAGGAGATGTG-3′ (sense) | 1458-1478 | 438 |
| 5′-AACGAGGAGAGGCTGAAGAAC-3′ (antisense) | 1875-1895 | ||
| 18s rRNA (X03205) | 5′-CCTGCGGCTTAATTTGACTC-3′ (sense) | 1230-1249 | 118 |
| 5′-AACTAAGAACGGCCATGCAC-3′ (antisense) | 1328-1347 |
Hypoxic Exposure
Rats were exposed to normobaric hypoxia (10% O2) for 0 (control), 6, 12, 18, or 24 hr, as previously described (Kato et al. 2010). Each rat was placed in a cage inside an acrylic chamber and adjusted to its environment for 30 min before hypoxic exposure. N2 gas was used to decrease O2 levels in the chamber and was flowed from an N2 gas cylinder to the chamber. O2 levels in the chamber were continuously monitored by a gas analyzer. In this system, O2 concentrations in the chamber were kept between 9.5% and 10.5% during exposure. CO2 levels in the chamber were also monitored during hypoxic exposure and were kept low (<1%). During each exposure, rats received water and food ad libitum.
Immunoblotting
For immunoblotting, four rats per each time point were used. A pair of CBs was collected from each rat and homogenized using a glass micro-homogenizer with liquid N2. Then, 25 µl RIPA buffer (10 mM Tris-HCl [pH 7.5], 1% Triton-X, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 1 mM EDTA) with a protease inhibitor cocktail (P8340; Sigma, St. Louis, MO) was added to each CB sample, and samples were incubated for 45 min on ice. Homogenates of CB samples were then added to an equal volume of 2× sample buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 0.002% bromophenol blue) containing 10% 2-mercaptoethanol and were denatured by boiling at 95C for 5 min. The loading sample of the CB contained total protein extracted from a pair of CBs from a rat. The methods of SDS polyacrylamide gel electrophoresis and electroblotting were described previously (Kato et al. 2010). Polyvinylidene fluoride membranes were blocked for 1 hr at room temperature in blocking buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, 0.1% Tween-20, 2.5% BSA) and incubated overnight at 4C with a monoclonal mouse antibody against DBH (1:100 dilution; sc-47707; Santa Cruz Biotechnology, Santa Cruz, CA) or a monoclonal mouse antibody against β-actin (1:100 dilution; A 4700; Sigma) as a loading control. Membranes were then rinsed with wash buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.1% Tween-20) and incubated for 1 hr at room temperature with horseradish peroxidase–conjugated donkey antibody against mouse IgG (1:10,000 dilution; 715-035-150, Jackson Immunoresearch Laboratories, West Grove, PA). After rinsing with wash buffer, visualization of immune complexes was carried out using ECL plus Western blotting detection reagents (GE Healthcare; Buckinghamshire, UK). Protein signals were detected using a CHEMIDOC XRS System with QuantityOne I-D analysis software (BioRad; Hercules, CA). The relative expression level of DBH protein in the CB between experimental groups was normalized to β-actin by densitometry using the ImageJ analysis program (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/).
Immunohistochemistry
For immunohistochemistry, four to six rats per each time point were used. Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (15 mg/kg) and transcardially perfused through the ascending aorta with Ringer’s solution (300 ml) and then with 4% paraformaldehyde in 0.1 M phosphate buffer containing 0.5% picric acid (pH 7.4; 300 ml). The bifurcation of the carotid artery was removed and further fixed with the same fixative for 2 to 3 hr at 4C. Tissues were then rinsed in PBS (pH 7.4), soaked in 30% sucrose in PBS, and frozen with O.C.T. compound medium (Sakura Finetek; Tokyo, Japan). Tissues were serially sectioned at a thickness of 10 µm using a cryostat (CM 1900; Leica, Wetzlar, Germany) and mounted on glass slides coated with chrome alum-gelatin.
