Abstract
The purpose of the trial was to determine the survival and incidence of secondary tumors in children with medulloblastoma receiving radiotherapy plus chemotherapy. Three hundred seventy-nine eligible patients with nondisseminated medulloblastoma between the ages of 3 and 21 years were treated with 2340 cGy of craniospinal and 5580 cGy of posterior fossa irradiation. Patients were randomized between postradiation cisplatin and vincristine plus either CCNU or cyclophosphamide. Survival, pattern of relapse, and occurrence of secondary tumors were assessed. Five- and 10-year event-free survivals were 81 ± 2% and 75.8 ± 2.3%; overall survivals were 87 ± 1.8% and 81.3 ± 2.1%. Event-free survival was not impacted by chemotherapeutic regimen, sex, race, age at diagnosis, or gender. Seven patients had disease relapse beyond 5 years after diagnosis; relapse was local in 4 patients, local plus supratentorial in 2, and supratentorial alone in 1. Fifteen patients experienced secondary tumors as a first event at a median time of 5.8 years after diagnosis (11 >5 y postdiagnosis). All non-CNS solid secondary tumors (4) occurred in regions that had received radiation. Of the 6 high-grade gliomas, 5 occurred >5 years postdiagnosis. The estimated cumulative 10-year incidence rate of secondary malignancies was 4.2% (1.9%–6.5%). Few patients with medulloblastoma will relapse ≥5 years postdiagnosis; relapse will occur predominantly at the primary tumor site. Patients are at risk for development of secondary tumors, many of which are malignant gliomas. This may become an increasing issue as more children survive.
Keywords: chemotherapy, medulloblastoma, radiotherapy, secondary tumors
Reported figures on event-free survival (EFS) and overall survival (OS) have slowly risen over the past 2 decades in pediatric cases of medulloblastoma, with multiple studies reporting 3- to 5-year EFS and OS rates of >70% in children with nondisseminated disease at time of diagnosis.1–5 Potential reasons for this apparent improvement in survival have been the routine employment of more aggressive surgery; more refined preoperative evaluations, resulting in a more pristine group of children with nondisseminated disease; and the use of adjuvant chemotherapy during and after radiotherapy.1–5 In past reports, especially those describing children receiving radiotherapy alone, late relapses, arbitrarily those occurring >5 years following diagnosis, were frequently reported.6–8 In addition, the frequency and impact of secondary tumors on both EFS and OS have been poorly characterized in children surviving medulloblastoma.6–8
In 2006, the results were reported of a phase III study of reduced-dose craniospinal radiation therapy (2400 cGy), standard local boost radiotherapy (total dose 5580 cGy), and adjuvant chemotherapy consisting of vincristine during radiotherapy and 1 of 2 cisplatin-containing postradiotherapy regimens.1 Five-year EFS and OS in this cohort of 379 patients were >80%, and the chemotherapy regimen received did not affect outcome. Since this initial report, both secondary tumors and late relapses have been encountered in children treated in this study. Reported for this patient population are long-term EFS, OS, pattern of disease relapse, and occurrence of secondary tumors.
Methods
Between December 1996 and December 2000, 421 patients with medulloblastoma were entered on our study. To be eligible, patients had to have histologically confirmed medulloblastoma and be between the ages of 3 and 21 years, inclusive, at the time of diagnosis.1 Patients were to have no evidence of disseminated disease on MRI of the entire brain and spine performed pre- or postoperatively or on cytological examination of lumbar cerebrospinal fluid performed between 5 days of surgery and the onset of radiation. Patients were to have <1.5 cm2 of residual tumor on postoperative imaging performed within 21 days, preferably within 72 h, of surgery. Patients with brainstem involvement were eligible for the study. Treatment must have begun within 31 days of definitive surgery. All institutions participating in this study had received approval from their institutional review boards, and age-appropriate informed consent/assent was obtained from each patient/parent/guardian.
