Abstract
Background. Loss of visual function differs between immune-mediated optic neuropathies and is related to axonal loss in the optic nerve. This study investigated the diagnostic and prognostic value of a biomarker for neurodegeneration, the neurofilament heavy chain (NfH) in three immune-mediated optic neuropathies. Methods. A prospective, longitudinal study including patients with optic neuritis due to multiple sclerosis (MSON, n = 20), chronic relapsing inflammatory optic neuritis (CRION, n = 19), neuromyelitis optica (NMO, n = 9), and healthy controls (n = 28). Serum NfH-SMI35 levels were quantified by ELISA. Findings. Serum NfH-SMI35 levels were highest in patients with NMO (mean 0.79 ± 1.51 ng/mL) compared to patients with CRION (0.13 ± 0.16 ng/mL, P = 0.007), MSON (0.09 ± 0.09, P = 0.008), and healthy controls (0.01 ± 0.02 ng/mL, P = 0.001). High serum NfH-SMI35 levels were related to poor visual outcome. Conclusions. Blood NfH-SMI35 levels are of moderate diagnostic and more important prognostic value in immune-mediated optic neuropathies. We speculate that longitudinal blood NfH levels may help to identify particular disabling events in relapsing conditions.
1. Introduction
An established biomarker for neurodegeneration are body fluid neurofilament (Nf) levels [1–3]. Damage to human central nervous system initiates a proteolytic cascade which causes release of Nf from neurons and axons into the extracellular fluid (ECF) [4]. Next, Nf move from the ECF into the cerebrospinal fluid (CSF) from where they diffuse into the systemic blood circulation. Therefore quantification of Nf from either body fluid permits to estimate the amount of neuroaxonal damage caused.
Cerebrospinal Fluid levels (CSF) of the Nf heavy chain (NfH SMI35) were higher in patients with a Clinically Isolated Syndrome including optic neuritis compared to control subjects and to correlate with disease activity [5] A Japanese study found the CSF NfHSMI35 concentration to be higher in patients with NMO (mean 0.75 ng) compared with the levels found in multiple sclerosis (MS, 0.09 ng/mL) [6]. This data is consistent with a European study [7]. Using a different analytical method, pNfH levels were however found to be comparable between patients with MS, NMO, spinal cord infarction, and controls [8]. Notably, CSF pNfH levels in this paper were also virtually absent from other conditions with known extensive axonal damage and high CSF NfHSMI35 levels suggesting a preanalytical or analytical problem.
Blood Nf heavy chain (NfH) levels have been shown to be elevated in patients with acute optic neuritis compared with control subjects, and its level correlates inversely with visual loss and the retinal nerve fibre thickness as assessed by retinal optical coherence tomography (OCT) [9–11].
The clinical spectrum of autoimmune ON includes Neuromyelitis Optica (NMO, Devic disease) and disease occurring as part of an Aquaporin 4 antibody spectrum (AQP4+), as well as Chronic Relapsing Inflammatory Optic Neuropathy (CRION) [12, 13]. Optic neuritis occurring as part of a multiple sclerosis spectrum (MSON) may present in exactly the same way as that occurring as part of an AQP4+ spectrum, whereas the treatment required for the first is very different to that required for the second [14]. The timing of steroid treatment in optic neuritis caused by NMO and other immune mediated optic neuropathies has been shown to be critical in the prevention of permanent retinal nerve fibre loss [15]. It is therefore of great use to identify cases of NMO from other immune mediated optic neuropathies.
This study investigated the diagnostic and prognostic value of plasma NfHSMI35 levels for differentiating NMO from non-NMO optic neuritis on the hypothesis that axonal loss in the optic nerve may be greater in the former.
2. Methods
2.1. Patients
Consecutive patients presenting with optic neuritis (ON) and healthy control subjects were recruited from The National Hospital of Neurology and Neurosurgery, Queen Square, St. Thomas' Hospital, and Moorfields Eye Hospital, all based in London, UK. Patients were classified into ON in the context of multiple sclerosis (MSON), CRION, and NMO as described [16, 17]. In all patients a diagnosis of optic neuritis was made clinically as described [9]. We exclude patients in whom transient visual disturbances were due to Uhthoff's phenomenon.
