Abstract
Glioblastomas (GBM) are characterized by resistance to chemotherapy and radiotherapy, and therefore, alternative therapeutic approaches are needed. TRAIL induces apoptosis in cancer but not in normal cells and is considered to be a promising anti-tumor agent. However, its short in vivo half-life and lack of efficient administration modes are serious impediments to its therapeutic efficacy. Nanoparticles (NP) have been used as effective delivery tools for various anticancer drugs. TRAIL was conjugated to magnetic ferric oxide NP by binding the TRAIL primary amino groups to activated double bonds on the surface of the NP. The effect of NP-TRAIL was examined on the apoptosis of glioma cells and self-renewal of glioma stem cells (GSCs). In addition, the ability of the NP-TRAIL to track U251 cell–derived glioma xenografts and to affect cell apoptosis, tumor volume, and survival among xenografted rats was also examined. Conjugation of TRAIL to NP increased its apoptotic activity against different human glioma cells and GSCs, as compared with free recombinant TRAIL. Combined treatment with NP-TRAIL and γ-radiation or bortezomib sensitized TRAIL-resistant GSCs to NP-TRAIL. Using rhodamine-labeled NP and U251 glioma cell–derived xenografts, we demonstrated that the NP-TRAIL were found in the tumor site and induced a significant increase in glioma cell apoptosis, a decrease in tumor volume, and increased animal survival. In summary, conjugation of TRAIL to NP increased its apoptotic activity both in vitro and in vivo. Therefore, NP-TRAIL represents a targeted anticancer agent with more efficient action for the treatment of GBM and the eradication of GSCs.
Keywords: apoptosis, glioblastoma, iron oxide, nanoparticles, TRAIL
The prognosis of glioblastoma (GBM), the most common malignant brain cancer, is extremely poor because of resistance to conventional therapies and to infiltration of tumor cells in the adjacent brain parenchyma.1,2 Current treatments include surgery, followed by radiation therapy and chemotherapy for residual disease.3 Despite best efforts, prognosis remains extremely poor, with median survival of 14 months.4 Innovative strategies to enhance drug delivery directly to infiltrating tumor cells is being sought to allow the eradication of residual tumor cells and glioma stem cells (GSCs).5
TRAIL, a type-II transmembrane homotrimeric protein that belongs to the TNF gene superfamily, is a promising candidate for cancer therapy because of its selective apoptotic effect in a wide variety of transformed cells without affecting normal cells.6,7 Despite its therapeutic merits and initial promise, TRAIL lacks clinical applicability because of short half-life and inefficient delivery, poor solubility, and an unfavorable pharmacokinetic profile.8,9 In addition, some glioma cells and, in particular, GSCs have been reported to exhibit resistance to the apoptotic effect of TRAIL.10
Nanoparticles (NP) are being widely used in the development of new approaches of drug delivery in cancer and can provide a platform for combined therapeutics with subsequent monitoring of response.11,12 NP-based delivery systems offer many benefits because of their tumor-targeting abilities, increased internalization efficiencies, and escape from multidrug resistance.13 Targeted delivery of NP could result in increased accumulation and retention of the NP at the tumor site, thus decreasing the systemic toxicity and increasing their therapeutic efficacy.14,15 Iron oxide NP are biodegradable, biocompatible, and relatively nontoxic. Moreover, they exhibit unique super-paramagnetic properties that allow their use as efficient MRI contrast agents.16
We used a unique type of uniform iron oxide NP of 70 nm mean hydrodynamic diameter. These NP were prepared by nucleation, followed by controlled growth of maghemite layers onto gelatin nuclei,17 and were studied for a variety of biomedical applications and uses.13,18–25
In this study, we describe an innovative method to combine the unique characteristics of TRAIL and that of the iron oxide NP in a single molecule, by covalently conjugating TRAIL to the surface of the iron oxide NP. Our results indicate that conjugation of TRAIL to the NP allows enhanced activity of TRAIL in glioma cells and GSCs and in a preclinical glioma animal model.
Methods
Synthesis of NP
NP of narrow size distribution were prepared by nucleation, followed by controlled growth of maghemite thin layers onto gelatine/iron oxide nuclei, as previously described.16,18 In brief, iron oxide NP were prepared by adding FeCl2 solution (10 mmol/5 mL 0.01N HCl) to 80 mL aqueous solution containing 200 mg gelatine, followed by NaNO2 solution (7 mmol/5 mL H2O). Next, NaOH aqueous solution (1 N) was added up to a pH of 9.5. This procedure was repeated 4 times at 10-min intervals. The formed magnetic NP were then washed from excess reagents with use of a high-gradient magnetic field (HGMF) technique. For this purpose, we used magnetic columns containing steel fibers using commercial magnetic columns (MACS µColumns, Miltenyi Biotech).
Fluorescent NP (FL-NP) were prepared similarly, replacing the gelatin with gelatin conjugated to the fluorescent dye rhodamine, as previously described.23
Activation of the NP was performed by adding divinyl sulfone (DVS) linker. In brief, 4.8 mg of DVS was added per 1 mg of NP in aqueous solution. The pH of the solution was then increased to a pH of 10.5 by adding triethylamine, and the NP were stored in a shaker at 60°C overnight. The DVS-activated NP were then washed using magnetic columns with bicarbonate buffer (0.1 M; pH, 8.3).
Recombinant human soluble TRAIL/Apo2L (168 aa polypeptide, Peprotech) was conjugated to the DVS-activated fluorescent or nonfluorescent NP through the DVS linker. In brief, 20 µg of TRAIL was added to 1 mL bicarbonate buffer (0.1 M; pH, 8.3) containing 1 mg of dispersed activated fluorescent or nonfluorescent NPs. The dispersion was then mixed at room temperature for 60 min. This process involves the binding, through Michael addition, of residual double bonds of the NP and primary amino groups of the TRAIL. Blocking of residual activated double bonds was then performed by adding glycine (1% w/v) and continuous mixing of the dispersion for an additional 30 min at room temperature. The excess unbound TRAIL and glycine was then washed using magnetic columns with PBS (pH, 7.4).
The amount of conjugated TRAIL was determined using an enzyme-linked immunosorbent assay kit (R&D).
Glioma Cell Lines
U87, A172, and U251 cells were obtained from ATCC. All cells were cultured in DMEM supplemented with 10% FBS (Hyclone), 2 mM L-glutamine, and 100 μg/mL streptomycin-penicillin (Invitrogen) at 37oC under 5% CO2.
U251 cells overexpressing GFP were obtained by stably transfecting the cells with a pEGFP-N1 plasmid (Clontech) by electroporation using the Nucleofector device program A027 (Amaxa Biosystems). A pool of cells selected in 750 ng/mL G418 was used in these studies.
