Abstract
Phloroglucinol (PG) is a phenolic compound isolated from Ecklonia cava, a brown algae abundant on Jeju island, Korea. Previous reports have suggested that PG exerts antioxidative and cytoprotective effects against oxidative stress. In this study, we confirmed that PG protected against small intestinal damage caused by ionizing radiation, and we investigated its protective mechanism in detail. Regeneration of intestinal crypts in the PG-treated irradiated group was significantly promoted compared with that in irradiated controls. The expression level of proapoptotic molecules such as p53, Bax, and Bak in the small intestine was downregulated and that of antiapoptotic molecules such as Bcl-2 and Bcl-XS/L was augmented in the PG-treated group. On histological observation of the small intestine, PG inhibited the immunoreactivity of p53, Bax, and Bak and increased that of Bcl-2 and Bcl-XS/L. These results demonstrate the protective mechanisms of PG in mice against intestinal damage from ionizing radiation, providing the benefit of raising the apoptosis threshold of jejunal crypt cells.
Keywords: phloroglucinol, apoptosis, crypt cell, ionizing radiation, immunohistochemistry
Radiation therapy is the medical use of ionizing radiation as a part of cancer treatment to control malignant cell proliferation and may be used as the primary therapy. As a therapeutic treatment, it provides survival and curative benefit, and as a palliative treatment, it provides local disease control or symptomatic relief where cure is not possible. Unfortunately, the radiation aimed at diseased cells has an adverse effect on surrounding healthy tissues (Weiss 1997). In the case of whole-body irradiation (WBI), intestinal crypts are particularly susceptible to the acute effects of radiation (Waselenko et al. 2004). Immediately after irradiation, intestinal crypt cells undergo apoptosis, and stem cells cannot recover as rapidly as necessary to restore intestinal villi. As a consequence, the heights of small intestinal villi are reduced, and blunting as well as subsequent functional incapacity occurs immediately after ionizing radiation (Somosy et al. 2002). These intestinal damages following an exposure to ionizing radiation are collectively called gastrointestinal (GI) syndrome.
It is essential to identify efficient, safe, and affordable radioprotectors for the prevention of radiation-induced damage in intestinal cells. Recently, several studies have been carried out to discover radioprotective agents from natural resources such as fruit, vegetables, and medicinal plants (Park and Pezzuto 2002; Stan et al. 2008), because of the toxic side effects of many synthetic compounds on human health. In particular, the Ecklonia species have been reported to exhibit radical scavenging activity (Kang HS et al. 2004), anticancer activity (Thomas and Kim 2011), and hematopoietic recovery activity (Park, Ahn, et al. 2008), and phlorotannin components were identified to be responsible for the biological activity. Phloroglucinol (1,3,5-trihydroxybenzene; PG), a monomeric building block of phlorotannin and a phenolic compound known to derive only from brown algae, has cytoprotective effects on oxidative stress–induced cell damage through catalase activation (Kang KA et al. 2010) and radioprotective effects on intestinal crypt cells after whole-body gamma-irradiation (Moon et al. 2008). Moon et al. (2008) suggested that PG protects mice from radiation damage by promoting the survival of jejunal crypts through the inhibition of apoptosis, but there has been no report on the molecular mechanisms of its protective effects in the small intestine of the mouse after ionizing irradiation. The primary objective of this study is to establish the molecular mechanism of the cytoprotective effects of PG following radiation by characterizing the difference in cellular localization and expression level of apoptosis-related proteins (P53, Bax, Bak, Bcl-2, and Bcl-XS/L) in intestinal crypt and epithelial cells using immunohistochemistry.
This study demonstrates that PG treatment enhances the protection and regeneration of small intestinal stem cells from damage caused by an exposure to lethal irradiation via inhibiting apoptosis.
Materials and Methods
Preparation and Treatment of Phloroglucinol
As described in our previous report (Ahn et al. 2007; Kang KA et al. 2010), PG was obtained from Dr. Nam Ho Lee (Jeju National University, Jeju, Korea). PG dissolved in phosphate-buffered saline (PBS) (pH 7.4) was injected intraperitoneally (IP) twice into mice at a dose of 10-mg/kg body weight first at 18 hr and then again at 2 hr before irradiation. An additional two groups of mice, sham-irradiated mice and irradiated mice injected IP with PBS, were treated as controls.
Irradiation of Animals with 60Co γ-ray
Six- to 8-week-old C57BL/6 mice (Orientbio; Sungnam, Korea) weighing 18 to 25 g were used in this study. All mice were acclimated upon arrival and housed in conventional animal facilities with an NIH-07–approved diet and water ad libitum at a constant temperature (23 ± 1C) and humidity (50 ± 10%), accredited by the Institutional Ethical Committee of Jeju National University. Mice were randomly separated into three groups (three mice per group): a sham-irradiated group, an irradiated control group, and a PG plus irradiation group. Each mouse was placed individually in a close-fitting Perspex box (3 × 3 × 11 cm) and given a single dose of 7 Gy (gray; SI unit) WBI at a dose rate of 1.5 Gy/min from a source-surface distance of 150 cm with a 60Co irradiator (Theraton-780 Teletherapy unit, Applied Radiological Science Institute, Jeju National University), as previously reported (Goel et al. 2007).
