Abstract
Mutations in isocitrate dehydrogenase 1 (IDH1) or 2 (IDH2) are found in a subset of gliomas. Among the many phenotypic differences between mutant and wild-type IDH1/2 gliomas, the most salient is that IDH1/2 mutant glioma patients demonstrate markedly improved survival compared with IDH1/2 wild-type glioma patients. To address the mechanism underlying the superior clinical outcome of IDH1/2 mutant glioma patients, we investigated whether overexpression of the IDH1R132H protein could affect response to therapy in the context of an isogenic glioma cell background. Stable clonal U87MG and U373MG cell lines overexpressing IDH1WT and IDH1R132H were generated, as well as U87MG cell lines overexpressing IDH2WT and IDH2R172K. In vitro experiments were conducted to characterize baseline growth and migration and response to radiation and temozolomide. In addition, reactive oxygen species (ROS) levels were measured under various conditions. U87MG-IDH1R132H cells, U373MG-IDH1R132H cells, and U87MG-IDH2R172K cells demonstrated increased sensitivity to radiation but not to temozolomide. Radiosensitization of U87MG-IDH1R132H cells was accompanied by increased apoptosis and accentuated ROS generation, and this effect was abrogated by the presence of the ROS scavenger N-acetyl-cysteine. Interestingly, U87MG-IDH1R132H cells also displayed decreased growth at higher cell density and in soft agar, as well as decreased migration. Overexpression of IDH1R132H and IDH2R172K mutant protein in glioblastoma cells resulted in increased radiation sensitivity and altered ROS metabolism and suppression of growth and migration in vitro. These findings provide insight into possible mechanisms contributing to the improved outcomes observed in patients with IDH1/2 mutant gliomas.
Keywords: glioma, isocitrate dehydrogenase 1, oxidative stress, IDH1, radiation
Diffuse gliomas are the most common type of primary adult brain tumor—with an annual incidence of ∼20 000 cases in the United States—and pose a significant health problem, as the vast majority remain incurable.1,2 Of these, glioblastoma multiforme (GBM; World Health Organization grade IV) is the most common and represents one of the most devastating human malignancies. Currently, the primary treatments for gliomas include surgical resection followed by radiotherapy (RT) and/or temozolomide (TMZ).
In 2008, a genome-wide somatic mutational analysis of glioblastomas revealed a subset with an isocitrate dehydrogenase 1 (IDH1) mutation at codon R132 (IDH1R132MUT).3 Subsequently, the IDH1 mutation was found in the majority of lower-grade and anaplastic diffuse gliomas.4,5 The IDH1 gene is located on chromosome 2 and encodes IDH1. IDH1 catalyzes the conversion of isocitrate to alpha-ketoglutarate (α-KG) and generates nicotine adenine disphosphonucleotide (NADPH) in the cytoplasm and peroxisomes.6 IDH1 is involved in lipid metabolism, glucose sensing, and defense against oxidative stress.7,8 At a much lower frequency, mutations in the functionally similar IDH2 gene have also been found in glioma patients. In addition to gliomas, the IDH1/2 mutation is found in a fraction of adult acute myelogenous leukemia (AML) patients.9,10 The most common IDH1 mutation is R132H (∼90%) with R132C and R132S found less commonly; the most common IDH2 mutation is R172K.8 Monoclonal antibodies have been derived for R132H11,12 and R132S.13
Since the identification of these mutations, there has been an explosion of interest in understanding how IDH1/2 mutation and the resulting alterations in cellular metabolism may contribute to tumor formation and behavior. Zhao and coworkers14 showed that the heterozygous IDH1 mutation dominantly inhibited the enzyme's activity, which led to the decreased formation of α-KG. The authors proposed that this contributed to gliomagenesis via increased expression of hypoxia-inducible factor 1α. Alternatively, Dang et al15 demonstrated that IDH1R132MUT protein possessed a novel enzyme activity, leading to the production of 2-hydroxyglutarate (HG). Elevated 2-HG has also been found in AML cells carrying IDH1 or IDH2 mutations.16,17 Recently, Koivunen et al18 demonstrated that the accumulation of 2-HG promoted transformation of human normal astrocytes, and multiple studies have shown that IDH1 mutant gliomas are associated with cytosine–phosphate–guanine (CpG) island hypermethylation.19
Patients with IDH1R132MUT gliomas have also been shown to have improved survival.5,20–22 The mechanism responsible for this improved survival is unclear, but it is possible that IDH1 mutant gliomas have a less aggressive phenotype and/or may respond better to standard therapies. One recent study showed that IDH1 or IDH2 mutations predicted response to TMZ and longer survival in low-grade gliomas.21 Another study showed that the mutation conferred improved prognosis independent of whether PCV (procarbazine, CCNU, vincristine) was given in addition to radiation.23 In that study the impact of IDH mutations on untreated tumors was limited. In AML, in contrast, the presence of the IDH1 mutation is an unfavorable prognostic factor.10,24
To evaluate the pathological consequence of the IDH1R132MUT mutation and how it might explain the improved survival seen in patients with IDH1R132MUT gliomas, we generated clonal U87MG glioma and U373MG glioma cell lines overexpressing the R132H mutant protein (IDH1R132H). Compared with control cells and cells overexpressing IDH1WT, IDH1R132H cells showed increased sensitivity to radiation in both U87MG cells and U373MG cells. This effect was also seen in U87MG cells overexpressing IDH2R172K, the most common glioma-associated IDH2 mutation. Examining the mechanism of radiosensitization, we found that U87MG-IDH1R132H cells also showed increased apoptosis and a heightened oxidative response to radiation. Interestingly, we also detected decreased proliferation at high cell density and decreased migration of U87MG-IDH1R132H cells. These findings suggest that the improved survival seen in patients with IDH1R132MUT tumors may result, in part, from direct effects of the IDH1R132MUT protein on proliferation, migration, and sensitivity to radiation.
Materials and Methods
Cell Culture
U87MG and U373MG glioma cells (obtained from Dr. Paul Mischel at UCLA, and originally from American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM)/F12 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen), 2 mm glutamine, and 100 units/mL of penicillin/streptomycin. Cells were dissociated with enzyme-free cell dissociation solution (Millipore) and cultured at 37°C and 5% CO2 in a 90% humidified tissue incubator. TMZ was obtained from the National Cancer Institute/National Institutes of Health (NCI/NIH) Developmental Therapeutics Program and dissolved in dimethyl sulfoxide (DMSO). N-acetyl cysteine (NAC; Sigma) was dissolved in phosphate buffered saline (PBS) (pH 7.4).