Sections were rinsed with PBS, incubated for 30 min with non-immune donkey serum (1:50 dilution), and rinsed with PBS. Sections were incubated overnight at 4C with monoclonal mouse antibody against DBH (1:4000 dilution; MAB308; Chemicon, Temecula, CA) together with polyclonal guinea pig antibody against synaptophysin (1:1000 dilution; Syn-GP-Af300-1; Frontier Science, Sapporo, Japan) as a marker protein for glomus cells. Sections were then rinsed with PBS and incubated for 90 min with Alexa Fluor 488–labeled donkey anti-mouse IgG (1:200 dilution; A21202; Invitrogen, Tokyo, Japan) and Cy3-labeled donkey anti–guinea pig IgG (1:100 dilution; 706-165-148; Jackson) at room temperature. After rinsing with PBS, coverslips were mounted onto glass slides with mounting medium. Digital images of each section were captured with a confocal laser scanning microscope (LSM 510; Carl Zeiss, Oberkochen, Germany) under the same conditions (e.g., pinhole, scan speed, and detection gain) and were used for the measurement of gray-scale intensity for DBH immunofluorescence (see Gray Scale for Dopamine β-hydroxylase Immunoreactivity). Controls of immunofluorescence for DBH were performed by omission of the primary antibody in the original reaction sequences.
Gray Scale for Dopamine β-hydroxylase Immunoreactivity
To analyze the immunoreactivity for DBH in glomus cells, the gray-scale intensity (GI; range 0–255: black = 0, white = 255) of DBH immunofluorescence was measured using the ImageJ analysis program. The cytoplasm of glomus cells was identified on micrographs of the section stained for synaptophysin. In gray-scale images of DBH-immunostained sections, the gray-scale intensity (256 shades of gray) of glomus cells was measured in each rat. Then, data from 50 glomus cells were randomly extracted from each rat and provided for statistical analysis. In the present study, glomus cells with GI greater than 0 were considered to be DBH immunopositive. The ratio of DBH-immunopositive glomus cells in each experimental group and the gray-scale intensity for DBH-immunopositive cells are given as mean ± SD. Statistical analysis for both the ratio of DBH-immunopositive glomus cells and the GI for DBH-immunopositive cells between experimental groups was done using the Kruskal-Wallis test, followed by the post hoc Holm method where applicable. Values less than 0.05 were considered significant.
Results
RT-PCR
PCR products of the predicted size (438 bp) for DBH were amplified from total RNA templates of the CB as well as both the superior cervical ganglion and adrenal gland as positive control organs (Fig. 1). PCR products with the predicted size (118 bp) for 18S rRNA were also detected in the three organs. In a negative control experiment, no product was found (data not shown). From these results, it was confirmed that mRNA for DBH is expressed within the CB.
Figure 1.
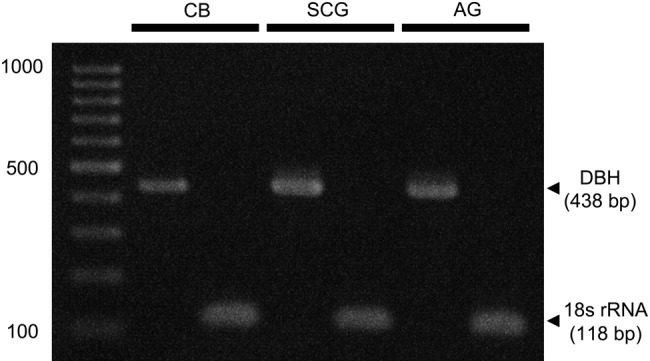
RT-PCR analysis for the expression of dopamine β-hydroxylase (DBH) mRNA in the carotid body (CB), superior cervical ganglion (SCG), and adrenal gland (AG). PCR products for DBH are visualized at an appropriate size (438 bp) in the CB as well as in the SCG and AG. 18S rRNA is used as an internal control. Far left lane indicates the DNA molecular marker.