Microcolony Survival Assay
To identify whether PG could protect intestinal stem cells (crypt cells) from damage by irradiation, hematoxylin and eosin (H&E) staining was performed. Small intestines were harvested from mice at 8.5 days after 7 Gy WBI and fixed in 10% buffered formalin. After fixation, tissues were vertically embedded in paraplast wax to prepare 5-µm sections for H&E staining. Following the intestinal crypt microcolony assay technique described by Withers and Elkind (1970), the number of regenerating crypts per circumference of the small intestine section was counted using a light microscope, and 50 crypt cells per intestinal section were recorded.
Western Blotting
To evaluate the molecular mechanism by which PG protects radiosensitive cells from irradiation-induced apoptosis, expression patterns of various proteins associated with apoptosis were studied in small intestines of mice at 1 day after 7 Gy WBI. Tissue lysates from small intestines were prepared, and cellular proteins (60 µg/well) were loaded onto 10% to 15% SDS-PAGE gels and immunoblotted onto a nitrocellulose membrane (Bio-Rad; Hercules, CA). The membranes were incubated with antibodies against p53 (1:1000 dilutions; Calbiochem, Darmstadt, Germany), Bax (1:500 dilutions; Cell Signaling, Beverly, MA), Bak (1:500 dilutions; Cell Signaling), Bcl-2 (1:1000 dilutions; Santa Cruz Biotechnology; Santa Cruz, CA), Bcl-XS/L (1:500 dilutions; Santa Cruz Biotechnology), and β-actin (1:1000 dilutions; Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase (HRP)–conjugated anti-rabbit or anti-mouse IgG (1:2000 dilutions; Santa Cruz, Biotechnology). The blots were developed by enhanced chemiluminescence reagents (iNtRON; Sungnam, Korea) according to the manufacturer’s instructions, and band densities were analyzed with MCID Analysis Evaluation 7.0 (Imaging Research; St. Catharines, Canada).
Immunohistochemical Staining
For immunohistochemical localization of apoptosis regulatory proteins, tissue sections were incubated with normal horse serum and reacted with antibodies against p53 (1:200 dilutions; Calbiochem), Bax (1:500 dilution), Bak (1:500 dilution), Bcl-2 (1:500 dilutions), and Bcl-XS/L (1:500 dilution) for 1 hr. Sections were then incubated with a biotinylated anti-mouse IgG secondary antibody (1:100 dilutions, Vector; Burlingame, CA), and HRP-labeled VECTASTAIN ABC Kit (Vector). HRP-binding sites were detected with 3,3′–diaminobenzidine (DAB; Vector) and were counterstained with H&E. Semiquantitative analysis was performed by using Image J 1.38× software. Two to three sections per animal were examined under a low-magnification microscope, and three lesions showing the most representative staining were analyzed under higher magnification to count positive cells.
Statistical Analyses
Results are reported as the mean ± standard error (SE). All results represent three separate experiments. Results were analyzed using one-way analysis of variance (ANOVA) followed by Student’s t-test, and p<0.05 was considered statistically significant.
Results
PG Enhances Regeneration and Survival of Intestinal Crypts
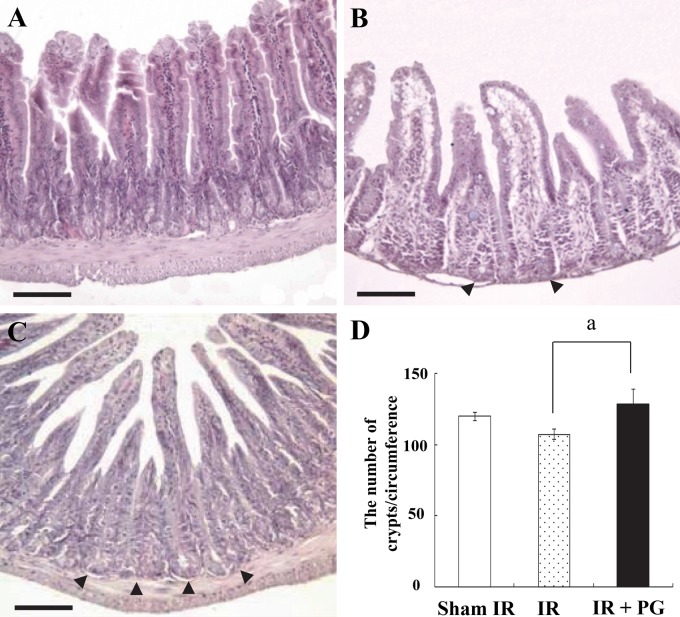
Because the gastrointestinal system is a major site of cell death induced by ionizing radiation, and because intestinal crypt cells are one of the most sensitive cells to irradiation, we investigated the potential of PG to protect intestinal crypt cells from radiation-induced apoptosis. As shown in Figure 1A, sham-irradiated controls exhibited a normal appearance, number and length of the mucosal villi, along with the intact organization of the deep crypt layer. However, the exposure to irradiation resulted in severe mucosal damage, as evidenced by the marked shortening in the length of villi as well as their fusion (Fig. 1B). Pretreatment with PG improved these changes to the mucosal villi: it remarkably restored villi length and appearance (Fig. 1C). Numbers of intestinal crypts per circumference in sham-irradiated, irradiated control, and PG plus irradiated groups were 119.9 ± 3.1, 107.6 ± 3.8, and 128.4 ± 28.6, respectively, corresponding to a 19.3% enlargement following PG treatment as compared with irradiated controls (Fig. 1D, p<0.05). These results show that PG enhanced the crypt cell survival by reducing intestinal stem cell damage after an exposure to irradiation.