GSCs and Enrichment of CD133+ Cells
All human materials were used in accordance with the policies of the institutional review board at Henry Ford Hospital. The generation of GSCs and the enrichment of CD133+ cells and their characterization have been recently described.26,27 In brief, GBM specimens were dissociated in 0.05% Trypsin/EDTA for 4 h at room temperature, followed by incubation in DMEM/F-12 medium containing 0.7 mg/mL ovomucoid. The tissue was then triturated mechanically with a fire-narrowed Pasteur cell pipette and filtered through a 40-mm mesh. Cells were density centrifuged in lympholyte-M and then maintained in neurosphere medium supplemented with 20 ng/mL EFG and 20 ng/mL FGFb. The enrichment of CD133+ cells was performed according to the MACS CD133 kit manual (Miltenyi Biotech). Spheroids were maintained in neurosphere medium and examined for the expression of CD44, Bmi-1, CD133, Musashi-1, Sox2 and nestin, self-renewal, and expression of astrocytic, oligodendrocytic, and neuronal markers after plating on poly-D-ornithine in serum-containing medium and for their tumorigenic potential in nude rats.27
For γ-radiation treatment, the GSCs were irradiated with 3 Gy 6 h prior to treatment with NP-TRAIL. Radiation exposure of the cell cultures was performed using a 5000 Ci Cesium (Cs-137) irradiator (Mark I:J.L. Shepherd and Associates). Bortezomib (100 nM) was administered concomitantly with TRAIL.
Neurosphere Formation Assay
The ability of CD133+ cells to form secondary neurospheres was determined as previously described.26,28 In brief, disaggregated cells were treated with the appropriate treatments, and cells were plated in 24-well plates at a density of 100 cells/well through limiting dilution. The number of neurospheres/well was determined 14 days thereafter for 8 different wells. Spheres that contained >20 cells were scored. Results are presented as percentage of maximal neurospheres formed in control untreated cells.
Western Blot Analysis
Cell lysates (30 μg protein) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with the primary antibody, specific reactive bands were detected using a goat anti-rabbit or goat anti-mouse IgG conjugated to horseradish peroxidase (BioRad), and the immunoreactive bands were visualized by the ECL Western blotting detection kit (Amersham).
Measurements of Cell Apoptosis
Cell apoptosis was measured by flow cytometry after propidium iodide (PI) staining as previously described.28 In brief, cells were treated according to the specific experiment. The cells were scraped and centrifuged with the supernatant medium at 3500 rpm for 5 min. Cells were resuspended in PBS and fixed in 70% ethanol on ice for 1 h. Fixed cells were washed with PBS and stained with PI (5 µg/mL) solution containing RNAse (50 µM). Cells were then analyzed on a Beckton-Dickinson FACS Caliber.
Caspase 8 Activity
Caspase 8 activity was measured using a colorimetric activity assay (Chemicon International) according to the manufacturer's instructions. In brief, glioma cells were treated with the different treatments for 3 h in the A172 cells or for 18 h in the U251 cells. The cells were collected and were centrifuged in lysis buffer (10 000x g). Equal protein samples were incubated with the caspase 8 substrate solution for 1 h at 37°C, and activity was measured at 405 nm using a microtiter plate reader.
Confocal Microscopy
For analysis of the internalization of the FL-NP-TRAIL, U251 cells were incubated with FL-NP-TRAIL (50 μg/mL) and were viewed using confocal microscopy (Nikon C1) with 63× magnification at an excitation wavelength of 488 nm at the beginning of the treatment and every 5 min thereafter. The internalization of the FL-NP-TRAIL reached plateau levels after 25 min of treatment.
Tumor Implantation and Nanoparticle Administration
Following Institutional Animal Care and Use Committee guidelines in an institutionally approved animal use protocol, female Nu/Nu rats were obtained through NCI Fredericks. All rats were 6–8-weeks-old at the time of tumor implantation.
Prior to tumor cell implantation, animals were anesthetized and prepared for sterile surgery. A 1-cm-long incision was made through the scalp, and a 26-gauge needle was used to gently puncture, by twisting, a hole through the skull 2 mm to the right and 2 mm to the dorsal of the midline. The animal was then placed into a stereotaxic device (Kopf) equipped with a micro-manipulator and a syringe holder. The needle was lowered 3 mm into the pre-made hole, raised 0.5 mm, and 5 × 105 U251 cells in a volume of 5 μL were slowly injected over a total of 2.5 min. The needle was left in place for 1 min, then slowly raised over 1 min. The animal was removed from the stereotaxic device, the hole was sealed with bone wax, and the scalp was sutured. The animal was then monitored for recovery. On day 7 after tumor implantation, NP, NP-TRAIL or 5 μL of PBS was implanted in the same manner in the ipsilateral hemisphere. At either selected time points or signs of morbidity, standard perfusion with saline followed by 10% formalin was performed. The brains were removed and placed in 10% neutral buffered formalin overnight for further processing. For visualization of fluorescently labeled tumors and NP, standard perfusion with saline was performed at specific time points; brains were then removed and snap-frozen for sectioning using a cryostat.
Histochemistry, Immunohistochemistry, NP Visualization, and Apoptosis Detection
Formalin-fixed tissues were embedded in paraffin, sectioned (6 μm), and stained with hematoxylin and eosin for histomorphological assessment. The 5-μm sections were dried in a 60°C oven for 1 h and routinely deparafinized to ddH20. Immunohistochemistry was performed using the Biocare Medical Nemesis 7200 stainer and reagents.
The sections were blocked with Biocare Sniper block for 7 min and incubated with primary antibody in Biocare diluent for 60 min. After buffer rinses, the sections were avidin-biotin blocked for 15 min. Following rinses, antigens were detected using universal link, followed by HRP (Biocare) and Betazoid DAB. Buffer rinses were then followed by ddH20 and 10 s counterstain with CAT hematoxylin. Control sections were processed omitting the primary antibody.
Gomori's iron reaction staining for NP visualization was done using 5-μm sections from formalin-fixed paraffin-embedded tissue. The 5-μm sections were dried in a 60°C oven for 1 h and routinely deparafinized to ddH20. Acid-cleaned glassware was used for all the remaining steps. Slides were immersed in equal parts 20% HCl and 10% aqueous potassium ferrocyanide for 10–20 min. Slides were then rinsed well in ddH20 and counterstained with Nuclear Fast Red for 2 min, followed by dehydration, and then cover slipped. Sections of spleen were used as positive controls for each staining.
Apoptosis staining of formalin-fixed paraffin-embedded tissue was performed using the Apoptag Peroxidase In Situ Apoptosis Detection Kit (Chemicon) according to the manufacturer's instructions. In brief, 5-μm sections from formalin-fixed paraffin-embedded tissue were dried in a 60°C oven for 1 h and routinely deparafinized. Tissue was then pretreated with proteinase K (20 μg/mL) at room temperature for 15 min. Slides were washed 2 times in ddH20, and endogenous peroxidase was quenched in 3.0% hydrogen peroxide in PBS at room temperature for 5 min. Slides were washed 2 times in PBS, and 75 μL/5 cm2 of equilibration buffer was added to each slide for at least 10 s. Excess liquid was tapped off, and 55 μL/5cm2 of TdT enzyme was added. Slides were incubated for 1 h and then washed in stop/wash buffer for 15 s with agitation, then at room temperature for 10 min. Next, the slides were washed in 3 changes of PBS for 1 min each, followed by incubation with 65 μL/5cm2 anti-digoxignenin conjugate for 30 min. After 4 changes of PBS for 2 min each, slides were incubated with 75 μL/5 cm2 peroxidase substrate for 3–6 min at room temperature. Slides were washed for a final 3 changes of ddH20 for 1 min each, incubated in ddH20 for 5 min, and then counterstained with methyl green for 10min at room temperature. They were then washed with ddH20, cleared with 100% N-butanol, dehydrated through xylene, and cover slips mounted with permount.