Serum samples were obtained by antecubital venopuncture. All samples were collected and processed at room temperature in polypropylene tubes within two hours after venopuncture. Samples were spun at 2000 g for 10 minutes. Samples were then stored in 500 uL aliquots in 1.5 mL Eppendorf tubes at −80°C.
Visual acuity (VA) was measured on Snellen charts and expressed in decimals. In cases of unilateral ON, the VA of the affected eye was recorded. In the case of bilateral ON, the VA of the worse eye was recorded. Poor vision was defined as hand movements only (6/60). National ethics permission for the study was sought by and granted to the Department of Neurology, Walton centre for Neurology and Neurosurgery, Liverpool (L9 7LJ), and informed consent was obtained from all subjects.
2.2. Neurofilament Test
Serum neurofilament levels (NfHSMI35) were measured in duplicates with the analyst being blinded to all other information using a standard in-house ELISA [18]. In brief, the mouse monoclonal antibody SMI35 was purchased from Sternberger Monoclonals Inc. This antibody is now available through Covance Research Products (Berkeley, CA, USA). Both, the secondary and tertiary antibodies were polyclonal and purchased from (Sigma, St. Louis, MO, USA; N 4142) and (DAKO, Copenhagen, Denmark, horseradish peroxidase (HRP)-labeled swine polyclonal anti-rabbit IgG). Adhering to a previously proposed nomenclature, we indicate the captured antibodies used for NfH quantification in superscript (NfHSMI35 for SMI35). The detection limit for the NfHSMI35 assay is 0.01 ng/mL. Serum NfHSMI35 levels above the highest value observed in the control group were classified as pathological.
2.3. Aquaporin 4 Test
Serum aquaporin 4 antibodies (AQP4) were tested at the Mayo Clinic laboratories by indirect immunofluorescence as described [19].
2.4. Statistical Analysis
All statistical analyses were performed using SAS (V9.1). For comparison of two variables the nonparametric Wilcoxon two-sample test was used. General linear model were used for comparison of more than two variables. Proportions of patients were compared using Fisher's exact test. A P value of 0.05 was accepted as significant.
3. Results
The demographic data is summarised in Table 1.
Table 1.
Patient characteristics. The median (numbers) are presented.
| CTRL | MSON | CRION | NMO | |
|---|---|---|---|---|
| N | 20 | 28 | 19 | 9 |
| Age | 34 | 33 | 45 | 29 |
| Followup | n/a | 5 | 65 | 67 |
| VA baseline | ≥1 | 0.1 | 0.008 | 0.01 |
| VA outcome | n/a | 0.67 | 0.1 | 0.33 |
| NfH (ng/mL) | 0.00 | 0.055 | 0.07 | 0.12 |
F: female, M: male, VA: visual acuity, CTRL: control subjects, MSON: multiple sclerosis optic neuritis, CRION: chronic relapsing isolated optic neuropathy, NMO: neuromyelitis optica.
3.1. Diagnostic Value of NfHSMI35
Serum NfHSMI35 levels were significantly different between the groups (F 3,56 = 4.05, P = 0.011). Serum NfHSMI35 levels were significantly higher in patients with NMO (mean 0.79 ± 1.51 ng/mL) compared to patients with CRION (0.13 ± 0.16 ng/mL, P = 0.007), MSON (0.09 ± 0.09, P = 0.008), and healthy controls (0.01 ± 0.02 ng/mL, P = 0.001, Figure 1).
Figure 1.