Figure 1.
Phloroglucinol (PG) protects small intestinal crypt cells from gamma ray irradiation-induced damage in mice. Mice were sacrificed and small intestines were obtained at 8.5 days after 7 Gy irradiation. (A) Sham-irradiated (IR) mice, (B) 7 Gy–irradiated mice, (C) PG (10 mg/kg body weight, intraperitoneally) plus 7 Gy-irradiated mice (bars = 120 µm). (D) The number of regenerating crypts per circumference of jejunal cross section from each group is presented in the columns. Values represent the mean ± SE (ap<0.05).
PG Modulated the Expression Levels of Apoptosis-Related Proteins in Small Intestine
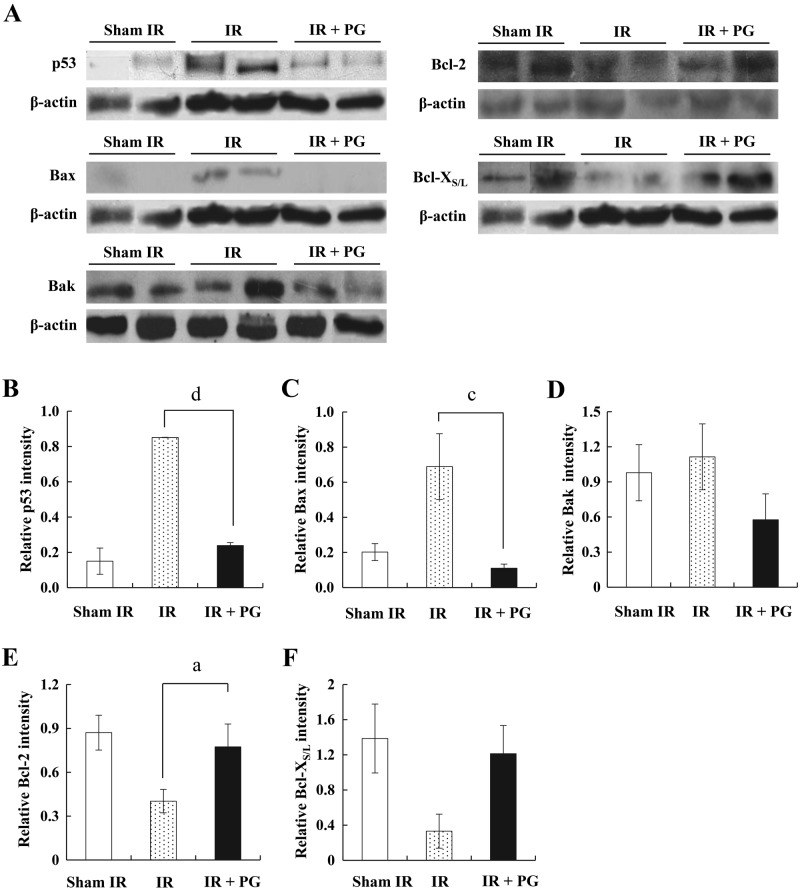
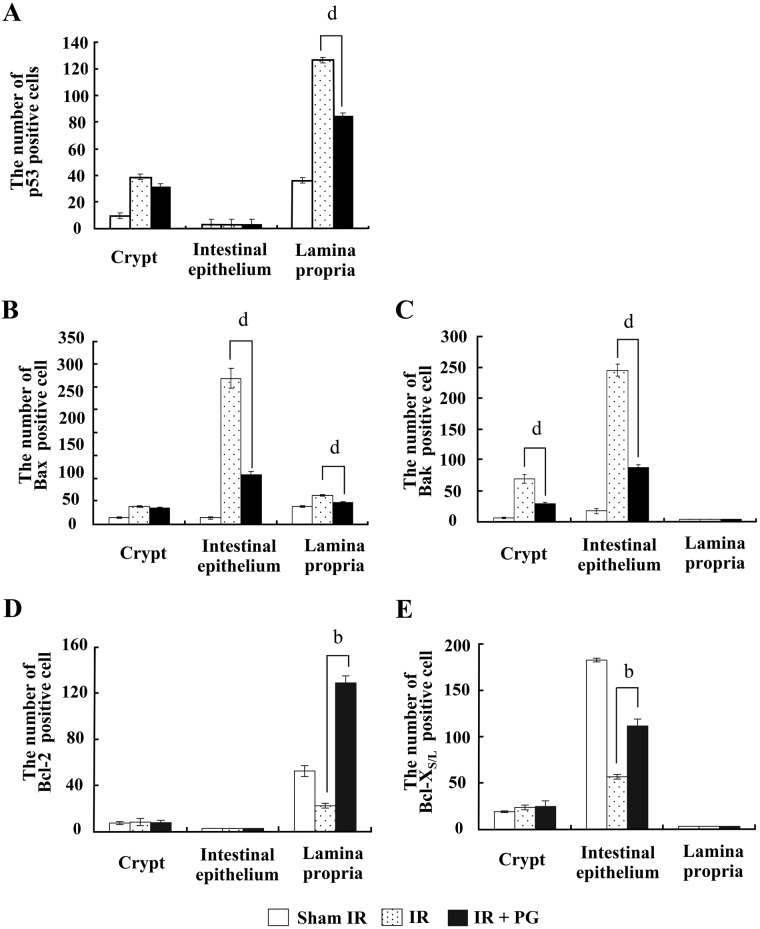
To understand the mechanism by which PG inhibits the apoptosis of jejunal crypt cells, we investigated the potency of PG to modulate apoptosis-related molecules (i.e., p53, Bax, Bak, Bcl-2, and Bcl-XS/L) in the small intestine. As shown in Figure 2A, an exposure to irradiation considerably increased the expression of proapoptotic proteins, such as p53, Bax, and Bak, whereas it decreased the expression of antiapoptotic Bcl-2 family proteins compared with that of sham-irradiated controls. However, PG treatment apparently curtailed the increase of p53, Bax, and Bak expression levels and the decrease of Bcl-2 and Bcl-XS/L expression levels. The expression level of p53 increased more than 5-fold in the irradiated controls compared with sham-irradiated controls but reduced 71.3% in the PG plus irradiated group against the irradiated controls (Fig. 2B; p<0.005). Bax protein expression in the PG-treated mice was similar, exhibiting a 88.6% decrease in the PG plus irradiated group as compared with the irradiated controls (Fig. 2C; p<0.05). Although a similar expression pattern was observed for Bak, no significant difference existed between PG-treated mice and irradiated controls (Fig. 2D). In contrast, Bcl-2 expression augmented about 2-fold in the PG plus irradiated group compared with irradiated controls, whereas irradiated controls presented a 53.7% decrease of Bcl-2 protein expression compared with sham-irradiated controls (Fig. 2E; p<0.05). In addition, Bcl-XS/L expression in the PG-treated mice was increased marginally compared with the irradiated controls, with no significant statistical difference observed (Fig. 2F). The protein expression levels were normalized against the level of β-actin. These results suggest that PG raised the apoptosis threshold in jejunal stem cells by modulating the expression levels of apoptosis-related molecules in gamma ray–irradiated mice.