Immunoblotting
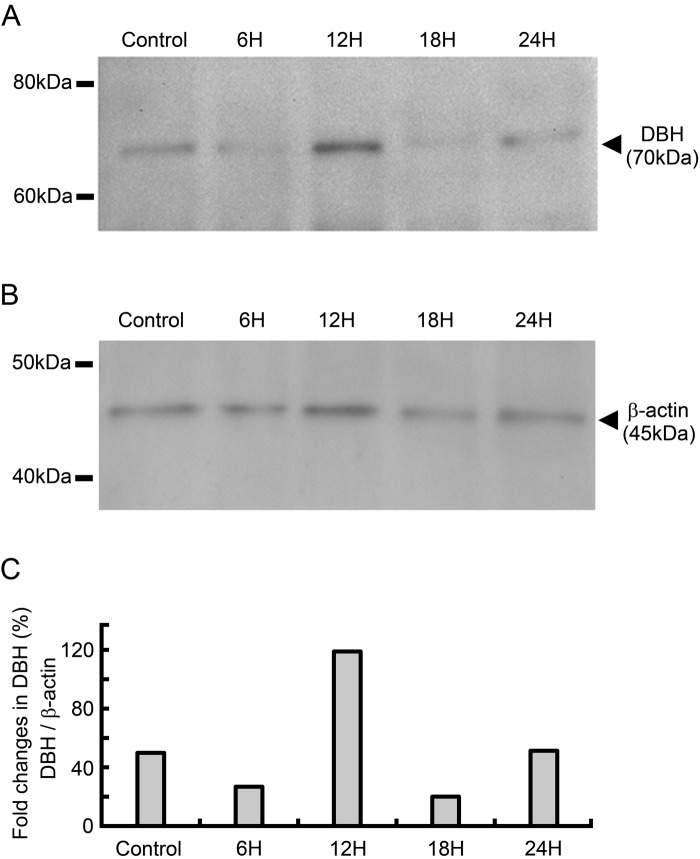
In control rats, DBH was identified as a single band with a molecular mass of approximately 70 kDa (Fig. 2A). Therefore, it was confirmed that DBH protein is expressed within the CB. In rats exposed to hypoxia for 6, 12, 18, and 24 hr, DBH was identified as a single band as in the case of control rats. Within the experimental groups, including controls, the signal intensity for DBH appeared to be the most intense in rats exposed to hypoxia for 12 hr relative to other experimental groups. β-Actin was identified as a single band with a molecular mass of approximately 45 kDa in all groups (Fig. 2B). The relative expression level of DBH protein in representative immunoblots is shown in Figure 2C. The value was 49.9% in the control group. In the groups exposed to hypoxia, the values were 26.8% for the 6-hr group, 118.9% for the 12-hr group, 20.0% for the 18-hr group, and 51.3% for the 24-hr group. These results suggest that DBH protein level in the CB is increased after hypoxic exposure for 12 hr.
Figure 2.
Immunoblotting for dopamine β-hydroxylase (DBH) in the carotid body (CB) of control rats and rats exposed to hypoxia for 6, 12, 18, and 24 hr. (A) Immunoblotting for DBH. Representative immunoblots are shown. The signal for DBH was visualized as a single band at approximately 70 kDa in the CB of all experimental groups. It is noteworthy that the signal intensity for DBH is more intense in rats exposed to hypoxia for 12 hr than in other experimental groups. (B) Immunoblotting for β-actin. The signal for β-actin was identified as a single band with a molecular mass of approximately 45 kDa in all experimental groups. (C) The relative expression level of DBH protein in representative immunoblots, normalized to β-actin. The value was 49.9% in the control group. In the groups exposed to hypoxia, the values were 26.8% for the 6-hr group, 118.9% for the 12-hr group, 20.0% for the 18-hr group, and 51.3% for the 24-hr group.