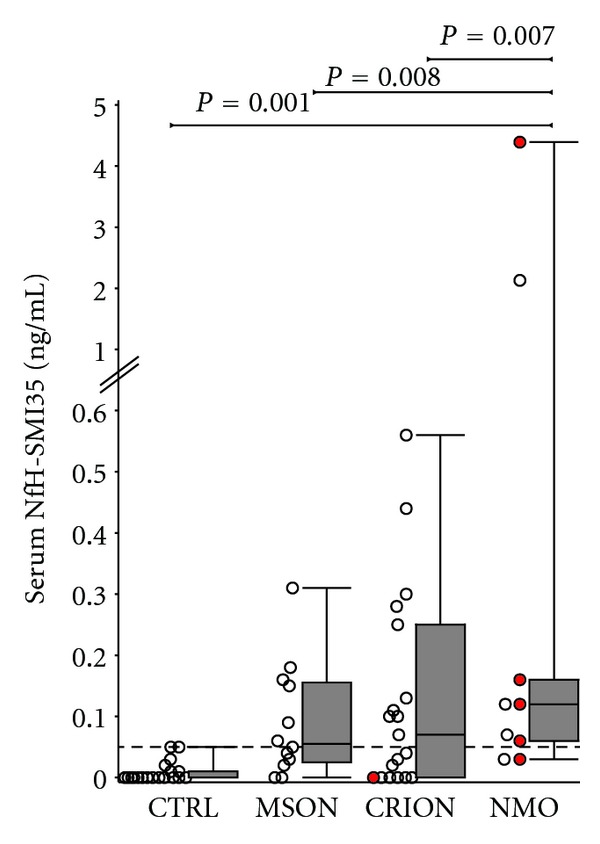
Serum NfHSMI35 levels are elevated in NMO compared to control subjects and other inflammatory optic neuropathies. Patients who were AQP4 seropositive are indicated in red.
From Figure 1 it is visible that the data was not normally distributed and serum NfHSMI35 levels were particularly high in two patients with NMO. For statistical rigorosity the data was therefore also analysed on a categorical level. The proportion of patients with NMO who had pathological levels (7/9) was significant larger compared to controls (0/20, P < 0.0001). Significance remained after the two NMO patients with particular high serum NfHSMI35 levels were removed (P < 0.0001).
Patients with MSON displayed the lowest levels of NfHSMI35 out of the three optic neuritis subtypes, although the difference between levels found in MSON and CRION was markedly smaller than that between these two groups and NMO on the one hand and healthy controls on the other.
3.2. Prognostic Value of NfHSMI35
Poor VA at onset (Figure 2) and at the last follow-up visit, (Figure 3) was associated with high serum NfHSMI35 levels in the pooled group analysis. The NfHSMI35 level at the onset of optic neuritis correlated stronger with VA than did the NfHSMI35 level at the last follow-up visit.
Figure 2.
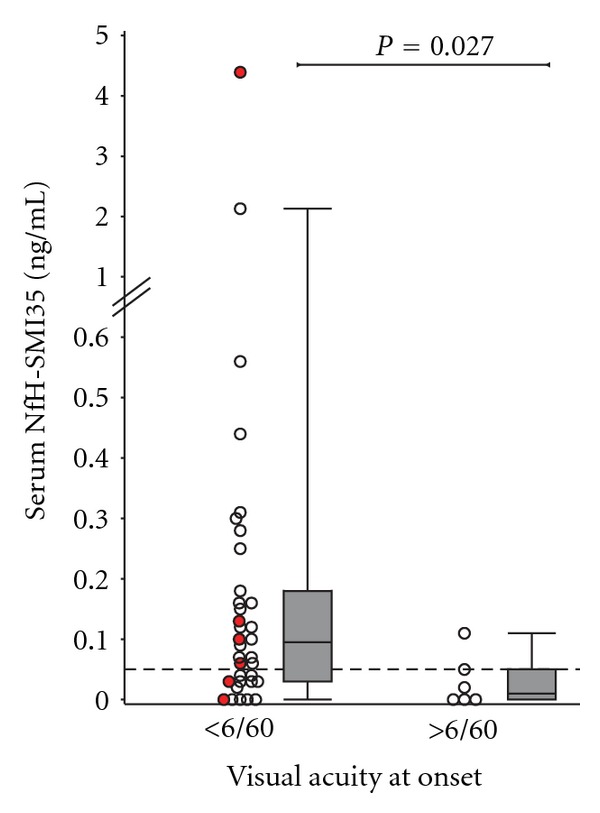
More severe loss of vision at onset is associated with higher serum NfHSMI35 levels.
Figure 3.