Imaging
Immunohistological and histochemical images were taken at room temperature using a Nikon Eclipse E800M microscope with ×10, ×20, and ×40 objectives connected to a Nikon DXM1200C digital camera, and were digitized using ACT-1C software on Dell Optiplex GX620 computers. Tiff images were imported into Adobe Photoshop for composite production.
Fluorescent images were taken at room temperature using a Nikon C1 with X4, X10, and X20 objectives connected to a digital camera. Bitmap images were imported into Adobe Photoshop for composite production.
Statistical Analysis
The results are presented as the mean values ± standard error of the mean. Data were analyzed using analysis of variance and a Student's t test. Student's t test (with correction for data sets with unequal variances) was done using Prism 4 (GraphPad Software). Kaplan-Meier curves and the log-rank test were used to compare differences between survival curves using R (version 2.12.0). The Student's t test was used for pairwise comparisons of mean survival between groups because no censoring was present.
Results
NP-TRAIL Exhibits a Stronger Apoptotic Effect in Glioma Cell Lines and GSCs, Compared With Free Recombinant TRAIL
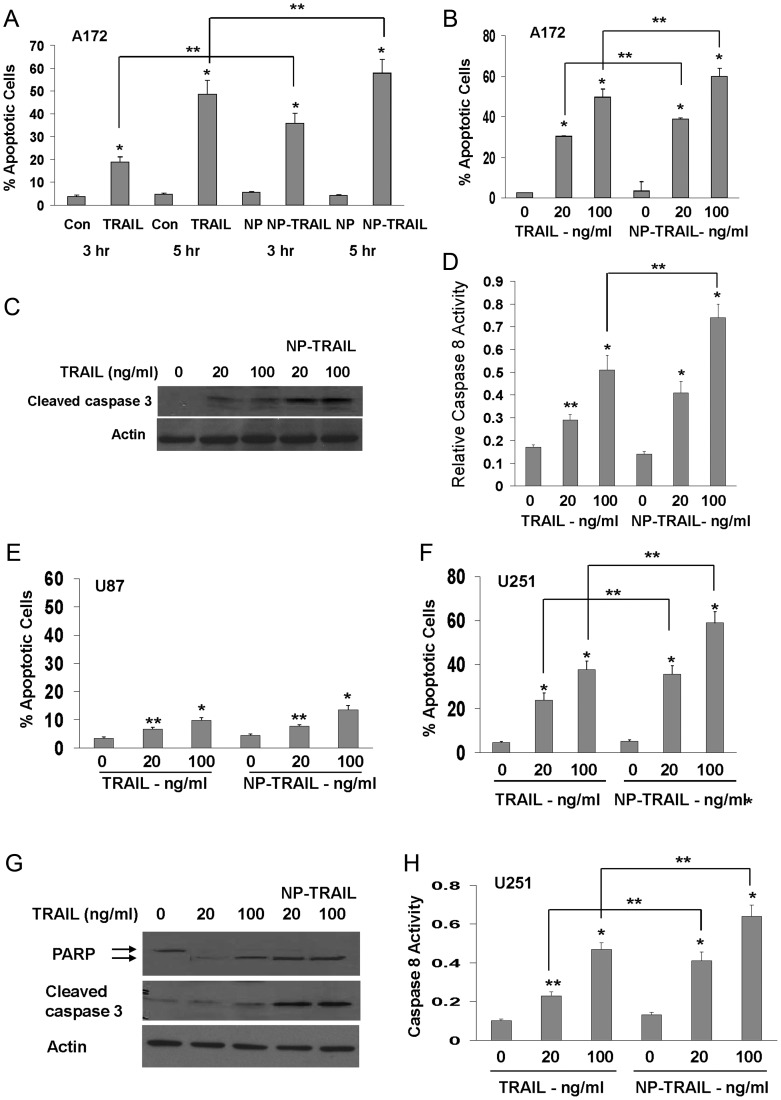
In these experiments, we studied the effect of TRAIL conjugated to NP (NP-TRAIL) on glioma cell lines and GSCs. We first examined the apoptotic potential of the NP-TRAIL, as compared with free TRAIL, by analyzing the effects of similar concentrations of TRAIL and NP-TRAIL (20 and 100 ng/mL) in the 3 different glioma cell lines: A172, U87, and U251. Cells were treated for 3 h and 5 h (A172 cells) or 24 h (U87 and U251 cells), and cell apoptosis was determined using PI staining and FACS analysis. We found that the A172 cells, which exhibit the highest sensitivity to TRAIL,29,30 displayed a stronger response to NP-TRAIL after 3 h of treatment, compared with free recombinant TRAIL (TRAIL – 15.1% and NP-TRAIL –30.3% increase in cell apoptosis, P < .05, Fig. 1A), whereas a more modest increased sensitivity to both concentrations of NP-TRAIL (20 and 100 ng/mL) was observed after 5 h (Fig. 1B). Similar results were obtained by measuring the levels of active caspase 3 (Fig. 1C) and the activity of caspase 8 (Fig. 1D). The U87 cells, which are relatively resistant to TRAIL, exhibited only a small apoptotic response to both TRAIL and NP-TRAIL and a small increased activity of the NP-TRAIL compared with free TRAIL (Fig. 1E). In contrast, the NP-TRAIL induced significantly larger degree of cell apoptosis in the U251 cells, as compared with the free TRAIL, for both concentrations examined as presented by PI staining and FACS analysis (33.2% and 19.4% for 20 ng/mL NP-TRAIL and TRAIL, respectively, and 53.8% and 33.2% for 100 ng/mL NP-TRAIL and TRAIL; P < .05; Fig. 1F), by levels of active caspase 3 and cleaved PARP (Fig. 1G) and by caspase 8 activity (Fig. 1H).
Fig. 1.
Conjugation of TRAIL to nanoparticles (NP-TRAIL) increases its apoptotic activity in glioma cells. The glioma cell lines A172 (A–D), U87 (E), and U251 (F–H) were treated with either 20 or 100 ng/mL TRAIL or similar concentrations of NP-TRAIL for different time points. Cell apoptosis was determined after 3 h for cells treated with 100 ng/mL TRAIL and NP-TRAIL or after 5 h (A–D) or 24 h thereafter (E–H) using PI staining and FACS analysis. Active caspase 3 (C and G) and cleaved PARP (G) were determined using Western blot after 5 h for A172 cells (C) and after 24 h of treatment for the U251 cells (G). Caspase 8 activity was determined as described in the methods after 3 h of treatment for the A172 cells (D) and after 18 h of treatment for the U251 cells (H). Results are representative of 3 different experiments that gave similar results. *P < .001 **P <.05.