Generation of Constructs
Full-length human wild-type IDH1 and IDH2 coding sequences were amplified from U87MG cells. The cDNA was fused in-frame with a FLAG tag at the C-terminus by the adapter primers (for IDH1: forward primer with the XhoI site: 5′-TACTCGAGATGTCCAAAAAAATCAGTGG-3′, reverse primer with the EcoRI site and FLAG tag: 5′-ACGAATTCTTACTTGTCATCGTCATCCTTGTAATCCATAAGTTTGGCCTG-3′; for IDH2: 5′-TACTCGAGATGGCCGGCTACCTGCG-3′, reverse primer with the EcoRI site and FLAG tag: 5′-ACGAATTCTTACTTGTCATCGTCATCCTTGTAATCCATCTGCCTGCCCAG-3′) and inserted into the XhoI- and EcoRI-linearized pLPCX vector. The IDH1R132H and IDH2R172K alterations were generated in pLPCX-IDH1-WT-FLAG and pLPCX-IDH2-WT-FLAG using the QuikChange method (Stratagene) with the following primer sequence: 5′R132H (5′-ACCTATCATCATAGGTCATCATGCTTATGGG-3′) and 3′R132H (5′-TGACCTATGATGATAGGTTTTACCCATCCAC-3′); 5′R172K (CCCATCACCATTGGCAAGCACGCCCATGG-3′) and 3′R172K (5′-TTGCCAATGGTGATGGGCTTGGTCCAGC-3′).
Stable Overexpression of IDH1WT, IDH1R132H, IDH2WT, and IDH2R172K Constructs in Glioma Cells
Using the FuGene HD transfection system (Roche), Plat-A cells were transfected with pLPCX (vector only), pLPCX-IDH1-WT-FLAG, pLPCX-IDH1-ΔR132H-FLAG, pLPCX-IDH2-WT-FLAG, and pLPCX-IDH2-ΔR172K-FLAG constructs. U87MG or U373MG cells were infected with retrovirus produced by Plat-A package cells in medium containing 8 μg/mL polybrene. Cells were then selected with puromycin (0.7 μg/mL for U87MG and 0.5 μg/mL for U373MG) after 48 h. For each construct, >10 single clonal cell lines were derived from the selected populations by limited dilution, and 2–4 representative cell lines per construct were selected for further experiments. All clones were maintained in culture media with puromycin. Protein expression was confirmed by western blot and immunofluorescence. For U87MG-control cells, U87MG-IDH1WT cells, and U87MG-IDH1R132H cells, 4 clones of each construct were selected based on expression level and were designated clones 1, 2, 3, and 4. Clones 1–4 were used in an assay by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma), and clones 1 and 2 were used in all other experiments. For U373MG-control cells, U373MG-IDH1WT cells, U373MG-IDH1R132H cells, U87MG-IDH2WT cells, and U87MG-IDH2R172K cells, 2 clones of each construct were selected based on expression level and were designated clones 1 and 2, which were used in all other experiments. All results represented the mean ± SEM for 4 or 2 clones. All clones for each construct had similar behavior in all experiments.
Immunofluorescence
Cells were grown on glass slides, fixed in 100% methanol at −20°C for 15 min, and stained with mouse monoclonal anti-FLAG M2 antibody (Sigma), goat anti-human IDH1 (Santa Cruz Biotech), mouse anti-human R132H mutant IDH1 (IDH1R132H) antibody (Dianova),11 fluorescein isothiocynate–conjugated goat anti-mouse immunoglobulin (Ig) G (Invitrogen), and Dy649-conjugated donkey anti-goat IgG (Jackson ImmunoResearch). Nuclei were counterstained with propidium iodide or 4′,6-diamidino-2-phenylindole (DAPI). Slides were mounted and imaged on an Olympus IX71 confocal microscope.
Western Blot
Total protein was isolated using radioimmunoprecipitation assay lysis buffer containing protease inhibitor, separated on 12% sodium dodecyl sulfate–polyacrylamide gel, and transferred to nitrocellulose membranes. Western blot was performed with mouse monoclonal anti-FLAG M2 antibody (Sigma), goat anti-human IDH1 (Santa Cruz Biotech), mouse anti-human IDH1R132H antibody (Dianova), mouse anti-human α-tubulin (Sigma), horse radish peroxidase (HRP)–conjugated goat anti-mouse IgG (Jackson ImmunoResearch), HRP-conjugated rabbit anti-goat IgG (Santa Cruz Biotech), and the SuperSignal West Pico Kit (Thermo Scientific).
2-HG Determination by Chromatography and Mass Spectrometry
Extracellular concentrations of 2-HG were measured using high-performance liquid chromatography (HPLC) or gas chromatography (GC) followed by mass spectrometry (MS). For HPLC-MS determination of extracellular 2-HG, 50-μL aliquots of cell media were transferred to sample tubes, and 250 µL of acetonitrile was added for sample extraction. An aliquot of the supernatant (50 µL) was diluted with water (250 µL) and injected (10 µL) onto a reverse-phase HPLC column (Phenomenex Synergi Polar-RP, 4.6 × 150 mm, 4-μm particle size) equilibrated in 80% of eluent A (water/formic acid 100/0.1, volume/volume [v/v]) and 20% of eluent B (acetonitrile/formic acid, 100/0.1, v/v). The chromatographic separation was achieved with gradient elution of 20%–70% eluent B in 4 min. The effluent from the column was directed to an electrospray ionization (ESI) source connected to a triple quadrupole mass spectrometer (API 5500 QTrap, Sciex; −5 kV ESI capillary voltage, 500°C source temperature, 80 m dwell time per channel, 5-m interchannel delay, unit mass resolution on both Q1 and Q3) operating in the negative-ion selected reaction monitoring mode, in which the intensity of the m/z 147→129 transition of 2-HG was monitored.
MTT Cell Viability Assay
The 96-well plates were coated with 0.1% gelatin before seeding cells. Cell viability was determined by MTT assay as previously described.25 Absorbance at 535 nm was measured on a Wallac Victor2 1420 plate reader (PerkinElmer) with a background reference filter at 660 nm.
Colony Formation Assay
Cell survival after radiation and TMZ treatment was assessed by colony formation assay. One thousand cells were seeded onto 60-mm dishes and incubated overnight. For radiation treatment, 7.43–7.49 Gy/min of irradiation was delivered to the cells on a rotating platform using a Mark-I Irradiator. TMZ treatment was performed as previously described.25 The cell culture medium was replaced with fresh medium containing TMZ (NCI/NIH Developmental Therapeutics Program) or vehicle only (DMSO) as control. Cells were incubated after irradiation or TMZ treatment for 14 days and then fixed with methanol and stained with 0.25% crystal violet. Colonies containing >30 cells were counted under a dissecting microscope. The results are reported as a percentage of the colonies in untreated cultures of each corresponding clone.
Apoptosis Analysis by Flow Cytometry
Apoptotic cells were stained with YO-PRO-1 (Invitrogen) per the manufacturer's instructions and assayed with flow cytometry. Briefly, cells were treated with radiation, cultured for 48 h, and then stained with 1 µM YO-PRO-1 and 10 µg/mL propidium iodide for 30 min before being harvested in trypsin and analyzed with flow cytometry.