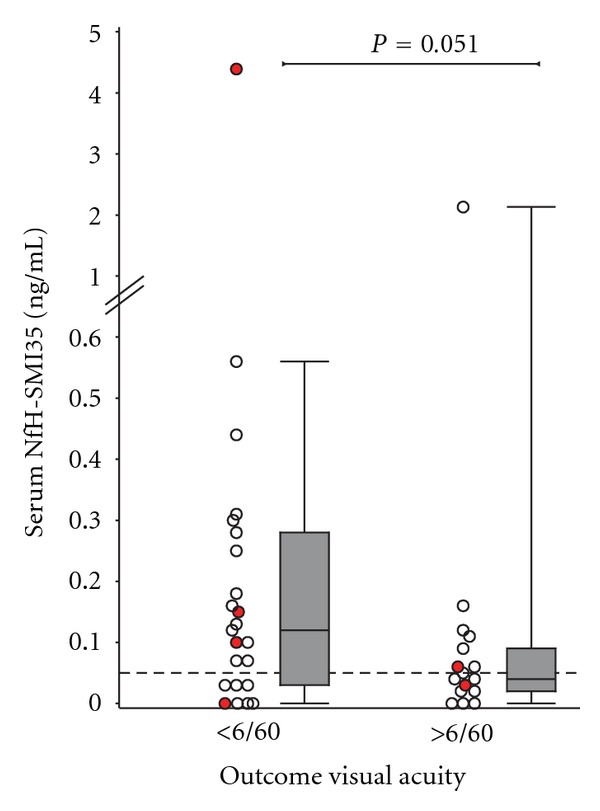
High serum NfHSMI35 levels are associated with poor visual outcome.
4. Discussion
To the best of our knowledge, this is the first published study reporting the levels of NfHSMI35 in the serum of patients with NMO and CRION. This study suggests that serum NfHSMI35 levels are of more prognostic then diagnostic value in the setting of acute immune mediated optic neuropathies.
Our results are comparable to data on plasma NfHSMI35 levels in acute ON, where the median level of plasma NfHSMI35 was found to be 0.17 ng/mL in patients with optic neuritis and 0.005 ng/mL in control subjects [9].
Our findings support the concept that axonal loss following optic neuritis is more extensive in patients with NMO compared to MSON [6]. This is consistent with the clinical observation of more server loss of visual function and retinal nerve fibres in patients with NMO [20]. Interestingly, loss of retinal nerve fibres as quantified by OCT was correlated to serum pNfH levels in patients participating in the Optic Neuritis Treatment Trial [11].
The correlation of high serum NfHSMI35 levels with poor visual recovery in the present study further supports the argument that axonal destruction with resulting retinal nerve fibre loss may be to blame for the permanent visual deficit after the attack. We speculate that longitudinal assessment of blood NfHSMI35 levels may help to identify more severely disabling events in relapsing conditions. It may be interesting to find out whether or not blood NfHSMI35 were therefore useful to improve prognostic accuracy in patients with AQP4 seropositive ON [17, 21] or in patients with NMO in whom isolated peaks in serial antibody titres suggest active disease [22].
A weakness of this study is that we did not have systematic spinal cord MRI performed in all patients. This may be relevant because high serum NfHSMI35 levels may also be caused by axonal loss due to concomitant myelitis in NMO which is clinically silent. In this context it is of note that the highest serum NfHSMI35 levels were found in an NMO patient who was also AQP4 seropositive. In total 4/5 (80%) of the AQP4 seropositive NMO patients had serum NfHSMI35 levels above the highest value observed in the control cohort (0.05 ng/mL, horizontal dotted line in Figures 2 and 3). This may require further investigation of blood NfHSMI35 levels, particularly during an acute relapses in NMO. Of note, removal of the two NMO patients with particular high serum NfHSMI35 levels did not change the statistical significance of the finding. It should be mentioned that elevated serum NfHSMI35 levels can also be observed in other neurological and nonneurological conditions such as cardiac arrest [23], cardiac surgery [24], traumatic brain injury [25], blast injury [26], subarachnoid haemorrhage [27], stroke [28], endcarotidectomy [29], and motor neuron disease [30, 31]. None of these were present in the patients reported here. Some of these studies presented longitudinal data which showed that serum NfHSMI35 levels peaked early after acute injury. Therefore an important shortcoming of our study was that the exact timing of sample acquisition in relation from onset of ocular pain to venopuncture was not collected systematically. This is an important point because onset of ocular pain may precede onset of visual loss and should be considered as time of onset in future studies. We cannot exclude the possibility that patients presenting with more severe loss of vision were recruited at an earlier time point in their disease which could have skewed the data.