GSCs are a rare population of cancer cells that play a role in the migration, resistance to therapy, and recurrence of GBM.31,32 Therefore, targeting these cells is extremely important therapeutically. We used 4 preparations of CD133+ cells that were generated from different GBM specimens (HF2355, HF2359, HF2414, and HF2485) as previously described.33 The cells were grown as spheroids, expressed CD133 and Sox2, and differentiated to the different neural lineages after plating on poly-D-ornithine coated plates in serum-containing medium. In addition, the cells exhibited a capacity for self-renewal and generated tumors that recapitulated the tumors of origin when injected intracranally.26,27
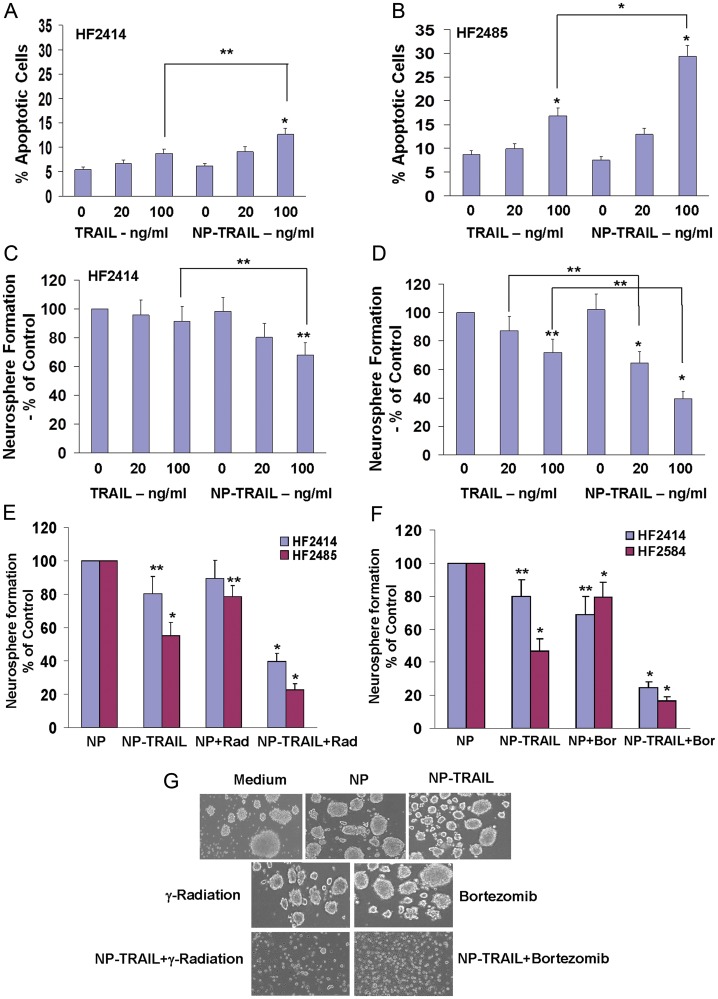
The 2 lines HF2355 and HF2359 were recently reported by us to exhibit resistance to TRAIL.33 We found that these cells also exhibited only marginal cell apoptosis in response to the NP-TRAIL (data not shown). In contrast, we found that both TRAIL and the NP-TRAIL induced a small degree of cell apoptosis in the HF2414 (8.7% in TRAIL and 12.8% in NP-TRAIL treated cells, Fig. 2A) and HF2485 (16.8% in TRAIL and 29.4% in NP-TRAIL-treated cells, Fig. 2B) GSCs, with the effects of the NP-TRAIL being significantly higher for the HF2485 cells. (P < .05).
Fig. 2.
NP-TRAIL induces apoptosis and decreases self-renewal of GSCs with and without γ-radiation and bortezomib. The GSCs HF2414 (A and C) and HF2485 (B and D) were treated with TRAIL (20 or 100 ng/mL) or with NP-TRAIL at similar concentrations. Cell apoptosis was determined after 48 h using PI staining and FACS analysis (A and B). Neurosphere formation assay was performed, and the number of neurospheres per well was quantified after 14 days for eight different wells (C and D). Spheres that contained more than 20 cells were scored, and the results are presented as percentage of maximal neurospheres formed in control untreated cells. For combined treatment of NP-TRAIL with γ-radiation (E and G) and bortezomib (F and G), cells were radiated with 3Gy and NP-TRAIL was added 6 h later. Bortezomib (100 nM) and NP-TRAIL (100 ng/mL) were added concomitantly. Neurosphere formation was determined after 14 days (E and F), and the morphology of the control and treated HF2414 GSCs was visualized after 3 days under a phase contrast microscope. All images are 20X (G). Results are representative of 3 different experiments or are the mean ± standard error of the mean of 3 experiments. *P < .001 **P < .05.
We further examined the effects of TRAIL and NP-TRAIL on the secondary neurosphere formation of these cells. We found that TRAIL induced a marginal decrease in the neurosphere formation in the HF2414 GSCs (Fig. 2C) and a small effect in the HF2485 GSCs (Fig. 2D). In contrast, the NP-TRAIL significantly decreased the neurosphere formation of both GSCs (Fig. 2C and D).
Because the GSCs exhibit only low sensitivity to TRAIL, we examined the effect of γ-radiation (3 Gy) and bortezomib (100 nM), which have been previously reported to increase the sensitivity of cancer cells to TRAIL.33 As presented in Fig. 2, both γ-radiation (Fig. 2E) and bortezomib (Fig. 2F) significantly increased the sensitivity of the GSCs to NP-TRAIL, as evident by the decreased self-renewal of both GSCs and by the morphology of the cells (Fig. 2G).
Fluorescent NP-TRAIL Accumulate in Glioma Cells and Induce Cell Apoptosis
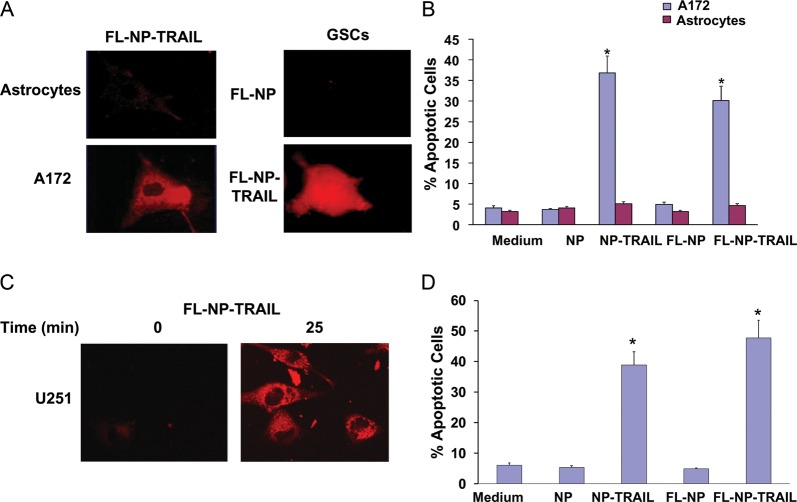
To further follow the localization and functions of the NP-TRAIL in the glioma cells, we generated fluorescent NP (FL-NP) and conjugated them to TRAIL. The A172 cells were incubated with 50 ng/mL FL-NP-TRAIL, and the fluorescence of the cells was followed using a fluorescent microscope. As presented in Fig. 3A, FL-NP-TRAIL accumulated in the A172 cells within 30 min, whereas no significant degree of fluorescence was observed in normal human astrocytes incubated with the FL-NP-TRAIL. Similarly, we found that the FL-NP-TRAIL accumulated in the HF2414 GSCs, whereas no accumulation of the control FL-NP was observed in these cells. Accordingly, the FL-NP-TRAIL induced cell apoptosis in the A172 cells, similar to the effects of the NP-TRAIL, and not in the normal human astrocytes (Fig. 3B).