Reactive Oxygen Species Measurement
Cellular ROS levels were quantitated using the oxidant-sensitive fluorescent dye 2′,7′-dichlorfluorescein-diacetate (DCFH-DA; Sigma) and a Wallac Victor2 1420 plate reader (PerkinElmer) as previously described.26 Briefly, cells were incubated with 10 μM DCFH-DA for 30 min at 37°C and then washed twice by using PBS before radiation or tert-butyl hydroperoxide (tert-BHP) exposure. Dichlorodihydrofluorescein (DCF) was measured 90 min (for radiation) or 10 min (for tert-BHP) after exposure by using the plate reader at excitation and emission wavelengths of 485 nm and 535 nm, respectively.
Soft Agar Growth Assays
Growth in soft agar was measured by colony assay as previously described.27 Briefly, 2-mL underlayers of 0.6% agar medium were prepared in 6-well plates by combining equal volumes of 1.2% Noble agar (Fisher) and DMEM with 20% FBS. Cells (2 × 103) were plated in 0.3% agar medium and cultured for 2–3 weeks. Colonies were then photographed, and colonies larger than 50 μm in diameter were counted in 5 random microscopic fields and tabulated.
Monolayer Cell Migration Scratch Assay
Cells were plated in 6-well plates coated with 0.1% gelatin to create a confluent monolayer. Scratch assay was performed as previously described.28 The scratch wound was observed using contrast microscopy (Olympus, IX41), and images were taken 0 h, 3 h, 6 h, 9 h, 12 h, and 24 h after the initial scratch. Scratch wound distance was quantitated using Adobe Photoshop software.
Transwell Migration Assay
Cell migration assays were performed essentially as described previously.29,30 Briefly, transwell plates (8-μm pore size) were coated with 100 μL gelatin (1 mg/mL) on the bottom side of the membrane and dried at room temperature. The transwell plates were assembled in a 24-well plate, and 1 × 105 cells were suspended in 100 μL FBS free culture media and added to each upper chamber. After a 5-h incubation period, the transwell plates and the nonmigrating cells in the top wells were removed. The migrating cells remaining on the bottom side of the transwell plates were fixed and stained with 0.25% crystal violet. Quantification of cell migration was done by calculating the area of migrated cells on the bottom chamber and was expressed as a percent of control.
Statistical Analyses
Statistical analysis was performed with GraphPad Prism 5 software. Statistical significance was determined using Student's t-test. Data are expressed as mean ± SEM, and the level of significance is set at P < .05, P < .01, and P < .001.
Results
Stable Expression of Wild-type and Mutant FLAG-tagged IDH1 and IDH2 Constructs in Glioma Cells
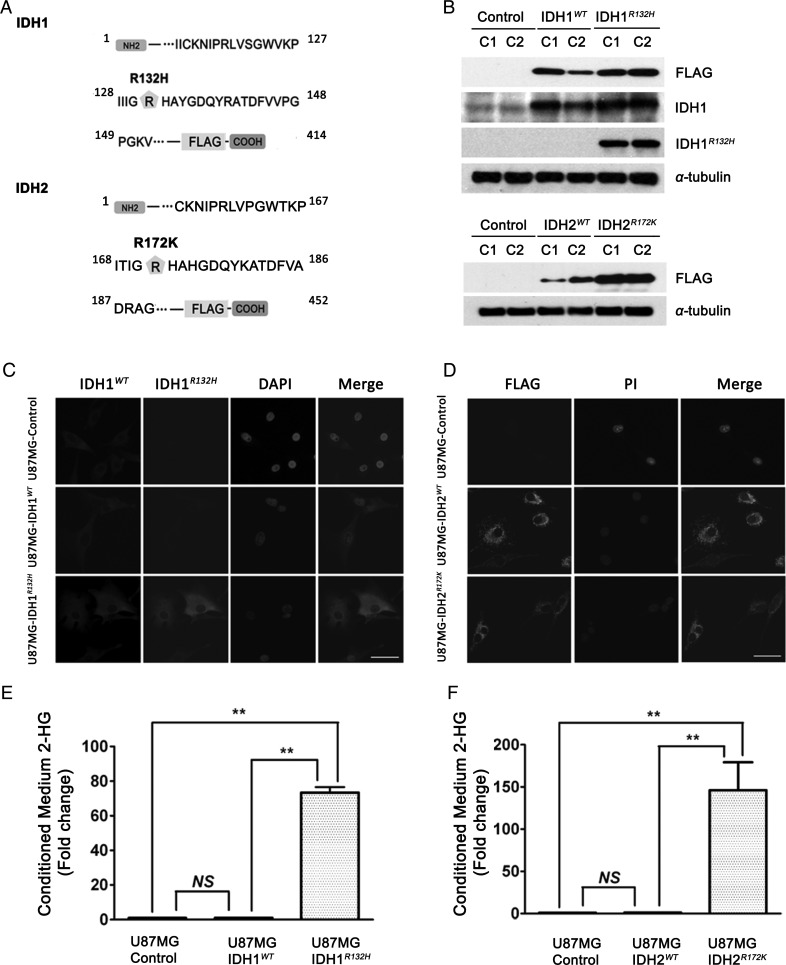
To determine the effects of overexpression of human glioma-associated IDH mutant proteins within an isogenic glioma cell context, we generated carboxy-terminus FLAG-tagged IDH1WT, IDH1R132H, IDH2WT, IDH2R172K, and control (vector only) constructs, stably expressed them in U87MG cells, and isolated clonal cell lines (U87MG-control, U87MG-IDH1WT, U87MG-IDH1R132H, U87MG-IDH2WT, and U87MG-IDH2R172K) (Fig. 1). The same FLAG-tagged IDH1WT and IDH1R132H constructs were also stably expressed and single-cell cloned in parallel with vector only in U373MG cells (U373MG-control, U373MG-IDH1WT, and U373MG-IDH1R132H) (Fig. 1A, Fig. S1). Western blot analysis with anti-FLAG, anti-IDH1, and mutant-specific anti-IDH1R132H antibodies confirmed the expression of the appropriate IDH1 protein products in the various cell lines. Similar confirmation of the IDH2 protein product expression was achieved by western blot analysis using an anti-FLAG antibody (Fig. 1B, Fig. S1A). By immunofluorescence, the steady-state cellular localization of the IDH1R132H mutant protein appeared indistinguishable from that of the IDH1WT protein, indicating that the mutations did not significantly alter the targeting of the IDH1 protein; similarly, the localization of the mutant IDH2R172K protein appeared identical to that of the IDH2WT protein (Fig. 1C and 1D). Using densitometric analysis of western blot, we found that for the selected clones, IDH1WT was overexpressed ∼3–4-fold over endogenous IDH1, and IDH1R132H was overexpressed by ∼5–6-fold in U87MG cells (Fig. S1B). The level of overexpression of IDH1WT or IDH1R132H was ∼2–3-fold more than endogenous IDH1 in U373MG cells (Fig. S1C).
Fig. 1.