Figure 2.
Western blot analysis of p53, Bax, Bak, Bcl-2, and Bcl-XS/L expression modulated by phloroglucinol (PG) in small intestines of mice 24 hr after gamma ray irradiation. (A) Expression levels of p53, Bax, Bak, Bcl-2, and Bcl-XS/L protein in small intestines of sham-irradiated (IR) mice (lanes 1), 7 Gy–irradiated mice (lanes 2 and 3), and 7 Gy–irradiated plus PG-treated mice (lanes 4 and 5) were analyzed by Western blot analysis. (B–F) Densitometric analysis of each molecule was evaluated (a,cp<0.05; dp<0.005). β-Actin expression was used to demonstrate that equal amounts of protein extracts were loaded.
PG Attenuated the Immunohistochemical Localization and Intensity of Proapoptosis-Related Proteins in the Intestine
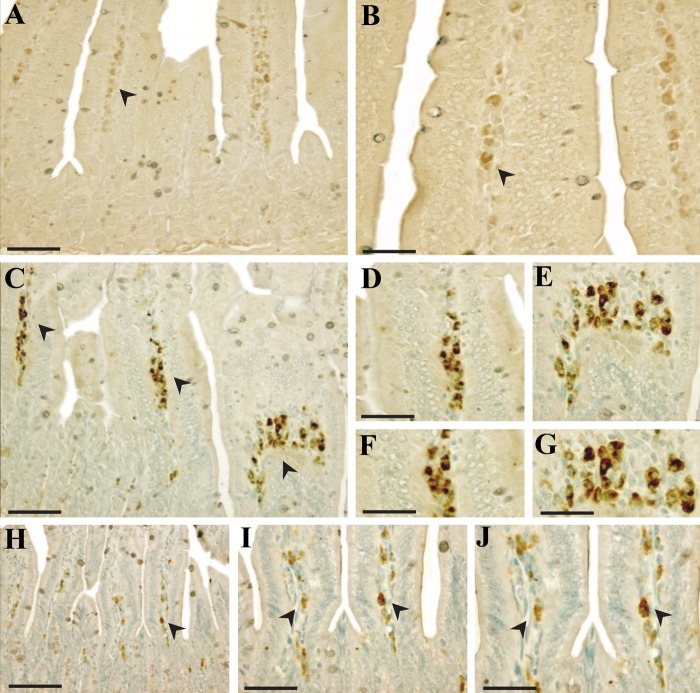
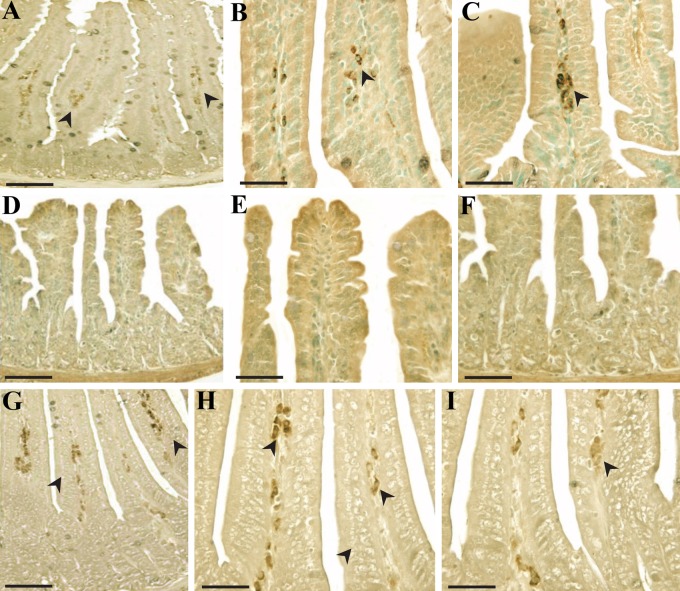
To pinpoint the apoptosis inhibition mechanism of PG, we investigated the localization of these regulatory proteins of apoptosis (p53, Bax, Bak, Bcl-2, and Bcl-XS/L) in intestines under PG-treated and untreated conditions using immunohistochemistry. As shown in Figures 3, 4, and 5, the immunoreactivities of p53, Bax, and Bak were markedly increased in small intestines of irradiated controls compared with sham-irradiated controls, but decreased following PG treatment. After an exposure to radiation, p53 immunoreactivity was revealed not only in the cytoplasm but also in the nucleus of intestinal cells, and immunoreactive p53-positive cells were highly expressed in the lamina propria (Fig. 3C–G) and crypt (data not shown) compared with the sham-irradiated control (Fig. 3A, B). The p53-positive cells in the small intestine after irradiation have been identified as stem cells near apoptotic cells in the crypt (Potten and Grant 1998). Coincident with this report, p53 immunoreactivity was enhanced in the crypt of irradiated controls compared with sham-irradiated mice (p=0.001), whereas PG treatment did not significantly affect p53 expression in the crypt. In contrast, PG treatment dramatically decreased