Another shortcoming of the study was that all patients were recruited through a busy routine UK NHS clinic. Therefore the timing of investigations and followup was less systematic than what would have been desirable and missing data limits the power of the present study. On the other hand the data presented may be more reflective for a hands on day-to-day neurological practise than a randomised clinical trial setting. Likewise, a limitation of the study is that assessment of low contrast VA was not performed [32]. Because recovery of low-contrast VA and colour vision following optic neuritis is poorer compared to high contrast VA, correlation analyses of serum NfH levels with these measures should be performed in future studies.
After this study was completed a number of methodological papers appeared in the literature showing differences in the analytical sensitivity of a range of AQP4 tests [33]. Using some of the more sensitive tests may have increased the proportion of AQP4 positive NMO cases in the present study.
In conclusion, blood NfHSMI35 levels are of moderate diagnostic and reasonable prognostic value in patients presenting with an immune-mediated optic neuropathy.
Acknowledgments
This data was presented at ECTRIMS 2008: Petzold A, Maggiore C, Plant GT. Serum neurofilament levels suggest axonal damage is more extensive in NMO than in CRION or MSON. World Congress on Treatment and Research in Multiple Sclerosis. Multiple Sclerosis 2008; 14:S285. This project was funded in part by Fight for Sight. The work described in this article is supported by University College London Comprehensive Biomedical Research Centre and the Moorfields Biomedical Research Centre.
References
- 1.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. Journal of the Neurological Sciences. 2005;233(1-2):183–198. doi: 10.1016/j.jns.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Dujmovic I. Cerebrospinal fluid and blood biomarkers of neuroaxonal damage in multiple sclerosis. Multiple Sclerosis International. 2011;2011:18 pages. doi: 10.1155/2011/767083.767083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gresle MM, Butzkueven H, Shaw G. Neurofilament proteins as body fluid biomarkers of neurodegeneration in multiple sclerosis. Multiple Sclerosis International. 2011;2011:7 pages. doi: 10.1155/2011/315406.315406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold A, Tisdall MM, Girbes AR, et al. In vivo monitoring of neuronal loss in traumatic brain injury: a microdialysis study. Brain. 2011;134(2):464–483. doi: 10.1093/brain/awq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brettschneider J, Petzold A, Junker A, Tumani H. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Multiple Sclerosis. 2006;12(2):143–148. doi: 10.1191/135248506ms1263oa. [DOI] [PubMed] [Google Scholar]
- 6.Miyazawa I, Nakashima I, Petzold A, Fujihara K, Sato S, Itoyama Y. High CSF neurofilament heavy chain levels in neuromyelitis optica. Neurology. 2007;68(11):865–867. doi: 10.1212/01.wnl.0000256820.26489.17. [DOI] [PubMed] [Google Scholar]
- 7.Petzold A, Marignier R, Verbeek MM, Confavreux C. Glial but not axonal protein biomarkers as a new supportive diagnostic criteria for Devic neuromyelitis optica? Preliminary results on 188 patients with different neurological diseases. Journal of Neurology, Neurosurgery and Psychiatry. 2011;82(4):467–469. doi: 10.1136/jnnp.2009.196550. [DOI] [PubMed] [Google Scholar]
- 8.Takano R, Misu T, Takahashi T, Sato S, Fujihara K, Itoyama Y. Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology. 2010;75(3):208–216. doi: 10.1212/WNL.0b013e3181e2414b. [DOI] [PubMed] [Google Scholar]
- 9.Petzold A, Rejdak K, Plant GT. Axonal degenaration and inflammation in acute optic neuritis. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75(8):1178–1180. doi: 10.1136/jnnp.2003.017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petzold A, Eikelenboom MJ, Keir G, et al. Axonal damage accumulates in the progressive phase of multiple sclerosis: three year follow up study. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(2):206–211. doi: 10.1136/jnnp.2004.043315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasol J, Feuer W, Yang C, Shaw G, Kardon R, Guy J. Phosphorylated neurofilament heavy chain correlations to visual function, optical coherence tomography, and treatment. Multiple Sclerosis International. 2010;2010:10 pages. doi: 10.1155/2010/542691.542691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION) Brain. 2003;126(2):276–284. doi: 10.1093/brain/awg045. [DOI] [PubMed] [Google Scholar]