Double Immunofluorescence
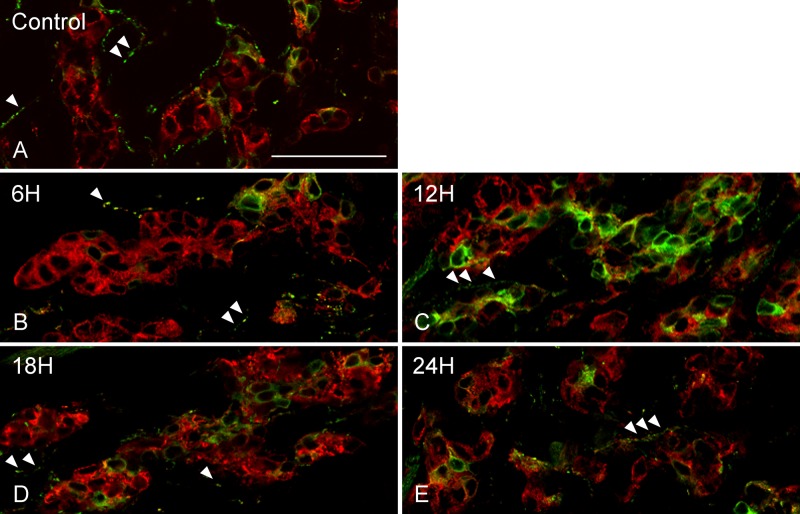
DBH immunoreactivity was observed in the cytoplasm of glomus cells. In control rats, DBH-immunopositive glomus cells were sparsely observed in the CB (Fig. 3A). The ratio of DBH-immunopositive glomus cells in control rats was 38.0 ± 3.7%. In the CB of rats exposed to hypoxia for 6 hr, apparent differences in DBH immunostaining properties were not observed compared with controls (Fig. 3B). However, in rats exposed to hypoxia for 12 hr, DBH-immunopositive glomus cells appeared to be higher in number and showed more intense DBH immunofluorescence than that of controls (Fig. 3C). In the CB of rats exposed to hypoxia for 18 and 24 hr, DBH-immunopositive glomus cells appeared to be lower in number than that of rats exposed to hypoxia for 12 hr (Fig. 3D, E). The ratios of DBH-immunopositive glomus cells in rats exposed to hypoxia for 6, 12, 18, and 24 hr were 42.3 ± 20.0%, 64.4 ± 11.1%, 55.0 ± 9.2%, and 53.2 ± 15.5%, respectively. Ratios were not significantly different among groups. In addition to glomus cells, varicose nerve fibers with DBH immunoreactivity were observed in the smooth muscle layer of blood vessels within the CB (Fig. 3A, arrowheads). Between experimental groups, including controls, there was no obvious difference in DBH-immunoreactive varicosities (Fig. 3A–E, arrowheads). There was no immunoreactivity in the section from which the anti-DBH antibody was omitted (data not shown). Immunoreactivity for synaptophysin was observed in the cytoplasm of glomus cells, and staining properties were similar between controls and hypoxic-exposed groups (Fig. 3A–E).
Figure 3.
Double immunofluorescence images of dopamine β-hydroxylase (DBH; green) and synaptophysin (red). Immunoreactivity for DBH was observed in the cytoplasm of glomus cells immunostained for synaptophysin. A few DBH-immunopositive glomus cells were scattered in the carotid body of controls (A) and rats exposed to hypoxia for 6 hr (B). In rats exposed to hypoxia for 12 hr, the number and fluorescent intensity of DBH-immunoreactive glomus cells were increased (C). DBH-immunopositive glomus cells were lower in number in rats exposed to hypoxia for 18 hr (D) and 24 hr (E) than that of rats exposed to hypoxia for 12 hr. DBH immunoreactivity was also observed in varicose nerve fibers (A–E, arrowheads), and there were no obvious differences in DBH-immunoreactive nerve fibers between experimental groups (A–E). Scale bars = 50 µm.
