Abstract
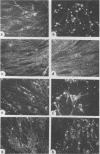
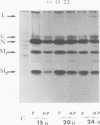
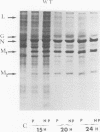
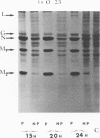
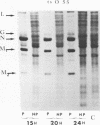
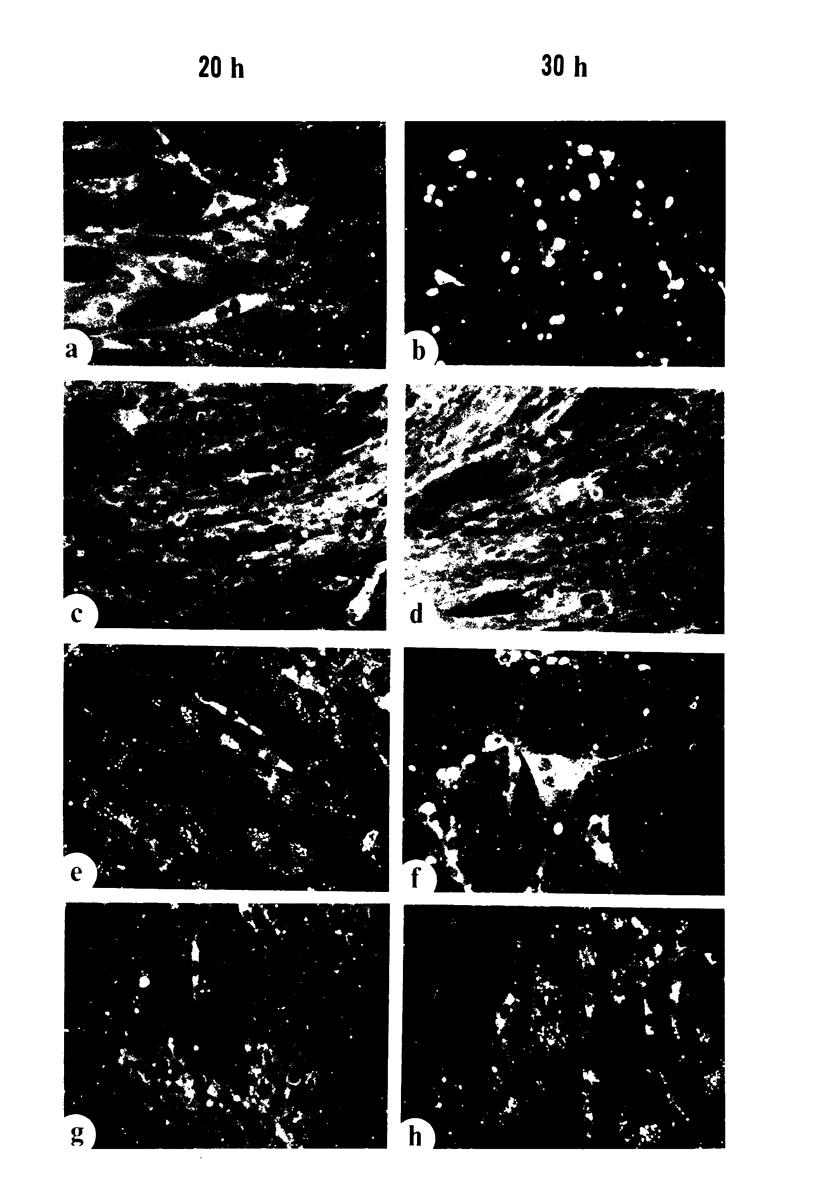
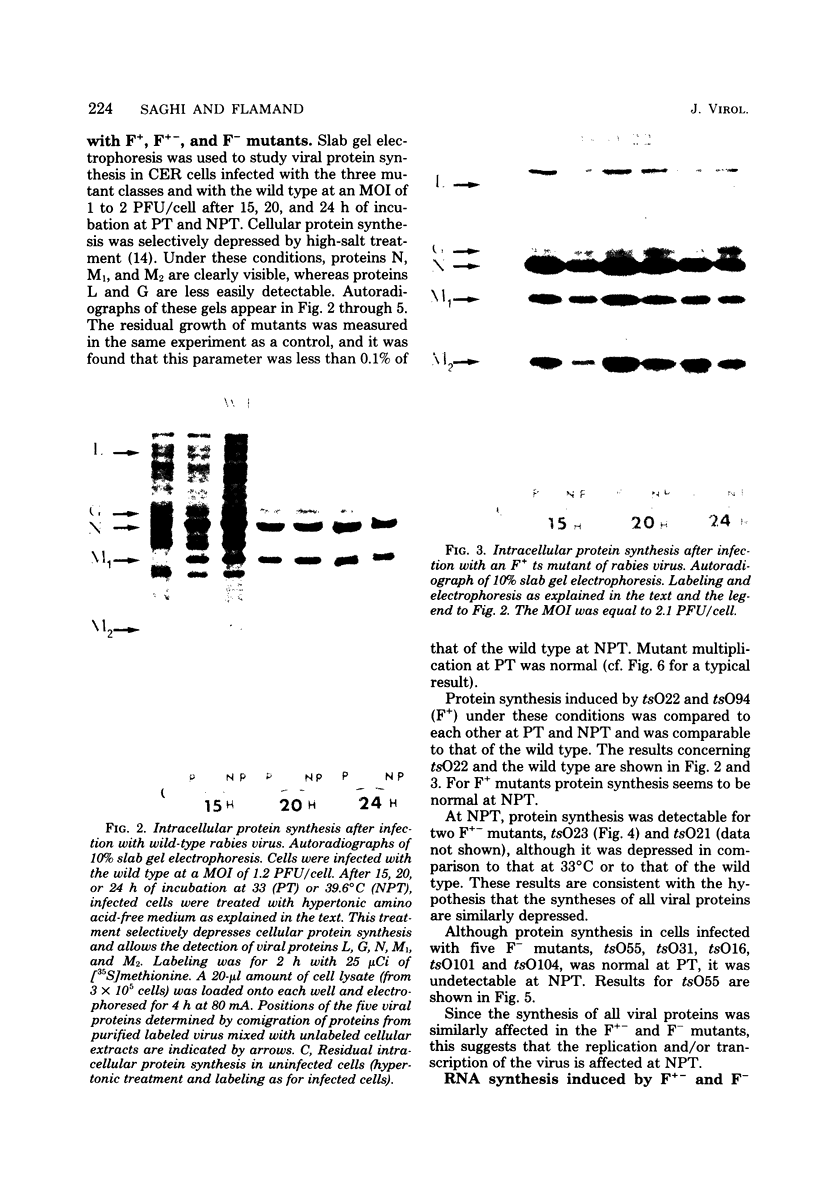
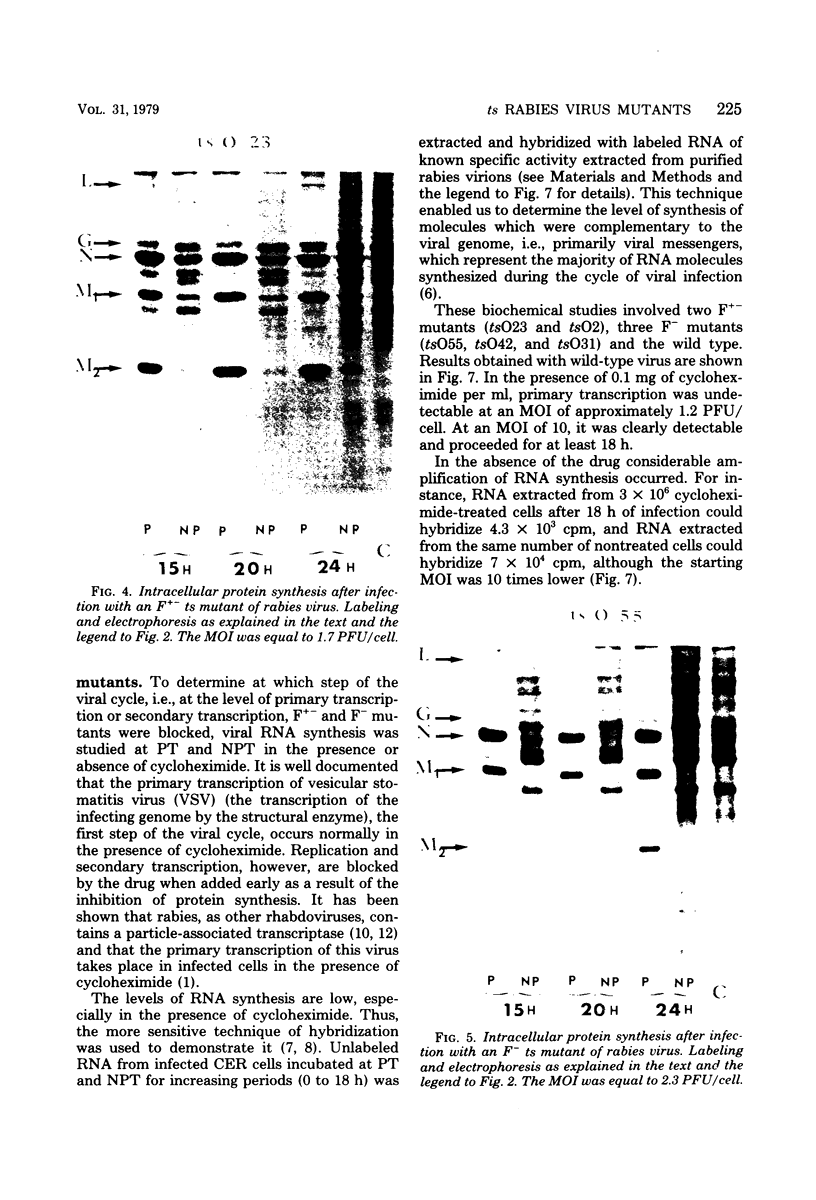
Biochemical characterization of 70 temperature-sensitive (ts) mutants of rabies virus has been done by following the appearance of viral proteins and RNA molecules in infected cells at both permissive and nonpermissive temperature. The presence or absence of the nucleocapsid protein (N) was demonstrated by treating infected cells with anti-N fluorescent antibodies. At 33 degrees C, all the mutants induced a fluorescence comparable to the wild type. At 39.6 degrees C, the mutants can be classified into three groups. Three mutants induced a fluorescence comparable to the wild type (F+ mutants); 54 mutants induced a faint fluorescence which was proportional to the multiplicity of infection and increased with time (F+- mutants). No fluorescence could be detected for the 13 remaining mutants (F- mutants). The synthesis of all viral proteins was