- 13.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. The Lancet Neurology. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 14.Beck RW, Gal RL. Treatment of acute optic neuritis: a summary of findings from the optic neuritis treatment trial. Archives of Ophthalmology. 2008;126(7):994–995. doi: 10.1001/archopht.126.7.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura M, Nakazawa T, Doi H, et al. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2010;248(12):1777–1785. doi: 10.1007/s00417-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 17.Petzold A, Pittock S, Lennon V, Maggiore C, Weinshenker BG, Plant GT. Neuromyelitis optica-IgG (aquaporin-4) autoantibodies in immune mediated optic neuritis. Journal of Neurology, Neurosurgery and Psychiatry. 2010;81(1):109–111. doi: 10.1136/jnnp.2008.146894. [DOI] [PubMed] [Google Scholar]
- 18.Petzold A, Keir G, Green AJE, Giovannoni G, Thompson EJ. A specific ELISA for measuring neurofilament heavy chain phosphoforms. Journal of Immunological Methods. 2003;278(1-2):179–190. doi: 10.1016/s0022-1759(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 19.Lennon PVA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. The Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 20.Green AJ, Cree BAC. Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(9):1002–1005. doi: 10.1136/jnnp.2008.166207. [DOI] [PubMed] [Google Scholar]
- 21.Jarius S, Frederikson J, Waters P, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. Journal of the Neurological Sciences. 2010;298(1-2):158–162. doi: 10.1016/j.jns.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131(11):3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rundgren M, Friberg H, Cronberg T, Romner B, Petzold A. Serial soluble neurofilament heavy chain in plasma as a marker of brain injury after cardiac arrest. Critical Care. 2012;16(2, article R45) doi: 10.1186/cc11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123(7):714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KJ, Scheff SW, Miller KM, et al. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. Journal of Neurotrauma. 2008;25(9):1079–1085. doi: 10.1089/neu.2007.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tisdall M, Petzold A. Comment on ‘chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model’. Science Translational Medicine. 2012;4(157, article 157le8) doi: 10.1126/scitranslmed.3004403. [DOI] [PubMed] [Google Scholar]
- 27.Lewis SB, Wolper RA, Miralia L, Yang C, Shaw G. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. Journal of Cerebral Blood Flow and Metabolism. 2008;28(6):1261–1271. doi: 10.1038/jcbfm.2008.12. [DOI] [PubMed] [Google Scholar]
- 28.Sellner J, Patel A, Dassan P, Brown MM, Petzold A. Hyperacute detection of neurofilament heavy chain in serum following stroke: a transient sign. Neurochemical Research. 2011;36(12):2287–2291. doi: 10.1007/s11064-011-0553-8. [DOI] [PubMed] [Google Scholar]
- 29.Sellner J, Petzold A, Sadikovic S, et al. The value of the serum neurofilament protein heavy chain as a biomarker for peri-operative brain Injury after carotid endarterectomy. Neurochemical Research. 2009;34(11):1969–1974. doi: 10.1007/s11064-009-9976-x. [DOI] [PubMed] [Google Scholar]
- 30.Lu C-H, Kalmar B, Malaspina A, Greensmith L, Petzold A. A method to solubilise protein aggregates for immunoassay quantification which overcomes the neurofilament “hook“ effect. Journal of Neuroscience Methods. 2011;195(2):143–150. doi: 10.1016/j.jneumeth.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Boylan KB, Glass JD, Crook JE, et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. doi: 10.1136/jnnp-2012-303768. 2012, Journal of Neurology, Neurosurgery and Psychiatry. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the multiple sclerosis functional composite. Neurology. 2003;61(10):1367–1373. doi: 10.1212/01.wnl.0000094315.19931.90. [DOI] [PubMed] [Google Scholar]
- 33.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78(9):665–671. doi: 10.1212/WNL.0b013e318248dec1. [DOI] [PMC free article] [PubMed] [Google Scholar]