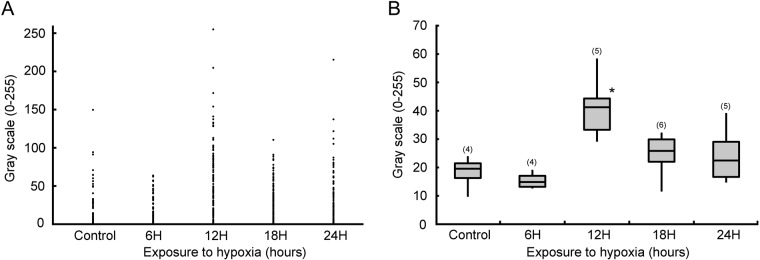
The scatter graph of glomus cells based on the GI for DBH immunoreactivity is shown in Figure 4A. In controls, plotted dots indicate glomus cells gathered below a GI of 100. In rats exposed to hypoxia for 6 hr, the distribution of plotted dots was similar to that of controls. In rats exposed to hypoxia for 12 hr, the number of glomus cells with intense DBH immunoreactivity (GI >100) was higher than that of controls. In the distribution of rats exposed to hypoxia for 18 and 24 hr, glomus cells with intense DBH immunoreactivity appeared to be lower than that of rats exposed to hypoxia for 12 hr.
Figure 4.
Measurement of the gray-scale intensity (GI) of dopamine β-hydroxylase (DBH) immunoreactivity in glomus cells. (A) Scatter graph based on the GI of DBH immunoreactivity in glomus cells. In controls and rats exposed to hypoxia for 6 hr, glomus cells gathered below a GI of 100. In rats exposed to hypoxia for 12 hr, the number of glomus cells with intense DBH immunoreactivity (GI >100) was increased. In rats exposed to hypoxia for 18 and 24 hr, the number of glomus cells with intense DBH immunoreactivity was lower than that of rats exposed to hypoxia for 12 hr. (B) Box plots showing the distribution of the GI in DBH-immunopositive glomus cells (GI ≠ 0) in each experimental group. GI values in DBH-immunopositive glomus cells were significantly higher in the rats exposed to hypoxia for 12 hr than that of control rats and rats exposed to hypoxia for 6, 18, and 24 hr (*p<0.05). The numbers on the top of each box plot indicate the numbers of rats provided for statistical analysis.
Box plots show the distribution of GI in DBH-immunopositive glomus cells in each experimental group (Fig. 4B). GI values in DBH-immunopositive glomus cells were significantly higher in rats exposed to hypoxia for 12 hr than that of control rats and rats exposed to hypoxia for 6 hr (p<0.05). GI values in the DBH-immunopositive glomus cells in the rats exposed to hypoxia for 18 and 24 hr were significantly lower than that of rats exposed to hypoxia for 12 hr (p<0.05). The GI value in DBH-immunopositive glomus cells in control rats was 18.2 ± 6.1, and values in rats exposed to hypoxia for 6, 12, 18, and 24 hr were 15.4 ± 3.0, 41.3 ± 11.4, 24.6 ± 7.6, and 24.4 ± 10.0, respectively.
Discussion
This study reveals for the first time that immunoreactivity for DBH is transiently increased in glomus cells of rat CBs by short-term hypoxia. The results of the present study show that DBH increases at a time point of 12 hr in glomus cells after rats are exposed to hypoxia and then decreases. Through immunoblotting, it has been reported that the level of DBH expression was transiently increased after 3 to 7 days in rat CBs during long-term hypoxic exposure (10% O2) (Hui et al. 2003). These results indicate that DBH is transiently increased in glomus cells under both short-term and long-term hypoxia. From the present study, it is suggested that NA biosynthesis is transiently facilitated in glomus cells at a time point of 12 hr under a short-term hypoxic condition. It has been reported that NA caused inhibition of Ca2+ currents in glomus cells (Almaraz et al. 1997; Overholt and Prabhakar 1999). Because an increase in intracellular Ca2+ concentrations in glomus cells leads to secretion of excitatory neurotransmitters (Buttigieg and Nurse 2004; Shirahata et al. 2007), it has been suggested that NA plays a role in the inhibition of excitatory neurotransmitter release from glomus cells, together with dopamine, and probably in inhibitory regulation of carotid sinus nerve discharge during hypoxia. In humans, it has been reported that minute ventilation increased from the start of isocapnic hypoxic exposure, decreased at a time point of 12 hr, and then increased again (Tansley et al. 1998). Given the time course of changes in minute ventilation, after the start of hypoxic exposure, it seems that dopamine in glomus cells is used for inhibitory regulation of enhanced carotid sinus nerve activity, and NA is also used to reinforce inhibitory regulation by dopamine. At the time point of 12 hr, it seems that NA biosynthesis is increased in glomus cells, and the enhanced carotid sinus nerve activity and ventilation receive inhibitory regulation by newly synthesized NA in addition to dopamine. After that, it appears that DBH is decreased, and thereby newly synthesized NA is reduced, perhaps to avoid excessive inhibition due to NA. Thus, it appears that NA from glomus cells contributes to the initial inhibitory regulation of carotid sinus nerve activity at an early stage of hypoxia.