Fig. 3.
Rhodamine-labeled NP-TRAIL (FL-NP-TRAIL) accumulate in glioma cells and induce cell apoptosis. The A172 glioma cells and the HF2414 GSCs were treated with FL-NP-TRAIL and control NP (FL-NP) for 30 min. The fluorescence of the cells was analyzed using a fluorescent microscope (A). The apoptotic effect of the FL-NP-TRAIL was analyzed in A172 cells and normal human astrocytes using PI staining and FACS analysis and was compared to the effect of non-fluorescent NP-TRAIL (B). U251 cells were treated with FL-NP-TRAIL (50 μg/mL), and confocal microscope analysis of the fluorescent NP internalization was performed at the beginning of the treatment and 25 min thereafter (C). U251 cells were treated with 50 μg/mL NP-TRAIL or FL-NP-TRAIL for 24h, and cell apoptosis was determined using PI staining and FACS analysis (D). The results are representative of three different experiments that gave similar results (A and C) or are the mean ± standard error of the mean of three different experiments (B and D). *P < .001.
We further analyzed the internalization of the FL-NP-TRAIL in the U251 cells using confocal microscopy. U251 cells were incubated with FL-NP-TRAIL (50 μg/mL) and were viewed at the beginning of the incubation and 25 min thereafter. As presented in Fig. 3C, the FL-NP-TRAIL accumulated in the cells after 25 min (Fig. 3C), whereas no accumulation of the FL-NP was observed up to 2h of incubation (data not shown). Incubation of the U251 cells with either NP-TRAIL or FL-NP-TRAIL for 24h induced a similar degree of cell apoptosis, as determined by PI staining and FACS analysis (Fig. 3D).
Fluorescent NP-TRAIL Accumulates in Tumor Cells In Vivo
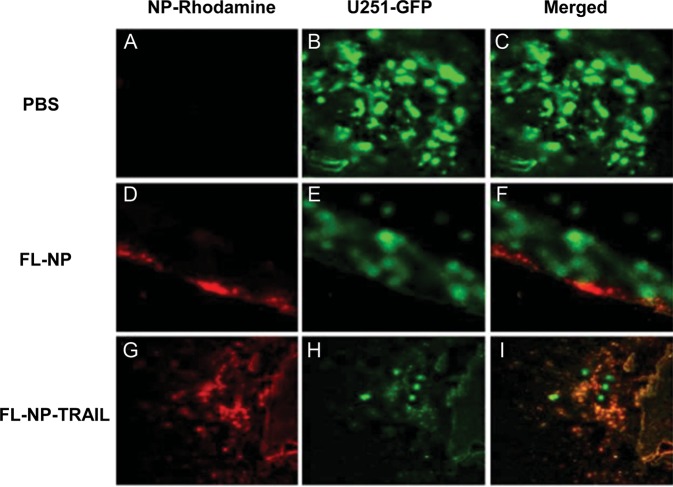
We next examined the ability of the FL-NP-TRAIL to accumulate in glioma cells in vivo. For these experiments, we generated glioma xenografts using U251 cells transfected with pEGFP-N1. Rhodamine-labeled NP or NP-TRAILs (0.25 μg) were implanted in the ipsilateral hemisphere 7 days after the GFP-U251 tumor cell implantation. Four days later, the animals were euthanized; brains were harvested and snap-frozen for sectioning and imaging. As can be seen in the bottom panel (Fig. 4G–I), the FL-NP-TRAIL (Fig. 4G) were not only found in areas of tumor mass (Fig. 4H), but they also colocalized with the GFP-labeled tumor cells (Fig. 4I). In contrast, the control FL-NP (Fig. 4D) were found near but did not co-localize with the tumor cells (Fig. 4F). No red fluorescence was observed in the PBS-treated animals (Fig. 4A).
Fig. 4.
FL-NP-TRAIL localize to tumor cells in U251 xenografts. Rhodamine-labeled control NP (FL-NP) or NP conjugated to TRAIL (FL-NP-TRAIL, 0.25 μg) were implanted directly within the tumor mass 7 days after GFP-U251 tumor cell implantation. Four days later, animals were euthanized, brains harvested and snap frozen for sectioning and imaging. As can be seen in the bottom panel, the FL-NP-TRAIL were not only found in areas of tumor mass, but they also colocalized within tumor cells (I), whereas the control NP (FL-NP) were found nearby, but did not colocalize with tumor cells (F). No red fluorescence was observed in the PBS-treated animals (C). All images are 20X. (A–C) – PBS treated; (D–F) – NP treated; (G–I) – NP-TRAIL treated. (A, D, G) – red channel; (B, E, H) – green channel; (C, F, I) – merged red and green channels. The results are representative of three different experiments that gave similar results.
NP-TRAIL Induces Apoptosis in Glioma Xenografts
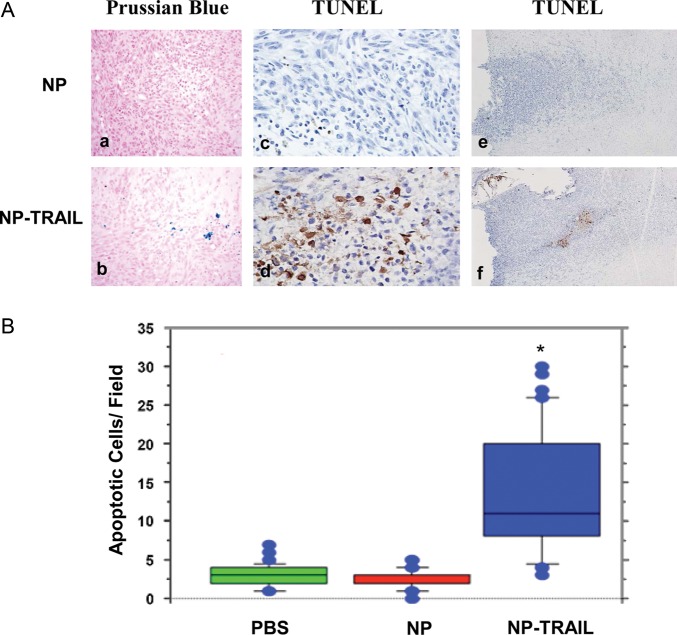
Because NP-TRAIL induces apoptosis in glioma cells in vitro, we examined the ability of the in vivo administered NP-TRAIL to induce cell apoptosis in the glioma xenografts. For these experiments, rats were implanted with the U251 cells intracranially and were treated with NP or NP-TRAIL (0.25 μg) after 7 days. The rats were euthanized at day 14, and cell apoptosis was analyzed using TUNEL staining. As presented in Fig. 5A: d,f), the NP-TRAIL induced a significant increase in cell apoptosis and TUNEL-positive cells tumor, as compared with NP-treated cells (Fig. 5A: c,e). Of interest, staining for NP in the tumor was observed for tumors treated with NP-TRAIL (Fig. 5A: b) but not for tumors treated with NP alone (Fig. 5A: a), further supporting the results shown in Fig. 4, that the NP were localized near but not in the tumor cells.