the immunoreactivity of p53 in the lamina propria (Fig. 3H–J and Fig. 6A; p<0.005). In addition, compared with sham-irradiated controls (Fig. 4A, B), Bax-positive cells were significantly increased in the irradiated controls, and the distribution of Bax was mostly in the cytoplasmic and perinuclear regions of cells in the intestinal epithelium (Fig. 4C) and lamina propria (Fig. 4D). Coupled with the reduced p53 immunoreactivity, the number of Bax-positive cells and the intensity of Bax in the PG-treated group was markedly decreased as compared with that of the irradiated controls (Fig. 4E, F and Fig. 6B; p<0.005). Accompanying these changes, more intense Bak immunoreactivity was exhibited in the cytoplasm of the intestinal epithelium (Fig. 5C, D) and crypts (Fig. 5E, F) in irradiated controls than in sham-irradiated controls (Fig. 5A, B). Bak expression in crypts was not restricted to the intestinal stem cells located at cell positions 3 to 5 in crypts of the small intestine (Watson and Pritchard 2000); rather, Bak expression was found across all crypt cells, including Paneth cells. Pretreatment with PG affected the immunoreactivity of Bak expressed in the intestinal epithelium (Fig. 5G) and crypts (Fig. 5H, I), leading to apparent reduction in expression (Fig. 6C; p<0.005). These results indicate that the decreased expression of p53, Bax, and Bak after PG treatment was closely related to the reduced number of apoptotic cells in the injured small intestines.
Figure 3.
p53 immunoreactivity in small intestines of mice 24 hr after gamma ray irradiation. (A, B) Sham irradiation, (C–G) 7 Gy irradiation, and (H–J) 7 Gy irradiation plus phloroglucinol (10 mg/kg) treatment. Bars = 60 µm (A–C, H–J) and 30 µm (D–G).
Figure 4.
Immunohistochemical reactivity and localization of Bax in small intestines of mice 24 hr after gamma ray irradiation. (A, B) Sham irradiation, (C, D) 7 Gy irradiation, and (E, F) 7 Gy irradiation plus phloroglucinol (10 mg/kg) treatment. Bars = 60 µm (A, C, E) and 30 µm (B, D, F).
Figure 5.
Immunohistochemical patterns of Bak in small intestines of mice obtained 24 hr after gamma ray irradiation. (A, B) Sham irradiation, (C–F) 7 Gy irradiation, and (G–I) 7 Gy irradiation plus phloroglucinol (10 mg/kg) treatment. Bars = 60 µm (A, C) and 30 µm (B, D, E–I).