Establishment of glioma stable cell lines overexpressing IDH1WT, IDH1R132H, IDH2WT, and IDH2R172K. (A) Schematic diagram of the IDH1-FLAG and IDH2-FLAG fusion protein amino acid sequence. The FLAG tag was linked to the C-terminus of IDH1 and IDH2. Amino acid residue 132 for IDH1 and 172 for IDH2 are indicated with ( ). (B) Representative western blot for FLAG-tagged IDH1WT and IDH1R132H or IDH2WT and IDH2R172K in stably transfected U87MG cells (2 representative independent clones for each construct, C1 and C2, are shown) using anti-FLAG, anti- IDH1WT, and anti-IDH1R132H antibodies. (C) Immunofluorescence showed the diffuse cytoplasmic distribution of IDH1WT and IDH1R132H proteins stained by anti-FLAG antibody (green), anti-IDH1WT antibody (red), and anti-IDH1R132H (green). Nuclei were counterstained with DAPI (blue). 40×, bar = 20μm (D) Immunofluorescence shows the punctate cytoplasmic distribution of IDH2WT and IDH2R172K proteins stained by anti-FLAG antibody (green), and nuclei were counterstained with propidium iodide (PI) (red), 40×, bar = 20μm. (E) ∼70-fold increase in 2-HG level was found in the conditioned medium of U87MG-IDH1R132H cells by HPLC-MS. Data were normalized by cell number and expressed as fold change relative to U87MG-control (mean ± SEM, n =3). **P < .01 compared with U87MG-control and U87MG-IDH1WT cells. (F) ∼140-fold increase in 2-HG level was found in the conditioned medium of U87MG-IDH2R172K cells by HPLC-MS. Data were normalized by cell number and expressed as mean fold change relative to U87MG-control (mean ± SEM, n =3). **P < .01 compared with U87MG-control and U87MG-IDH2WT cells.
). (B) Representative western blot for FLAG-tagged IDH1WT and IDH1R132H or IDH2WT and IDH2R172K in stably transfected U87MG cells (2 representative independent clones for each construct, C1 and C2, are shown) using anti-FLAG, anti- IDH1WT, and anti-IDH1R132H antibodies. (C) Immunofluorescence showed the diffuse cytoplasmic distribution of IDH1WT and IDH1R132H proteins stained by anti-FLAG antibody (green), anti-IDH1WT antibody (red), and anti-IDH1R132H (green). Nuclei were counterstained with DAPI (blue). 40×, bar = 20μm (D) Immunofluorescence shows the punctate cytoplasmic distribution of IDH2WT and IDH2R172K proteins stained by anti-FLAG antibody (green), and nuclei were counterstained with propidium iodide (PI) (red), 40×, bar = 20μm. (E) ∼70-fold increase in 2-HG level was found in the conditioned medium of U87MG-IDH1R132H cells by HPLC-MS. Data were normalized by cell number and expressed as fold change relative to U87MG-control (mean ± SEM, n =3). **P < .01 compared with U87MG-control and U87MG-IDH1WT cells. (F) ∼140-fold increase in 2-HG level was found in the conditioned medium of U87MG-IDH2R172K cells by HPLC-MS. Data were normalized by cell number and expressed as mean fold change relative to U87MG-control (mean ± SEM, n =3). **P < .01 compared with U87MG-control and U87MG-IDH2WT cells.
Consistent with prior reports,15 we found, using HPLC-MS, that U87MG-IDH1R132H cells generated a concentration increase in 2-HG of ∼70-fold in conditioned medium compared with U87MG-control and U87MG-IDH1WT cells (Fig. 1E). These results were independently confirmed using GC-MS (Fig. S2A). Using HPLC-MS, similarly increased 2-HG levels were seen in the conditioned medium from U373MG-IDH1R132H (Fig. S2B) and U87MG-IDH2R172K cells (Fig. 1F).
IDH1R132H and IDH2R172K Overexpression Increases Sensitivity to Radiation Treatment
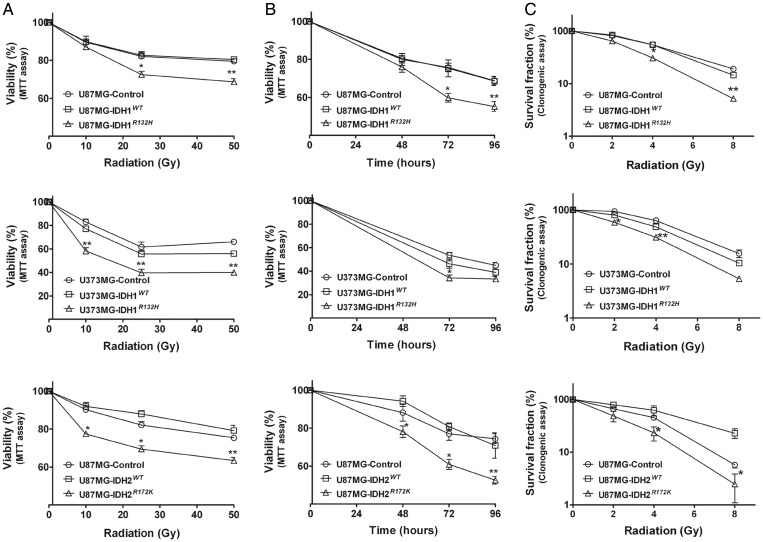
Given the clinical observation that patients with IDH1/2 mutant tumors have prolonged survival, we hypothesized that one contributing factor could be that IDH1R132H tumors are more sensitive to treatment. To test this hypothesis, we treated the isogenic U87MG-control, U87MG-IDH1WT, and U87MG-IDH1R132H clonal cell lines with radiation, a common treatment modality for glioma. Following escalating doses of radiation, MTT cell viability (Fig. 2A and 2B) and colony formation assays (Fig. 2C) showed that the U87MG-IDH1R132H cells exhibited dose- and time-dependent increased loss of viability compared with U87MG-control and U87MG-IDH1WT cell lines. To determine whether this effect could be observed in another isogenic glioma cell context, we performed similar experiments on U373MG cells, which notably differ from U87MG cells in harboring mutant p53 and phosphatase and tensin homolog (PTEN). In both the MTT assay (Fig. 2A and 2B) and the colony forming assay (Fig. 2C), we found that U373MG-IDH1R132H cells also demonstrated increased sensitivity to radiation compared with U373MG-control and U373MG-IDH1WT cells, albeit to a slightly less degree. This may reflect lower levels of overexpression of IDH1R132H in U373MG cells compared with U87MG cells (Fig. S1A and S1C). To determine whether overexpression of IDH2R172K, the most common glioma-associated IDH2 mutation, was also associated with radiosensitization, we tested U87MG-control, U87MG-IDH2WT, and U87MG-IDH2R172K cells. In both MTT (Fig. 2A and 2B) and colony forming assays (Fig. 2C), we observed that U87MG-IDH2R172K cells also showed increased sensitization to radiation similar to that seen for U87MG-IDH1R132H cells.
Fig. 2.