In contrast to DBH, we reported that immunoreactivity for tyrosine hydroxylase was increased in CB glomus cells of rats exposed to hypoxia for 12 hr and that these increased levels were maintained at 18 and 24 hr (Kato et al. 2010). Hypoxia-inducible factor 1 (HIF-1) is known to be one of the major transcriptional factors associated with hypoxia (Powell and Fu 2008). It has been reported that immunoreactivity for HIF-1α was enhanced in the nuclei of CB glomus cells of rats exposed to hypoxia for 1 day (Tipoe and Fung 2003). Because it is known that the promoter region of the tyrosine hydroxylase gene has a hypoxia-response element (HRE) (Paulding et al. 2002), the enhanced expression of tyrosine hydroxylase would be maintained by increased HIF-1 in CB glomus cells during short-term hypoxic exposure. On the other hand, it is known that HRE is not present in the promoter region of DBH (Sabban and Kvetnanský 2001), suggesting that HIF-1 is not responsible for the transcription of DBH mRNA. Alternatively, it is known that cyclic AMP-response element (CRE) is contained in the promoter region of the DBH gene (Sabban and Kvetnanský 2001). It is suggested that CRE is essential for the transcription of the DBH gene, identified through the use of human neuroblastoma SK-N-BE(2)C (Ishiguro et al. 1993) and SK-N-SH-TFM cell lines (Lamouroux et al. 1993). Therefore, it is considered that DBH expression in the present study is regulated by cyclic AMP-response element binding protein (CREB), via CRE. Actually, it was confirmed by immunohistochemistry that hypoxic exposure to rats for 9 min increased phosphorylated CREB in the nucleus of glomus cells (Wang and Bisgard 2002). Using rat pheochromocytoma cells (PC12), Beitner-Johnson and Millhorn (1998) reported the time course of phosphorylated CREB expression during short-term hypoxia by immunoblotting. They observed that the increase in phosphorylated CREB in the cells peaked at 6 hr, followed by a small decrease at 24 hr compared with 6 hr. In part, this time course of phosphorylated CREB corresponds to that of DBH in the present study, although there is a difference in cell type. Thus, it could be possible that the difference in the time course of expression between DBH and tyrosine hydroxylase is due to differences in the transcriptional factors responsible for the expression of each enzyme.
It has been reported that glomus cells are divided into cell subpopulations based on the size of dense-cored vesicles and/or innervations (McDonald and Mitchell 1975; Hansen 1981) and α-bungarotoxin binding affinity (Chen and Yates 1984). In the present study, both DBH-immunopositive glomus cells and DBH-immunonegative glomus cells were observed in all experimental groups based on GI values. Concerning DBH immunoreactivity in glomus cells, another study also reported that both DBH-immunopositive glomus cells and DBH-immunonegative glomus cells were observed in the CB of rats at all stages during 0 to 10 weeks (Oomori et al. 2002). Therefore, it may be that there are two types of glomus cells: one expresses DBH to synthesize NA, and the other does not; although, it is unclear whether the presence or absence of DBH expression is associated with the cell subpopulation previously reported.