Figure 6.
Quantitative analysis of immunohistochemistry for cells positive for p53, Bax, Bak, Bcl-2, and Bcl-XS/L in intestinal sections (b,dp<0.005). Cells were counted as described in Materials and Methods. Results are the mean and standard error for each experimental group.
PG Augmented the Immunohistochemical Localization and Intensity of Antiapoptosis-Related Proteins in Intestine
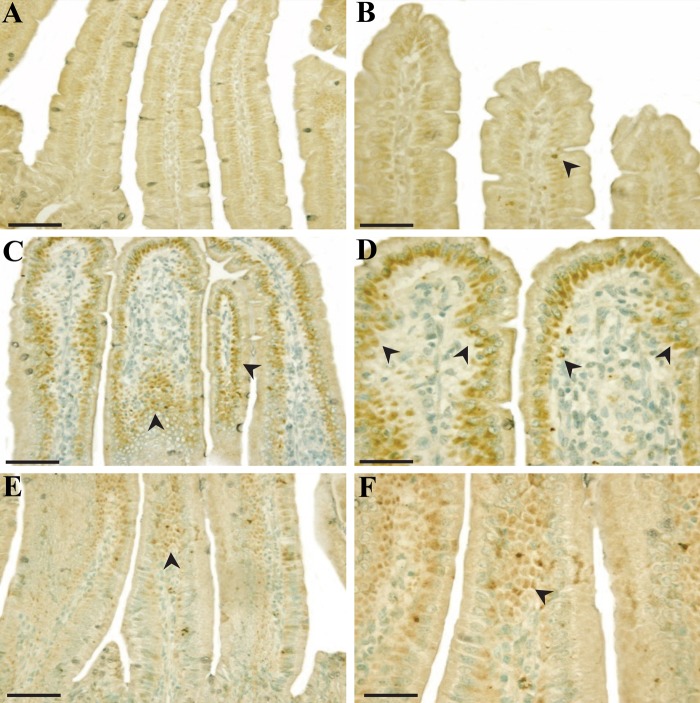
Bcl-2 and Bcl-XS/L immunoreactive stainings were found only in the cytoplasm of intestinal cells. Merritt et al. (1995) reported Bcl-2 was absent in crypts of the small intestine at cell positions 4 to 5 (the stem cell region), and the removal of the bcl-2 gene had no effect on the level of radiation-induced apoptosis in the small intestine. Coincident with this report, Bcl-2 immunoreactivity was weak in crypts (Fig. 7A), and notable positive staining for Bcl-2 was easily demarcated in the laminar propria (Fig. 7B, C). Besides, Bcl-XS/L–positive cells were preferentially present in intestinal epithelium, with few present in crypts (Fig. 8A–C). Furthermore, staining intensities of antiapoptotic Bcl-2 and Bcl-XS/L were weak in the laminar propria (Fig. 7D–F) and in the intestinal epithelium (Figure 8D–F) in irradiated controls compared with that of sham-irradiated controls. However, PG treatment significantly increased the intensities of Bcl-2 (Fig. 7G–I) and Bcl-XS/L (Fig. 8G–I) in the lamina propria (Fig. 6D; p<0.005) and in the intestinal epithelium (Fig. 6E; p<0.005). Thus, the overexpression of Bcl-2 and Bcl-XS/L in immunocytes in the laminar propria and intestinal epithelium after PG treatment might be clearly involved in the repair of gastrointestinal cells. These results suggest that PG presumably inhibited the apoptosis of the small intestine by regulating the expression of proapoptotic p53, Bax, and Bak and antiapoptotic Bcl-2 and Bcl-XS/L after gamma ray irradiation.
Figure 7.
Immunoreactivity of Bcl-2 in small intestines of mice 24 hr after gamma ray irradiation. (A–C) Sham irradiation, (D–F) 7 Gy irradiation, and (G–I) 7 Gy irradiation plus phloroglucinol (10 mg/kg) treatment. Bars = 60 µm (D, F, G) and 30 µm (A–C, E, H, I).
Figure 8.
Immunohistochemical reactivity and localization of Bcl-XS/L in small intestines of mice 24 hr after gamma ray irradiation. (A–C) Sham irradiation, (D–F) 7 Gy irradiation, (G–I) 7 Gy irradiation plus phloroglucinol (10 mg/kg) treatment. Bars = 60 µm (A, D, G) and 30 µm (B, C, E, F, H, I).
Discussion
The small intestinal crypt stem cells are particularly susceptible to ionizing radiation due to their high rate of proliferation (Potten and Grant 1998) and, infrequently, several chemotherapy or radiotherapy agents can cause severe injury by destroying epithelial barriers of the intestine. Generally, the survival of stem cells and their ability to generate an effective level of competence are crucial to survival after WBI (Goel et al. 2007). Surviving crypt stem cells can populate to refill the number of viable crypts and multipotent crypt stem cells divide throughout life, giving rise to the epithelial lineages. Therefore, the crypt cells play essential functions in mucosal regeneration after radiation injury (Potten et al. 1995). In this study, pretreatment of mice with PG profoundly restored the villi length and the appearance of the intestine, and increased the number of intestinal crypt cells, indicating that PG protected intestinal crypt cells from damage caused by irradiation.