Overexpression of IDH1R132H or IDH2R172K increased the sensitivity of glioma cells to radiation treatment. (A) Cells were treated with various doses of radiation and incubated for 48 h for U87MG cells or 72 h for U373MG cells. Viability was quantitated with MTT assay and expressed as mean percentage of untreated control cells (mean ± SEM, n =3). *P < .05, **P < .01 compared with control, IDH1WT or IDH2WT cells. (B) Cells were treated with 25 Gy of radiation for different incubation times. Viability was quantitated with MTT assay and expressed as mean percentage of untreated control cells (mean ± SEM, n = 3). *P < .05, **P < .01 compared with control, IDH1WT or IDH2WT cells. (C) Equal number of cells was seeded into 60-mm dishes and treated with different doses of radiation 24 h later. After 14 days, the cells were fixed and stained with 0.25% crystal violet. Mean clonogenic survival of cells after radiation was plotted on a logarithmic scale (mean ± SEM, n =3). *P < .05, **P <.01 compared with control, IDH1WT or IDH2WT cells.
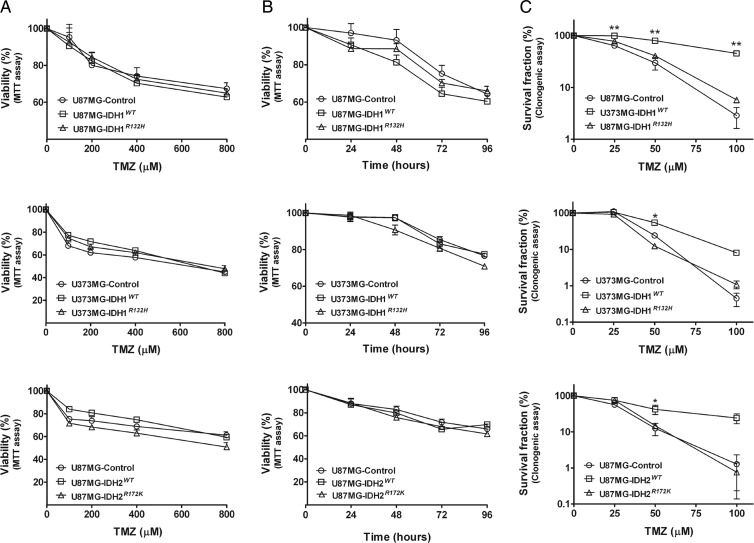
IDH1R132H and IDH2R172K Overexpression Does Not Affect TMZ Sensitivity, Whereas IDH1WT and IDH2WT Overexpression Appears Protective Against TMZ
Since the alkylating agent, TMZ, is also commonly used in the treatment of gliomas, we sought to determine whether overexpression of IDH1R132H altered sensitivity to TMZ. After exposing U87MG-control, U87MG-IDH1WT, and U87MG-IDH1R132H cells to varying concentrations of TMZ, we found, using MTT assays, that there were no differences in viability among the 3 cell lines, in either a dose- (Fig. 3A) or a time-dependent manner (Fig. 3B). Similar lack of differences was seen among U373MG-control, U373MG-IDH1WT, and U373MG-IDH1R132H (Fig. 3A and 3B). When measured by clonogenic assay, we also found that TMZ sensitivity was similar between U87MG-control and U87MG-IDH1R132H cells. Interestingly, however, U87MG-IDH1WT cells showed increased survival percentage when treated with TMZ, compared with U87MG-control and U87MG-IDH1R132H cells, suggesting a protective effect of overexpressed IDH1WT protein against TMZ cytotoxicity (Fig. 3C). We observed this same pattern in U373MG cells, in which U373MG-IDH1WT cells demonstrated decreased TMZ sensitivity by clonogenic assay compared with either U373MG-IDH1R132H or U373MG-control cells (Fig. 3C). In addition, when we performed the same experiments on U87MG-control, U87MG-IDH2WT, and U87MG-IDH2R172K cells, we similarly observed no difference among any of the cell lines on MTT assay (Fig. 3A and 3B), whereas the colony forming assay also showed decreased TMZ sensitivity of the U87MG-IDH2WT cells (Fig. 3C).
Fig. 3.
IDH1R132H and IDH2R172K overexpression did not affect TMZ sensitivity. (A) Cells were treated with various doses of TMZ for 72 h. Viability was quantitated with MTT assay and expressed as mean percentage of untreated control cells (mean ± SEM, n =3). (B) Cells were treated with 400 μM of TMZ for different periods of time. Viability was quantitated with MTT assay and expressed as mean percentage of untreated control cells (mean ± SEM, n =3). (C) Clonogenic survival of cells after TMZ treatment was plotted on a logarithmic scale (mean ± SEM, n =3). *P < .05, **P < .01 compared with control, IDH1R132H or IDH2R172K cells.
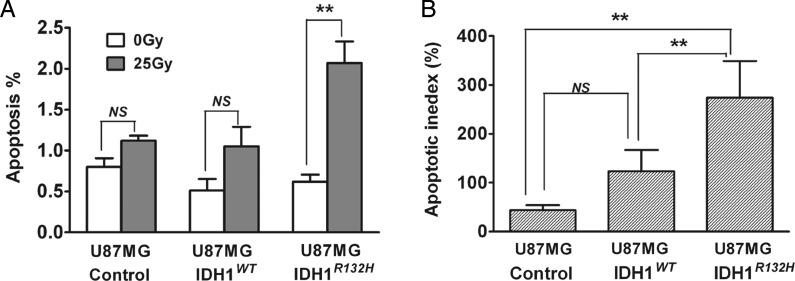
U87MG-IDHR132H Cells Show Significant Increase in Apoptosis Following Radiation Treatment
Using the U87MG-control, U87MG-IDH1WT, and U87MG-IDH1R132H cells, we investigated molecular mechanisms mediating the effect of overexpression of IDHR132H protein on increasing radiation sensitivity. To determine whether increased radiation sensitivity was accompanied by increased apoptotic index, we assessed the apoptotic index in the various U87MG cell lines using the fluorescent dye YO-PRO-1. Following radiation, measurement of YO-PRO-1 staining by flow cytometry showed that U87MG-IDH1R132H cells had an increased apoptotic index compared with U87MG-control and U87MG-IDH1WT cells (Fig. 4). In comparison, all of the various U87MG cells demonstrated similar increases in apoptotic index upon treatment with paclitaxel, used as a positive control (Fig. S3).
Fig. 4.
U87MG-IDHR132H cells showed significant increase in apoptosis following radiation treatment. (A) Apoptosis was assessed by YO-PRO-1 and propidium iodide (PI) staining after 48 h radiation treatment (0 Gy or 25 Gy). Harvested cells were evaluated by flow cytometry. Column plot summarized 3 independent experiments, each performed with triplicates (mean ± SEM, n =3). **P < .01 compared with U87MG-control and U87MG-IDH1WT cells. (B) Apoptosis % was normalized to U87MG-control cells treated with 0 Gy and expressed as mean apoptotic index (mean ± SEM, n =3). **P< .01 compared with U87MG-control and U87MG-IDH1WT cells.