Within the CB, it is known that NA is released not only from glomus cells but also from sympathetic nerve endings innervating the CB (Almaraz et al. 1997). In the present study, DBH immunoreactivity was observed in varicosities around blood vessels. It was reported that electrical stimulation of the preganglionic sympathetic trunk caused reductions in blood flow within the CB (O’Regan 1981). This finding suggests that NA from sympathetic nerve endings could regulate blood flow within the CB. In the present study, there were no obvious differences in DBH immunoreactive varicosities within the CB between all experimental groups. By means of immunohistochemistry, it was reported that the density of substance P, calcitonin gene-related peptide, and vasoactive intestinal polypeptide-immunoreactive varicose nerve fibers was increased in the rat CB under long-term hypoxic conditions for 2 weeks or more, except for neuropeptide Y (Kusakabe et al. 1998, 2003). Therefore, the increase may be observed in DBH-immunoreactive varicose nerve fibers within the CB when the duration of hypoxic exposure is prolonged.
In conclusion, the present study demonstrates that the immunoreactivity for DBH is transiently enhanced in glomus cells of rat CB by short-term hypoxia. This finding suggests that NA biosynthesis is transiently facilitated in glomus cells at an early stage of hypoxia.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This study was partly supported by grants-in aid from the Japan Society for the Promotion of Science to KK (No. 24.419) and YY (No. 22580330).
References
- Almaraz L, Pérez-García MT, Gómez-Nino A, González C. 1997. Mechanisms of α2-adrenoceptor-mediated inhibition in rabbit carotid body. Am J Physiol. 272:628–637 [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Millhorn DE. 1998. Hypoxia induces phosphorylation of the cyclic AMP response element-binding protein by a novel signaling mechanism. J Biol Chem. 273:19834–19839 [DOI] [PubMed] [Google Scholar]
- Benot AR, López-Barneo J. 1990. Feedback inhibition of Ca2+ currents by dopamine in glomus cells of the carotid body. Eur J Neurosci. 2:809–812 [DOI] [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. 2004. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 322:82–87 [DOI] [PubMed] [Google Scholar]
- Chen IL, Yates RD. 1984. Two types of glomus cell in the rat carotid body as revealed by alpha-bungarotoxin binding. J Neurocytol. 13:281–302 [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. 1994. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 74:829–898 [DOI] [PubMed] [Google Scholar]
- Hanbauer I, Karoum F, Hellstrom S, Lahiri S. 1981. Effects of hypoxia lasting up to one month on the catecholamine content in rat carotid body. Neuroscience. 6:81–86 [DOI] [PubMed] [Google Scholar]
- Hansen JT. 1981. Chemoreceptor nerve and type A glomus cell activity following hypoxia, hypercapnia, or anoxia: a morphological study in the rat carotid body. J Ultrastruct Res. 77:189–198 [DOI] [PubMed] [Google Scholar]
- Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, Guo SZ, Sachleben LR, Haycock JW, Gozal D, et al. 2003. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension. 42:1130–1136 [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Kim KT, Joh TH, Kim KS. 1993. Neuron-specific expression of the human dopamine beta-hydroxylase gene requires both the cAMP-response element and a silencer region. J Biol Chem. 268:17987–17994 [PubMed] [Google Scholar]
- Kato K, Yamaguchi-Yamada M, Yamamoto Y. 2010. Short-term hypoxia increases tyrosine hydroxylase immunoreactivity in rat carotid body. J Histochem Cytochem. 58:839–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou YR, Ernsberger P, Cragg PA, Cherniack NS, Prabhakar NR. 1991. Role of α2-adrenergic receptors in the carotid body response to isocapnic hypoxia. Respir Physiol. 83:353–364 [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Hayashida Y, Matsuda H, Gono Y, Powell FL, Ellisman MH, Kawakami T, Takenaka T. 1998. Hypoxic adaptation of the peptidergic innervation in the rat carotid body. Brain Res. 806:165–174 [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Hirakawa H, Matsuda H, Kawakami T, Takenaka T, Hayashida Y. 2003. Peptidergic innervation in the rat carotid body after 2, 4, and 8 weeks of hypocapnic hypoxic exposure. Histol Histopathol. 18:409–418 [DOI] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. 2006. Oxygen sensing in the body. Prog Biophys Mol Biol. 91:249–286 [DOI] [PubMed] [Google Scholar]