shown to be normal for F+ mutants, indicating that transcription and replication of the virus were normal and that the ts lesion was located in a protein which is not directly required for those functions. The synthesis of all viral proteins was similarly decreased for F+- mutants and undetectable for the F- mutants. This suggests that the ts lesion affects the transcription and/or replication of the virus. By annealing techniques it was demonstrated that the F+- mutants were able to perform some amount of secondary transcription at nonpermissive temperature. No secondary transcription occurred with F- mutants. When detectable (i.e., at higher multiplicity of infection), primary transcription of F- mutants was normal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Ermine A., Flamand A. Rna syntheses in BHK21 cells infected by rabies virus. Ann Microbiol (Paris) 1977 May-Jun;128A(4):477–488. [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. In vivo synthesis of RNA by vesicular stomatitis virus and its mutants. J Mol Biol. 1974 Jul 25;87(1):31–53. doi: 10.1016/0022-2836(74)90558-0. [DOI] [PubMed] [Google Scholar]
- Flamand A., Bishop D. H. Primary in vivo transcription of vesicular stomatitis virus and temperature-sensitive mutants of five vesicular stomatitis virus complementation groups. J Virol. 1973 Dec;12(6):1238–1252. doi: 10.1128/jvi.12.6.1238-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Delagneau J. F., Bussereau F. An RNA polymerase activity in purified rabies virions. J Gen Virol. 1978 Jul;40(1):233–238. doi: 10.1099/0022-1317-40-1-233. [DOI] [PubMed] [Google Scholar]
- Flamand A., Delagneau J. F. Transcriptional mapping of rabies virus in vivo. J Virol. 1978 Nov;28(2):518–523. doi: 10.1128/jvi.28.2.518-523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Pese D., Bussereau F. Effect of actinomycin D and cytosine arabinoside on rabies and VSV multiplication. Virology. 1977 May 1;78(1):323–327. doi: 10.1016/0042-6822(77)90103-9. [DOI] [PubMed] [Google Scholar]
- Kawai A. Transcriptase activity associated with rabies virion. J Virol. 1977 Dec;24(3):826–835. doi: 10.1128/jvi.24.3.826-835.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Rabies virus protein synthesis in infected BHK-21 cells. J Virol. 1977 Apr;22(1):102–112. doi: 10.1128/jvi.22.1.102-112.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]