U87MG-IDH1R132H Cells Show Increased ROS Levels Upon Radiation Treatment
Given that IDH1 functions to generate NADPH, we hypothesized that impaired NADPH production resulting in increased susceptibility to oxidative stress might contribute to the increased radiation sensitivity in U87MG-IDH1R132H cells. To investigate whether IDH1R132H alters ROS metabolism, we measured levels of cellular oxidants using the fluorescent dye DCFH-DA, which is retained in cells following de-esterification and becomes fluorescent when oxidized. We found that basal DCF fluorescence level was lower in U87MG-IDH1R132H cells compared with U87MG-control and U87MG-IDH1WT cells by using the plate reader (Fig. 5A) and fluorescence-activated cell sorting (Fig. S4). However, after radiation treatment, the U87MG-IDH1R132H cells showed an accentuated elevation in ROS level compared with both U87MG-control and U87MG-IDH1WT cells, which had little increase in DCF signal after radiation treatment (Fig. 5B). Similar oxidant response was shown when cells were treated with tert-BHP, a mimic of lipid hydroperoxides, with increased levels detected in U87MG-IDH1R132H cells compared with either U87MG-control or U87MG-IDH1WT cells (Fig. S5).
Fig. 5.
U87MG-IDHR132H cells showed increased oxidative response to radiation. Cells were incubated with the oxidant sensitive fluorescent dye DCFH-DA for 30 min before exposure to radiation, and ROS level was measured 90 min later by the plate reader. (A) The baseline ROS levels were measured by DCF assay and expressed as mean fold change relative to U87MG-control cells (mean ± SEM, n = 3). *P < .05, **P< .01 compared with U87MG-control and U87MG-IDH1WT cells. (B) The ROS level after radiation treatment was measured by DCF assay and expressed as mean fold change relative to untreated U87MG-control, U87MG-IDH1WT, and U87MG-IDH1R132H cells (mean ± SEM, n =3). *P < .05 compared with U87MG-control and U87MG-IDH1WT cells.
N-Acetyl Cysteine Protects U87MG-IDH1R132H Cells From Radiation
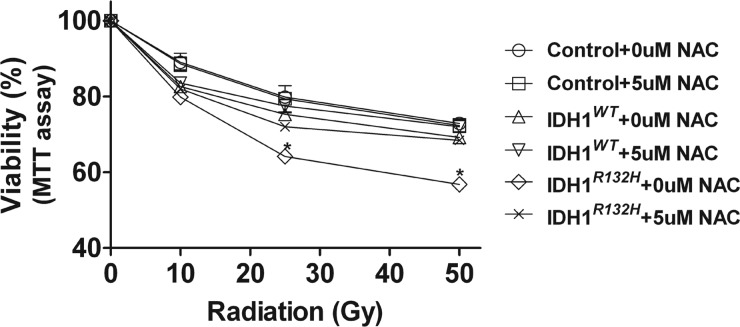
To determine whether addition of an ROS scavenger can protect the U87MG-IDHR132H cells from radiation, we pre-incubated cells with 5 μM NAC for 4 h before exposure to different doses of radiation and determined cell viability 48 h later by MTT assay. The choice of the low dose of 5 μM NAC incubation was determined as a concentration with minimal effects on viability of the various cell lines (Fig. S6). We observed that the increased radiosensitivity of U87MG-IDH1R132H was abrogated in the presence of 5 μM NAC. In contrast, this dose of NAC did not affect the radiosensitivity of either U87MG-IDH1WT or U87MG-control cells (Fig. 6).
Fig. 6.
ROS scavenger protected the U87MG-IDHR132H cells from radiation. Cells were pretreated with NAC for 4 h before exposure to different doses of radiation. Viability was determined 48 h later by MTT assay and expressed as mean percentage of untreated U87MG-control cells (mean ± SEM, n= 3). *P < .05, **P < 0.01 compared with U87MG-control and U87MG-IDH1WT cells.
U87MG-IDH1R132H Cells Show Decreased Cell Proliferation at High Growth Density, Growth in Soft Agar, and Glioma Migration
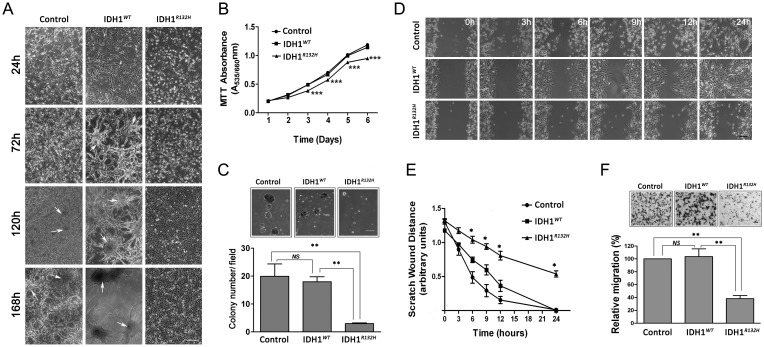
During routine cell culture, we observed that the U87MG-IDH1R132H cells appeared more cuboidal than U87MG-IDH1WT cells and that the mutant cells appeared to grow more slowly in culture. At higher cell densities, U87MG-IDH1R132H cells maintained a monolayer, whereas the U87MG-control and U87MG-IDH1WT cells formed multilayered cell clusters (Fig. 7A). The MTT proliferation assays showed that U87MG-IDH1R132H cells grew more slowly than U87MG-control and U87MG-IDH1WT cells at higher cell density (Fig. 7B). These findings were confirmed by direct cell counting (Fig. S7). We measured colony formation of the cells in soft agar (non-adherent) for the various cell lines and found that the U87MG-IDH1R132H cells had significantly decreased colony formation ability as well as smaller colony size than U87MG-control and U87MG-IDH1WT cells (Fig. 7C). Based on the altered growth characteristics demonstrated by the U87MG-IDH1R132H cells, we sought to investigate whether the IDH1R132H protein could suppress cell migration using a wound-healing assay to study directional cell migration in vitro.28 As seen qualitatively in Fig. 7D, U87MG-IDH1R132H cells showed decreased migration. Quantitative assessment of these assays clearly show decreased cell migration in the U87MG-IDH1R132H cells compared with U87MG-control and U87MG-IDH1WT cells (Fig. 7E). Similar results showing decreased migration of U87MG-IDH1R132H cells were obtained with the transwell migration assay (Fig. 7F).
Fig. 7.