Recently, Moon et al. (2008) reported that PG has protective effects against radiation-induced damage in the intestine by estimating the survival of the jejunal crypt and the apoptotic fragmentation of intestinal cells using H&E staining. However, little is known about the protective capacity of PG in intestinal stem cells from radiation damage and its molecular mechanism in relation to the inhibition of apoptosis. It is well recognized that ionizing radiation that causes apoptosis in intestinal cells is largely due to the p53 proapoptotic molecule (Clarke et al. 1994), and gamma ray–irradiated p53 null mice have shown resistance to apoptosis in the small intestine (Merritt et al. 1997). In general, p53 is activated by single- and double-strand breaks (DSBs) from irradiation (Fei and El-Deiry 2003) and, subsequently, activated p53 stimulates Bax and Bak, proapoptotic members of the Bcl-2 family (Maiuri et al. 2007). Bax, normally present in the cytosol as a soluble monomeric protein, then translocates to the mitochondrial outer membrane in response to an apoptotic signal from p53 activation. Activated p53 also stimulates the proapoptotic mitochondrial membrane protein Bak. The interaction between p53 and Bax/Bak forms oligomeric complexes of Bax and Bak and accelerates the rate of apoptosis by releasing cytochrome c from mitochondria (van Delft and Huang 2006). Previous studies reported that Bax and Bak expression is upregulated during p53-dependent apoptosis (Miyashita and Reed 1995), and both induce apoptosis (Nataraj et al. 1995; Leu et al. 2004). In this study, we observed that the expression level and localization of p53, Bax, and Bak were significantly downregulated in the PG-treated irradiation group compared with irradiation controls. Previous reports demonstrated that p53 is primarily expressed in crypts of the small intestine after irradiation (Potten and Grant 1998), and a few apoptotic cells in villi are tissue-resident leukocytes (Schuller et al. 2007). On the other hand, Bax expression in the small intestine of normal mice is noted mainly along the epithelial lining, including absorptive enterocytes as well as goblet cells, and on crypt stem cells together with Paneth cells (Krajewski, Krajewska, Shabaik, Miyashita, et al. 1994). In the lamina propira, Bax is expressed on myocytes but not on fibroblasts and lymphocytes (Krajewski, Krajewska, Shabaik, Miyashita, et al. 1994). In the present study, p53 and Bax expression showed significant changes in not only crypt stem cells and intestinal epithelium but also in lamina propria after irradiation. Furthermore, Bak expression showed a significant change in the overall intestinal epithelial cells (IECs) and in crypt cells, including Paneth cells.
Furthermore, we examined the consequence of PG treatment on the localization of antiapoptotic members of Bcl-2 family proteins, Bcl-2 and Bcl-XS/L, which play an important role in determining the ultimate sensitivity or resistance of cells to various stimuli that induce apoptosis (Cory et al. 2003). In particular, Bcl-2 and Bcl-XS/L are the two most important antiapoptotic members of the Bcl-2 family and can antagonize apoptosis by preventing the signal transmission of proapoptotic genes (MacManus and Linnik 1997; van Delft and Huang 2006). The expression level and immunohistochemical localization of antiapoptotic molecules Bcl-2 and Bcl-XS/L were greater in PG-treated mice than in irradiated controls, which implies that antiapoptotic properties of Bcl-2 and Bcl-XS/L interrupted by ionizing radiation were restored by PG treatment. Indeed, Bcl-2 was reported to be preferentially expressed on the upper portion of the epithelial lining in the small intestine of normal mice (Krajewski, Krajewska, Shabaik, Miyashita, et al. 1994). However, we confirmed that Bcl-2 was expressed primarily on the lamina propria rather than on IECs, consistent with our previous report (Park, Lee, et al. 2008). Furthermore, although strong Bcl-XS/L immunoreactivity was found in absorptive epithelial cells and goblet cells, together with cells at the crypt base (Krajewski, Krajewska, Shabaik, Wang, et al. 1994), a large number of Bcl-XS/L–positive cells were detected in the epithelial lining rather than in the crypt in our study. In view of this disagreement, which was possibly caused by a difference in experimental conditions (e.g., portion of small intestine, dose of irradiation, and analysis time points), specific patterns of the apoptosis-related molecule expression in the lamina propria in the small intestine after irradiation need further clarification. Our results suggest that expression patterns of apoptosis-related molecules in irradiated small intestine can be profoundly influenced by PG treatment. The existence of differing staining patterns poses an intriguing question worthy of pursuit in future studies.
In conclusion, our results reveal that PG has a radioprotective capacity, and we propose that the beneficial effect is exerted by limiting the damage from radiation-induced apoptosis by blocking the activation of p53, Bax, and the Bak-dependent pathway and modulating the levels of Bcl-2 and Bcl-XS/L.