- Lamouroux A, Houhou L, Biguet NF, Serck-Hanssen G, Guibert B, Icard-Liepkalns C, Mallet J. 1993. Analysis of the human dopamine beta-hydroxylase promoter: transcriptional induction by cyclic AMP. J Neurochem. 60:364–367 [DOI] [PubMed] [Google Scholar]
- McDonald DM, Mitchell RA. 1975. The innervations of glomus cells, ganglion cells and blood vessels in the rat carotid body: a quantitative ultrastructural analysis. J Neurocytol. 4:177–230 [Google Scholar]
- Nurse CA. 2005. Neurotransmission and neuromodulation in the chemosensory carotid body. Auton Neurosci. 120:1–9 [DOI] [PubMed] [Google Scholar]
- Olson EB, Vidruk EH, McCrimmon DR, Dempsey JA. 1983. Monoamine neurotransmitter metabolism during acclimatization to hypoxia in rats. Respir Physiol. 54:79–96 [DOI] [PubMed] [Google Scholar]
- Oomori Y, Murabayashi H, Ishikawa K, Miyakawa K, Nakaya K, Tanaka H. 2002. Neuropeptide Y- and catecholamine-synthesizing enzymes: immunoreactivities in the rat carotid body during postnatal development. Anat Embryol (Berl). 206:37–47 [DOI] [PubMed] [Google Scholar]
- O’Regan RG. 1981. Responses of carotid body chemosensory activity and blood flow to stimulation of sympathetic nerves in the cat. J Physiol. 315:81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholt JL, Prabhakar NR. 1999. Norepinephrine inhibits a toxin resistant Ca2+ current in carotid body glomus cells: evidence for a direct G protein mechanism. J Neurophysiol. 81:225–233 [DOI] [PubMed] [Google Scholar]
- Paulding WR, Schnell PO, Bauer AL, Striet JB, Nash JA, Kuznetsova AV, Czyzyk-Krzeska MF. 2002. Regulation of gene expression for neurotransmitters during adaptation to hypoxia in oxygen-sensitive neuroendocrine cells. Microsc Res Tech. 59:178–187 [DOI] [PubMed] [Google Scholar]
- Pequignot JM, Cottet-Emard JM, Dalmaz Y, Peyrin L. 1987. Dopamine and norepinephrine dynamics in rat carotid body during long-term hypoxia. J Auton Nerv Syst. 21:9–14 [DOI] [PubMed] [Google Scholar]
- Powell FL, Fu Z. 2008. HIF-1 and ventilatory acclimatization to chronic hypoxia. Respir Physiol Neurobiol. 164:282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. 2006. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 91:17–23 [DOI] [PubMed] [Google Scholar]
- Sabban EL, Kvetnanský R. 2001. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 24:91–98 [DOI] [PubMed] [Google Scholar]
- Shirahata M, Balbir A, Otsubo T, Fitzgerald RS. 2007. Role of acetylcholine in neurotransmission of the carotid body. Respir Physiol Neurobiol. 157:93–105 [DOI] [PubMed] [Google Scholar]
- Tansley JG, Fatemian M, Howard LS, Poulin MJ, Robbins PA. 1998. Changes in respiratory control during and after 48 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol. 85:2125–2134 [DOI] [PubMed] [Google Scholar]
- Tipoe GL, Fung ML. 2003. Expression of HIF-1α, VEGF and VEGF receptors in the carotid body of chronically hypoxic rat. Respir Physiol Neurobiol. 138:143–154 [DOI] [PubMed] [Google Scholar]
- Wakai J, Kizaki K, Yamaguchi-Yamada M, Yamamoto Y. 2010. Differences in tyrosine hydroxylase expression after short-term hypoxia, hypercapnia or hypercapnic hypoxia in rat carotid body. Respir Physiol Neurobiol. 173:95–100 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Bisgard GE. 2002. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc Res Tech. 59:168–177 [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhong H, Vollmer C, Nurse CA. 2000. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol. 525:143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]