U87MG-IDHR132H protein overexpression decreased cell proliferation, growth in soft agar, and migration. (A) Phase contrast microscopy pictures of U87MG-control, U87MG-IDH1WT cells, and U87MG-IDH1R132H cells in culture. Clusters are found at higher densities U87MG-control, U87MG-IDH1WT cells (indicated by white arrows). 4×, bar = 100 μm (B) Cell proliferation was determined by MTT assay and expressed as mean absorbance value at 535 nm with reference at 660 nm (mean ± SEM, n =3). ***P < .001 compared with U87MG-control and U87MG-IDH1WT cells. (C) Soft agar colony formation assay was performed by counting individual colonies (>50 µm) in 5 random microscopic fields, and representative micrographs are shown. The quantification of colony numbers are shown as mean ± SEM (n =3). 4×, bar = 100 μm, **P < .01 compared with U87MG-control and U87MG-IDH1WT cells. (D) and (E) Monolayer cell migration scratch assay. Representative phase micrographs of U87MG-control, U87MG-IDH1WT, and U87MG-IDHR132H cells at 0 h, 3 h, 6 h, 9 h, 12 h, and 24 h after monolayer wounding are shown in D. Monolayer scratch wound distances were quantitated in (E) and expressed as mean ± SEM (n = 3). 4×, bar = 100 μm, *P < .05 compared with U87MG-control and U87MG-IDH1WT cells. (F) Cell migration quantitated with a transwell migration assay. U87MG-control, U87MG- IDH1WT, and U87MG-IDH1R132H cells were plated in the transwell chamber for 5 h before migrated cells were fixed, stained, and counted. Top: representative micrographs of migrated cells. Bottom: migrated cell number is quantitated and expressed as mean percentage of U87MG-control (mean ± SEM, n =3). 4×, bar = 100 μm, **P < .01 compared with U87MG-control and U87MG-IDH1WT cells.
To determine whether overexpression of IDH1R132H had similar effects in U373MG cells, we compared proliferation and migration among U373MG-control, U373MG-IDH1WT, and U373MG-IDH1R132H. In contrast to what we found with U87MG cells, we did not observe decreased growth (Fig. S8A) and migration in U373MG-IDH1R132H (Fig. S8B), suggesting that these effects are cell context dependent. The slightly lower lever of overexpression of IDH1R132H provides another possible explanation for the lack of effect on U373MG cells (Fig. S1B and S1C).
Discussion
Using an isogenic U87MG model system, we demonstrated that overexpression of the IDH1R132H mutant protein enhanced the cytotoxic effect of radiation exposure, possibly through altered ROS metabolism. There was also similar radiosensitization in cells overexpressing IDH2R172K. These findings are consistent with improved survival in patients with IDH1R132H or IDH2R172K mutant tumors because radiation is the most common therapeutic treatment for glioma patients. For example, in a recent study by the European Organisation for Research and Treatment of Cancer (EORTC), patients with tumors harboring IDH1 or IDH2 mutations showed similarly improved outcomes whether they received RT or RT/PCV.23 From these data, the authors concluded that IDH mutation is a prognostic marker but not predictive of PCV response. Since all patients received RT, an interpretation of these data is that IDH1R132H predicts improved response to RT. In addition, our data showed that IDH1R132H protein did not sensitize U87MG cells to TMZ and that, interestingly, overexpression of IDH1WT increased resistance of U87MG cells to TMZ. Thus far, retrospective clinical studies have reported conflicting results regarding TMZ response in IDH1 mutant gliomas. For example, Houillier and coworkers21 found that IDH1R132MUT predicted response to TMZ in low-grade gliomas, while Dubbkink et al22 found that IDH1R132MUT predicted overall survival but not response to TMZ. Definitive clinical evidence on whether IDH1R132MUT predicts and/or influences response to RT or TMZ may be derived from the results of an ongoing randomized prospective trial that compares RT-only with TMZ-only treatments (the EORTC 22033-26033 trial).31
In terms of the mechanism of enhanced radiosensitivity in the U87MG-IDH1R132H cell lines, data from the current study suggest that mutant IDH1 may result in increased ROS levels and a heightened oxidative response to radiation exposure in the U87MG-IDH1R132H cells. It is possible that the observed increased oxidative stress in IDH1 mutant cells is due to decreased buffering of ROS from lower available levels of NADPH. Previous studies have shown that IDH-mediated NADPH production and overall NADPH levels are reduced in IDH1R132H glioblastomas in situ.20 Similarly, it has been previously shown that mitochondrial IDH-regulated radiation induced apoptosis in vitro, as demonstrated by increased radiation sensitivity in cells treated with mitochondrial IDH small interfering (si)RNA.32 In another study, this effect was also shown in vivo based on increased radiosensitivity in mice fed oxalomalate, an inhibitor of IDH1.29 Interestingly, we found that the basal (untreated) ROS levels were lower in the U87MG-IDH1R132H cell lines. While it is possible that this lower basal DCFH-DA staining may somehow represent an artifact, the apparent decreased level of ROS could result from lower basal levels of oxidant generation from slower cellular metabolism. The lower basal ROS level may also contribute to the decreased proliferation observed in U87MG-IDH1R132H cells.33–36 Although supported by the current study, the mechanism(s) by which IDH1R132H overexpression results in increased sensitivity to radiation are unclear and warrant further studies.
Several points regarding our in vitro model must be considered that may limit the clinical relevance of the current results. First, the “overexpression” model used here has an approximately 5–6-fold increased mutant protein expression compared with endogenous IDH1WT expression (Fig. S1A and S1B). In patient glioma samples, IDH1R132MUT is heterozygous, which would suggest a 1:1 ratio of IDH1WT: IDH1R132MUT gene dosage. Presently, “naturally occurring” cell lines derived from a patient tumor harboring the IDH1R132MUT have been elusive, with only one reported cell line derived from an oligodendroglioma.37 Existing studies have all utilized an overexpression system similar to that used here.14,15,38 Further experiments using cell lines with varying levels of overexpression may be useful to estimate the effects of gene dosage. Second, the isogenic U87MG cell model represents a specific genetic background: the salient molecular features of the U87MG cells include IDH1WT,39 methylated MGMT,40 p53WT,41 EGFRWT,42 and PTEN null.43 This raises the possibility that our results are specific to the U87MG genetic background. In order to investigate whether radiosensitization was specific to U87MG cells, we performed similar experiments in U373MG cells that had a background of IDH1WT,39 methylated MGMT,40 p53MUT,41 EGFRWT,44 and PTEN null43 and found similar radiosensitization by overexpression of IDH1R132H in U373MG cells. These results support the notion that radiosensitization of glioma cells by overexpression of IDH1R132H may apply to the varied genetic backgrounds seen in the clinic.
In addition, similar to other reported results,45 we found that U87MG-IDH1R132H cells demonstrated reduced baseline growth in soft agar, migration, and growth at higher cell density. Similar to our results in U87MG cells, it has been shown that siRNA knockdown of endogenous IDH1WT results in lower growth in vitro in SF188 cells, a pediatric glioblastoma cell line that is p53MUT and PTENWT.46 However, we did not observe these effects in the context of U373MG cells, suggesting that these effects are cell context dependent, although there is a possibility that U373MG cells did not show these effects on basal growth because of lower dosage of IDH1R132H overexpression than in the U87MG cells. Overall, these data suggest that lowered basal growth and migration might be other mechanisms for improved outcomes, albeit in only selected IDH1 mutant tumors.