Fig. 5.
NP-TRAIL induce cell apoptosis in U251 xenografts. Nude rats were implanted with U251 cells. On day 7, NP or NP-TRAIL (0.25 μg) were administered in the same manner in the ipsilateral hemisphere. Animals were then euthanized on day 14, perfused, and their brains harvested and hemotoxylin and eosin (H&E) stained. Magnification 400X (A). Sections were stained for the presence of iron-labeled nanoparticles (blue stain) in NP alone (a) or in NP-TRAIL (b). Cell apoptosis was determined by TUNEL staining as described in the methods: NP alone (c, e) and NP-TRAIL (d, f). The quantification of TUNEL-positive cells in the NP or NP-TRAIL-treated xenografts was determined in at least 10 different areas containing the highest levels of positive staining, and the number of apoptotic cells per field was determined (B). The results are representative of three different experiments that gave similar results (A) or the means (with outliners) ± standard error of the mean (B). *P < .001.
Quantification of the TUNEL-positive tumor cells in rats treated with PBS, NP alone, or NP TRAIL clearly demonstrated a significant increase in the number of the apoptotic cells, as compared with the PBS- and NP-treated rats (Fig. 5B).
NP-TRAIL Decreases Tumor Bolume and Prolongs Animal Survival
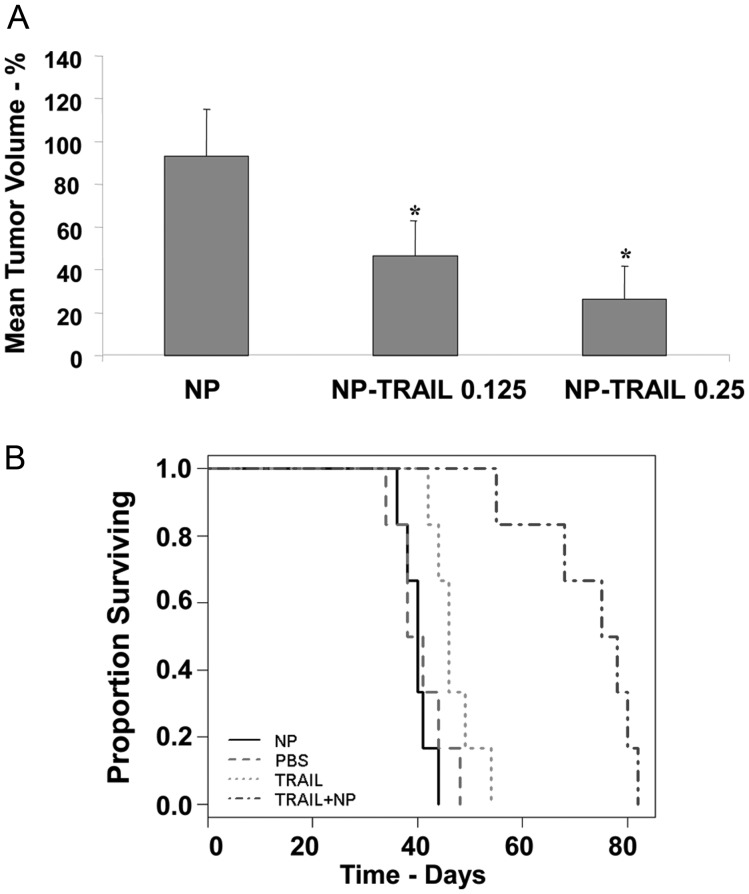
We then examined the effect of the NP-TRAIL on tumor volume and on the survival of the xenograft-bearing rats. Animals were implanted with human U251 cells intracranially and treated with NP alone or NP-TRAIL at day 7. Animals were euthanized at day 21, and brain tissue was harvested and sectioned for volume determination. Slides were photodocumented with identical settings for all slides used in this experiment. As presented in Fig. 6A, NP-TRAIL at both concentrations significantly decreased the tumor volume, as compared with xenografts that received NP alone or PBS (data not shown).
Fig. 6.
NP-TRAIL decrease tumor volume and prolong animal survival. Animals were implanted with human U251 tumor xenograft intracranially, followed 7 days later with administration of NP-TRAIL (0.25 μg), NP-TRAIL (0.125 μg), or NP alone (A). Animals were euthanized at day 21 and their brain tissue harvested and sectioned for volume determination. Slides were photodocumented with identical settings for all slides used in this determination. Volume was determined by measuring greatest width and length of tumor for every 15th 5-μm slice. Volume for each slice was determined and multiplied by the number of slices to the next measured slice until the edge of the tumor was reached. Tumor volume is given as arbitrary units (A). The results are representative of three different experiments that gave similar results. *P < .001. For analyzing the effect of the NP-TRAIL on animal survival, PBS, NP, TRAIL (1 μg), and NP-TRAIL (0.25 μg) were implanted in the ipsilateral hemisphere 7 days after U251 glioma cell implantation. Animals (n = 6 per group) were observed for signs of distress and/or morbidity and were euthanized at that time. Kaplan-Meier survival curves and two-sample t-tests of average survival show that TRAIL exerted only a marginal effect on animal survival, whereas NP-TRAIL significantly prolonged survival over that of control PBS-treated animals and NP alone or TRAIL-treated animals (B). The results are representative of four different experiments that gave similar results. NP-TRAIL vs all other groups P < .001; TRAIL vs PBS and NP P < .05.
We next examined the effects of TRAIL and NP-TRAIL on animal survival using U251 xenografts. The cells were implanted intracranially, and PBS, TRAIL, NP, and NP-TRAIL were administered 7 days thereafter (Fig. 6B). Animals were observed for signs of distress and/or morbidity and were euthanized at that time. NP-TRAIL significantly prolonged survival over that of TRAIL- and NP-treated animals (log-rank P < .001), with median survival of 76.5 days, compared with 46.0 days for TRAIL (P = .0002) and 40.0 days for NP-treated animals (P = .0065). Collectively, these results indicate that the conjugation of TRAIL to the NP increased the apoptotic activity of TRAIL, its ability to target glioma cells in vivo, and its therapeutic effect in glioma xenografts.