Acknowledgments
The authors thank T. H. Chung for editorial assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was a part of the project titled 2012 Jeju Sea Grant (D10900612H41000110), funded by the Ministry of Land, Transport and Maritime Affairs of Korea, and supported by the Basic Science Research Program (2011-0006016), funded by the Ministry of Education, Science and Technology of Korea.
References
- Ahn G-N, Kim K-N, Cha S-H, Song C-B, Lee J, Heo M-S, Yeo I-K, Lee N-H, Jee Y-H, Kim J-S, et al. 2007. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol. 226:71–79 [Google Scholar]
- Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH. 1994. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma- irradiation. Oncogene. 9:1767–1773 [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. 2003. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 22:8590–8607 [DOI] [PubMed] [Google Scholar]
- Fei P, El-Deiry WS. 2003. P53 and radiation responses. Oncogene. 22:5774–5783 [DOI] [PubMed] [Google Scholar]
- Goel HC, Prakash H, Ali A, Bala M. 2007. Podophyllum hexandrum modulates gamma radiation–induced immunosuppression in Balb/c mice: implications in radioprotection. Mol Cell Biochem. 295:93–103 [DOI] [PubMed] [Google Scholar]
- Kang HS, Chung HY, Kim JY, Son BW, Jung HA, Choi JS. 2004. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch Pharm Res. 27:194–198 [DOI] [PubMed] [Google Scholar]
- Kang KA, Zhang R, Chae S, Lee SJ, Kim J, Jeong J, Lee J, Shin T, Lee NH, Hyun JW. 2010. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation–induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem Biol Interact. 185:215–226 [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. 1994. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 145:1323–1336 [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Wang HG, Irie S, Fong L, Reed JC. 1994. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 54:5501–5507 [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. 2004. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 6:443–450 [DOI] [PubMed] [Google Scholar]
- MacManus JP, Linnik MD. 1997. Gene expression induced by cerebral ischemia: an apoptotic perspective. J Cereb Blood Flow Metab. 17:815–832 [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. 2007. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752 [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Allen TD, Potten CS, Hickman JA. 1997. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 14:2759–2766 [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Hickman JA. 1995. Differential expression of bcl-2 in intestinal epithelia: correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 108(Pt 6):2261–2271 [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 80:293–299 [DOI] [PubMed] [Google Scholar]
- Moon C, Kim SH, Kim JC, Hyun JW, Lee NH, Park JW, Shin T. 2008. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother Res. 22:238–242 [DOI] [PubMed] [Google Scholar]
- Nataraj AJ, Trent JC, Ananthaswamy HN. 1995. p53 gene mutations and photocarcinogenesis. Photochem Photobiol. 62:218–230 [DOI] [PubMed] [Google Scholar]
- Park E, Ahn GN, Lee NH, Kim JM, Yun JS, Hyun JW, Jeon YJ, Wie MB, Lee YJ, Park JW, et al. 2008. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 582:925–930 [DOI] [PubMed] [Google Scholar]
- Park E, Lee NH, Joo HG, Jee Y. 2008. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochem Biophys Res Commun. 372:792–797 [DOI] [PubMed] [Google Scholar]
- Park EJ, Pezzuto JM. 2002. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 21:231–255 [DOI] [PubMed] [Google Scholar]
- Potten CS, Grant HK. 1998. The relationship between ionizing radiation–induced apoptosis and stem cells in the small and large intestine. Br J Cancer. 78:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Owen G, Hewitt D, Chadwick CA, Hendry H, Lord BI, Woolford LB. 1995. Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut. 36:864–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller BW, Rogers AB, Cormier KS, Riley KJ, Binns PJ, Julius R, Hawthorne MF, Coderre JA. 2007. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. Int J Radiat Oncol Biol Phys. 68:205–210 [DOI] [PubMed] [Google Scholar]
- Somosy Z, Horvath G, Telbisz A, Rez G, Palfia Z. 2002. Morphological aspects of ionizing radiation response of small intestine. Micron. 33:167–178 [DOI] [PubMed] [Google Scholar]
- Stan SD, Kar S, Stoner GD, Singh SV. 2008. Bioactive food components and cancer risk reduction. J Cell Biochem. 104:339–356 [DOI] [PubMed] [Google Scholar]
- Thomas NV, Kim SK. 2011. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ Toxicol Pharmacol. 32:325–335 [DOI] [PubMed] [Google Scholar]
- van Delft MF, Huang DC. 2006. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 16:203–213 [DOI] [PubMed] [Google Scholar]
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, et al. 2004. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 140:1037–1051 [DOI] [PubMed] [Google Scholar]
- Watson AJ, Pritchard DM. 2000. Lessons from genetically engineered animal models, VII: apoptosis in intestinal epithelium: lessons from transgenic and knockout mice. Am J Physiol Gastrointest Liver Physiol. 278:G1–G5 [DOI] [PubMed] [Google Scholar]
- Weiss JF. 1997. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect. 105(Suppl 6):1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers HR, Elkind MM. 1970. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 17:261–267 [DOI] [PubMed] [Google Scholar]