In summary, our results indicate that overexpression of the IDH1R132H protein can sensitize glioma cells to radiation and, in the context of U87MG cells, exerts suppression of growth and migration. These in vitro results are consistent with the clinical observation that patients with IDH1R132H tumors have improved survival. If validated in patients, these findings may have broader implications regarding the possibility of therapeutically targeting IDH1-mediated pathways. Additional in vitro and in vivo studies are needed to further elucidate the pathophysiology and clinical implication of IDH1 mutation.
Supplementary Material
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (K08CA124479 to A.L.); University of California/Cancer Research Coordinating Committee (to A. L.); and American Brain Tumor Association Basic Research Fellowship (to S.L.); American Association of Neurological Surgeons Neurosurgery Research and Education Foundation Fellowship sponsored by the Section on Tumors (to A.P.C.); Congress of Neurological Surgeons Wilder Penfield Fellowship (to A. P. C.).
Supplementary Material
Acknowledgments
We thank Dr. Jeff M Bronstein for kindly providing access to his flow cytometry equipment, Dr. Hongwei Dong for providing access to his confocal microscope, Dr. Paul Mischel for providing the U87MG and U373MG cell lines, and Dr. Yi Sun for providing the Plat-A packaging cells. Authors Sichen Li and Arthur P. Chou contributed equally to this work.
Conflict of interest statement. R. C., Y. D., and H. S. P. are employees of Roche/Genentech.
References
- 1.Louis DN, Holland EC, Cairncross JG. Glioma classification: a molecular reappraisal. Am J Pathol. 2001;159(3):779–786. doi: 10.1016/S0002-9440(10)61750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA. 1999;96(18):10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shechter I, Dai P, Huo L, Guan G. IDH1 gene transcription is sterol regulated and activated by SREBP-1a and SREBP-2 in human hepatoma HepG2 cells: evidence that IDH1 may regulate lipogenesis in hepatic cells. J Lipid Res. 2003;44(11):2169–2180. doi: 10.1194/jlr.M300285-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 11.Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2009;20(1):245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato Y, Jin G, Kuan CT, McLendon RE, Yan H, Bigner DD. A monoclonal antibody IMab-1 specifically recognizes IDH1R132H, the most common glioma-derived mutation. Biochem Biophys Res Commun. 2009;390(3):547–551. doi: 10.1016/j.bbrc.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko MK, Tian W, Takano S, et al. Establishment of a novel monoclonal antibody SMab-1 specific for IDH1-R132S mutation. Biochem Biophys Res Commun. 2011;406(4):608–613. doi: 10.1016/j.bbrc.2011.02.102. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;465(7300):966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross S, Cairns RA, Minden MD, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207(2):339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17(3):225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483(7390):484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. doi: 10.1200/JCO.2010.33.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleeker FE, Atai NA, Lamba S, et al. The prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119(4):487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 22.Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73(21):1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- 23.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16(5):1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 24.Schnittger S, Haferlach C, Ulke M, Alpermann T, Kern W, Haferlach T. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116(25):5486–5496. doi: 10.1182/blood-2010-02-267955. [DOI] [PubMed] [Google Scholar]
- 25.Remington M, Chtchetinin J, Ancheta K, Nghiemphu PL, Cloughesy T, Lai A. The L84F polymorphic variant of human O6-methylguanine-DNA methyltransferase alters stability in U87MG glioma cells but not temozolomide sensitivity. Neuro Oncol. 2009;11(1):22–32. doi: 10.1215/15228517-2008-080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan XS, Zhou Z, Kennedy AR. Adaptation of the dichlorofluorescein assay for detection of radiation-induced oxidative stress in cultured cells. Radiat Res. 2003;160(6):622–630. doi: 10.1667/3099. [DOI] [PubMed] [Google Scholar]
- 27.Freshney RI. Culture of Animal Cells: A Manual of Basic Technique. 3rd ed. New York: Wiley-Liss, Inc.; 1994. pp. 166–169. [Google Scholar]
- 28.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Kim SY, Kil IS, Park JW. Regulation of ionizing radiation-induced apoptosis by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem. 2007;282(18):13385–13394. doi: 10.1074/jbc.M700303200. [DOI] [PubMed] [Google Scholar]
- 30.Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met) J Cell Biochem. 2000;78(3):465–475. doi: 10.1002/1097-4644(20000901)78:3<465::aid-jcb11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Musat E, Roelofs E, Bar-Deroma R, et al. Dummy run and conformity indices in the ongoing EORTC low-grade glioma trial 22033–26033: First evaluation of quality of radiotherapy planning. Radiother Oncol. 2010;95(2):218–224. doi: 10.1016/j.radonc.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Park JW. Oxalomalate regulates ionizing radiation-induced apoptosis in mice. Free Radic Biol Med. 2007;42(1):44–51. doi: 10.1016/j.freeradbiomed.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Velarde V, de la Cerda PM, Duarte C, et al. Role of reactive oxygen species in bradykinin-induced proliferation of vascular smooth muscle cells. Biol Res. 2004;37(3):419–430. doi: 10.4067/s0716-97602004000300007. [DOI] [PubMed] [Google Scholar]
- 34.Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21(2):71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 35.Han MJ, Kim BY, Yoon SO, Chung AS. Cell proliferation induced by reactive oxygen species is mediated via mitogen-activated protein kinase in Chinese hamster lung fibroblast (V79) cells. Mol Cells. 2003;15(1):94–101. [PubMed] [Google Scholar]
- 36.Le Belle JE, Orozco NM, Paucar AA, et al. Proliferative Neural Stem Cells Have High Endogenous ROS Levels that Regulate Self-Renewal and Neurogenesis in a PI3K/Akt-Dependant Manner. Cell Stem Cell. 2010;8(1):59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly JJ, Blough MD, Stechishin OD, et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10) Neuro Oncol. 2010;12(7):745–755. doi: 10.1093/neuonc/noq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poulos NE, Farmer AA, Chan KW, Stanbridge EJ. Design of a novel bicistronic expression vector with demonstration of a p16INK4-induced G(1)-S block(1) Cancer Res. 1996;56(8):1719–1723. [PubMed] [Google Scholar]
- 41.Van Meir EG, Kikuchi T, Tada M, et al. Analysis of the p53 gene and its expression in human glioblastoma cells. Cancer Res. 1994;54(3):649–652. [PubMed] [Google Scholar]
- 42.Fenstermaker RA, Ciesielski MJ. Deletion and tandem duplication of exons 2–7 in the epidermal growth factor receptor gene of a human malignant glioma. Oncogene. 2000;19(39):4542–4548. doi: 10.1038/sj.onc.1203802. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 44.Guo D, Hildebrandt IJ, Prins RM, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci USA. 2009;106(31):12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bralten LB, Kloosterhof NK, Balvers R, et al. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69(3):455–463. doi: 10.1002/ana.22390. [DOI] [PubMed] [Google Scholar]
- 46.Wise DR, Ward PS, Shay JE, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA. 2011;108(49):19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.