Discussion
TRAIL, a death receptor agonist, is characterized by a selective apoptotic effects on tumor cells;34 however, its bioavailability and lack of efficient administration modes hampers its clinical application.35 In this study, we demonstrated the in vitro and in vivo anti-tumor effects of a unique and uniform type of iron oxide NP conjugated to TRAIL for the treatment of glioma.
The results of the in vitro studies demonstrate that the NP-TRAIL are effective in inducing cell death of glioma cells and GSCs. We further showed that, in some cell lines and GSCs, the NP-TRAIL were even more potent than free TRAIL in inducing cell death. These data indicate that the conjugation of TRAIL to the NP does not interfere with the apoptotic effect of TRAIL and can even increase it. Indeed, other proteins, such as GDNF,19 thrombin,36 and factor VII,37 were also tested for bioactivity after conjugation to this type of iron oxide NP and were shown to maintain their bioactivity.
In addition to inducing cell death, the NP-TRAIL also synergized with γ-radiation and bortezomib in their cytotoxic effect on GSCs, as observed in the in vitro studies, suggesting that these treatments can sensitize resistant cells to the NP-TRAIL similar to their effects on recombinant TRAIL.33,38
Using fluorescent NP conjugated to TRAIL, we demonstrated the accumulation and internalization of the NP-TRAIL into glioma cells and GSCs but not in normal human astrocytes. Accordingly, the interaction of the fluorescent NP-TRAIL and their internalization into the glioma cells was followed by the induction of cell apoptosis, whereas no effect was observed in the astrocytes.
We further studied the accumulation and effects of NP-TRAIL in vivo using the well-established U251 xenograft as a glioma model.39 Similar to the in vitro results, we demonstrated that the fluorescent NP-TRAIL accumulated in the tumor site and were found in the majority of the xenograft cells. In contrast, the control fluorescent NP accumulated in the margin of the tumor and did not localize in the tumor cells. We further demonstrated that the administered NP-TRAIL induced cell apoptosis in the glioma xenografts, whereas no apoptotic effect was exerted by the control NP, suggesting that the NP-TRAIL can efficiently accumulate in the tumor cells and induce cell death in vivo and in vitro. Moreover, the results of the survival study clearly documented that the NP-TRAIL were more effective in decreasing the tumor volume and in prolonging the survival of the xenograft-bearing mice than higher concentrations of recombinant TRAIL. Of importance, the injection of the NP-TRAIL to tumor-free control rats did not result in any cytotoxic effects or changes in animal survival, suggesting that, similar to their in vitro effects, the NP-TRAIL induce cell death only in tumor cells.
Other types of NP and liposomes were used to deliver TRAIL to glioma tumors.40–42 However, these compounds differ from the described NP-TRAIL, because the technology used in those studies is based on the encapsulation of TRAIL within a nano-vehicle followed by slow release after intravenous injection. Thus, TRAIL is released from the vehicle prior to engagement with the targeted cancer cell receptors, which might decrease its activity on the tumor cells. In another study, the delivery of TRAIL gene using cationic albumin–conjugated pegylated NP was used to elevate TRAIL levels in BALB/c mice bearing intracranial C6 glioma cell-derived xenografts, with some delay in tumor growth.43 The NP-TRAIL described in this work represents a new approach in which TRAIL is directly conjugated to the NP and inflicts its apoptotic affect on the tumor cells as a single nano-TRAIL molecule. Because NP-TRAIL internalizes in the tumor cells, the NP can be further engineered to carry additional anti-tumor drugs, which may further increase the anti-tumor effect of TRAIL or sensitize resistant cells to this NP-conjugated ligand. We previously showed that direct injection of this type of NP to healthy rat brains had no apparent toxicity in the rats, with 80%–90% clearance after 40 days, as measured by MRI.12
Previous studies reported that glioma cells that were relatively resistant to TRAIL could be sensitized to TRAIL by treatment with subtoxic levels of ionizing radiation10,44 and with several compounds, such as resveratrol, roscovitine, cisplatin, troglitazone, bortezomib, MG-132, and temozolomide.45–49 Indeed, we recently reported that glioma cells and GSCs can be sensitized to the apoptotic effect of TRAIL using the proteasome inhibitors MG-132 and bortezomib.33 We now further demonstrate that GSCs can be sensitized to NP-TRAIL after treatment with subtoxic levels of bortezomib or low levels of ionizing radiation. Because the standard of care for patients with GBM includes radiation and chemotherapeutic agents, intratumor injection of NP-TRAIL may potentially result in a synergistic anti-tumor effect.
Collectively, these results indicate that the conjugation of TRAIL to the NP increases the apoptotic activity of TRAIL in glioma cells and GSCs in vitro, its ability to target glioma cells in vivo, and its therapeutic effect in glioma xenografts. Moreover, our in vitro studies demonstrated that radiation and proteasome inhibitors sensitize resistant tumor cells and GSCs to NP-TRAIL.
Funding
This work was supported by the William and Karen Davidson Fund, The Lori and Alan Zekelman Fund, Hermelin Brain Tumor Center, and Nanothera Ltd.
Acknowledgments
We thank Ana deCarvalho and Laura Hasselbach for their help with the glioma stem cells and the animal studies. Benny Perlstein and Susan A. Finniss contributed equally to this work.
Conflict of interest statement. None declared.
References
- 1.Parsons DW, Jones SN, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–4124. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 5.Van Meir EG, Hadjipanayis CG, Norden AD, Shu H-K, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA: A Cancer J Clinicians. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy? Apoptosis. 2009;14:607–623. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang S. The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene. 2008;27:6207–6215. doi: 10.1038/onc.2008.298. [DOI] [PubMed] [Google Scholar]
- 9.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi L, Bellail AC, Rossi MR, et al. Heterogeneity of primary glioblastoma cells in the expression of caspase-8 and the response to TRAIL-induced apoptosis. Apoptosis. 2011;16:1150–1164. doi: 10.1007/s10495-011-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhojani MS, Van Dort M, Rehemtulla A, Ross BD. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol Pharmaceutics. 2010;7:1921–1929. doi: 10.1021/mp100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong X, Mattingly CA, Tseng MT, et al. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69:3918–3926. doi: 10.1158/0008-5472.CAN-08-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlstein B, Ram Z, Daniels D, et al. Convection-enhanced delivery of maghemite nanoparticles: increased efficacy and MRI monitoring. Neuro Oncol. 2008;10:153–161. doi: 10.1215/15228517-2008-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Controlled Release. 2010;145:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances Enzyme Reg. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 16.Stephen ZR, Kievit FM, Zhang M. Magnetite nanoparticles for medical MR imaging. Materials Today. 2011;14:330–338. doi: 10.1016/S1369-7021(11)70163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margel S, Gura S. 2003. Nucleation and growth of magnetic metal oxide nanoparticles and its use. EP Patent 1,088,315. [Google Scholar]
- 18.Corem-Salkmon E, Ram Z, Daniels D, et al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int J Nanomed. 2011;6:1595–1602. doi: 10.2147/IJN.S23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, et al. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Ziv-Polat O, Topaz M, Brosh T, Margel S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials. 2010;31:741–747. doi: 10.1016/j.biomaterials.2009.09.093. [DOI] [PubMed] [Google Scholar]
- 21.Skaat H, Shafir G, Margel S. Acceleration and inhibition of amyloid-2- fibril formation by peptide-conjugated fluorescent-maghemite nanoparticles. J Nanoparticle Res. 2012;12:1–14. [Google Scholar]
- 22.Skaat H, Margel S. Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-[beta] fibrils detection and removal by a magnetic field. Biochem Biophys Res Commun. 2009;386:645–649. doi: 10.1016/j.bbrc.2009.06.110. [DOI] [PubMed] [Google Scholar]
- 23.Perlstein B, Lublin Tennenbaum T, Marom I, Margel S. Synthesis and characterization of functionalized magnetic maghemite nanoparticles with fluorescent probe capabilities for biological applications. J Biomed Mats Res Part B: Applied Biomats. 2008;92:353–360. doi: 10.1002/jbm.b.31521. [DOI] [PubMed] [Google Scholar]
- 24.Gordon T, Perlstein B, Houbara O, Felner I, Banin E, Margel S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2011;374:1–8. [Google Scholar]
- 25.Ziv O, Avtalion RR, Margel S. Immunogenicity of bioactive magnetic nanoparticles: Natural and acquired antibodies. J Biomed Mats Res. 2008;85:1011–1021. doi: 10.1002/jbm.a.31518. [DOI] [PubMed] [Google Scholar]
- 26.Lomonaco SL, Finniss S, Xiang C, et al. The induction of autophagy by λ3ג€ radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125:717–722. doi: 10.1002/ijc.24402. [DOI] [PubMed] [Google Scholar]
- 27.de Carvalho AC, Nelson K, Lemke N, et al. Gliosarcoma stem cells undergo glial and dmesenchymal differentiation in vivo. Stem Cells. 2010;28:181–190. doi: 10.1002/stem.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomonaco SL, Finniss S, Xiang C, et al. Cilengitide induces autophagy-mediated cell death in glioma cells. Neuro Oncol. 2011;13:857–65. doi: 10.1093/neuonc/nor073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okhrimenko H, Lu W, Xiang C, et al. Role of tyrosine phosphorylation and cleavage of PKCδ in its protective effect against tumor necrosis factor-related apoptosis inducing ligand-induced apoptosis. J Biol Chem. 2005;280:23643–23652. doi: 10.1074/jbc.M501374200. [DOI] [PubMed] [Google Scholar]
- 30.Okhrimenko H, Lu W, Xiang C, Hamburger N, Kazimirsky G, Brodie C. Protein kinase C-epsilon regulates the apoptosis and survival of glioma cells. Cancer Res. 2005;65:7301–7309. doi: 10.1158/0008-5472.CAN-05-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venere M, Fine HA, Dirks PB, Rich JN. Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer's hierarchy. Glia. 2011;59:1148–1154. doi: 10.1002/glia.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stiles CD, Rowitch DH. Glioma stem cells: A midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Kahana S, Finniss S, Cazacu S, et al. Proteasome inhibitors sensitize glioma cells and glioma stem cells to TRAIL-induced apoptosis by PKC [epsilon]-dependent downregulation of AKT and XIAP expressions. Cell Signal. 2011;23:1348–1357. doi: 10.1016/j.cellsig.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Lostao L, Marzo I, Anel A, Naval J. Targeting the Apo2L/TRAIL system for the therapy of autoimmune diseases and cancer. Biochem Pharmacol. 2012;83:1475–83. doi: 10.1016/j.bcp.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Nitsch R, Bechmann I, Deisz RA, et al. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Lancet. 2000;356:827–828. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 36.Ziv O, Lublin-Tennenbaum T, Margel S. Synthesis and characterization of thrombin conjugated λ3-€ 5Fe2O3 magnetic nanoparticles for hemostasis. Advanced Eng Mat. 2009;11:B251–B260. [Google Scholar]
- 37.Shafir G, Galperin A, Margel S. Synthesis and characterization of recombinant factor VII conjugated magnetic iron oxide nanoparticles for hemophilia treatment. J Biomed Mat Res. 2009;91:1056–1064. doi: 10.1002/jbm.a.32296. [DOI] [PubMed] [Google Scholar]
- 38.Nagane M, Cavenee WK, Shiokawa Y. Synergistic cytotoxicity through the activation of multiple apoptosis pathways in human glioma cells induced by combined treatment with ionizing radiation and tumor necrosis factor-related apoptosis-inducing ligand. J Neurosurg. 2007;106:407–416. doi: 10.3171/jns.2007.106.3.407. [DOI] [PubMed] [Google Scholar]
- 39.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim SM, Kim TH, Jiang HH, et al. Improved biological half-life and anti-tumor activity of TNF-related apoptosis-inducing ligand (TRAIL) using PEG-exposed nanoparticles. Biomaterials. 2011;32:3538–3546. doi: 10.1016/j.biomaterials.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Kim TH, Jiang H-H, Youn YS, et al. Preparation and characterization of Apo2L/TNF-related apoptosis-inducing ligand–loaded human serum albumin nanoparticles with improved stability and tumor distribution. J Pharmaceutical Sci. 2010;100:482–491. doi: 10.1002/jps.22298. [DOI] [PubMed] [Google Scholar]
- 42.Guo L, Fan L, Pang Z, et al. TRAIL and doxorubicin combination enhances anti-glioblastoma effect based on passive tumor targeting of liposomes. J Controlled Release. 2000;154:93–102. doi: 10.1016/j.jconrel.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Lu W, Sun Q, Wan J, She Z, Jiang X-G. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 44.Ciusani E, Croci D, Gelati M, et al. In vitro effects of topotecan and ionizing radiation on TRAIL/Apo2L-mediated apoptosis in malignant glioma. J Neurooncol. 2005;71:19–25. doi: 10.1007/s11060-004-9180-4. [DOI] [PubMed] [Google Scholar]
- 45.Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
- 46.Ding L, Yuan C, Wei F, et al. Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-FLIP. Canver Invest. 2011;29:511–520. doi: 10.3109/07357907.2011.605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultze K, Bock B, Eckert A, et al. Troglitazone sensitizes tumor cells to TRAIL-induced apoptosis via down-regulation of FLIP and survivin. Apoptosis. 2006;11:1503–1512. doi: 10.1007/s10495-006-8896-3. [DOI] [PubMed] [Google Scholar]
- 48.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 49.Hingtgen S, Ren X, Terwilliger E, Classon M, Weissleder R, Shah K. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and temozolomide. Mol Cancer Ther. 2008;7:3575–3585. doi: 10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]