Preoperative and postoperative MRI studies were centrally analyzed for 409 (97%) of the 421 patients for evaluation of extent of disease and amount of postoperative residual disease. Eligibility was based on institutional review, except when central review revealed unequivocal evidence of dissemination or excess residual disease, in which case, for analysis, patients were considered ineligible. If, on central review, studies were considered incomplete or not interpretable because of movement or other artifacts, patients were considered incompletely assessable but remained eligible for analysis. Central pathologic review was performed on 358 (85%) of the cohort by 1 of 2 neuropathologists.
After central review, 379 patients (including 66 who, on evaluation, had no evidence of excess residual or metastatic disease but whose studies could not be fully evaluated because of poor quality or incompleteness of submission) were deemed eligible for analysis. Patient characteristics have been noted in a previous report.1 Two hundred twenty-three patients were male and 156 were female. Seventeen percent of patients (n = 65) were 3–4 years of age, 51% (n = 193) were 5–9 years of age, and 32% (n = 121) were >15 years of age.
Treatment
A dose of 2340 cGy of craniospinal radiation with a posterior fossa boost of 3240 cGy (total dose 5580 cGy) was prescribed in fractions of 180 cGy per day, 5 days per week. Treatment to the craniospinal axis was not to exceed 20 days, and the entire treatment was to be completed within 51 days. The boost volume included the entire posterior fossa with a 1-cm margin around the tentorium or the tumor. Both parallel opposing fields and conformal radiation therapy techniques were allowed. Spinal treatment was as outlined previously.1
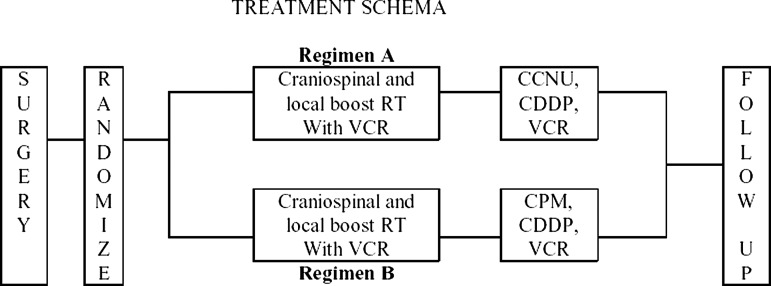
After surgery, eligible patients were randomized to receive either 8 cycles of regimen A or regimen B of chemotherapy, as previously described (see Fig. 1). Patients on both regimens were treated with weekly vincristine during radiotherapy (1.5 mg/m2, maximum 2 mg, maximum 8 doses). Regimen A consisted of CCNU, cisplatin, and vincristine. Regimen B consisted of cisplatin, cyclophosphamide, and vincristine. Dose modifications for toxicity were as have been previously published.1
Fig. 1.
Treatment schema.
Statistical Consideration
Patients were randomly assigned to 1 of the 2 experimental regimens at the time of study enrollment, stratified by age and brainstem involvement. The primary endpoint for analysis was time to a treatment-failure event (EFS) measured from the time of study enrollment. An event was defined as death from any cause, or the first occurrence of relapse, progressive disease, or development of a secondary tumor. The secondary endpoint was time to death from any cause or the first occurrence of, from which actuarial survival probability was computed. (Refer to the original article for details of statistical design of the trial.) Nonparametric EFS and survival curves were computed using product-limit (Kaplan–Meier) estimates, with standard errors via the Greenwood formula. Cumulative incidence of secondary tumors over time was calculated by the method proposed by Gray. Fisher's exact test was used to detect the relationship between years of relapse and the type of relapse.
Secondary tumors and relapse determinations
Patients were considered to have relapse or secondary tumors based on institutional determinations. All neuroradiographic studies demonstrating relapse or secondary tumors were centrally received. Pathologic confirmation of a secondary tumor was mandatory for inclusion, but pathologies were not centrally reviewed. As regards determining tumor relapse, pathologic confirmation was not mandatory and relapse could be diagnosed based on neuroradiographic interpretation by the treating institution.
Results
Overall Outcome
Data collection was halted 10 years after entry of the last patient on study. At time of analysis of the 379 eligible patients, the median follow-up for the 312 patients who were alive was 9.7 years (range, 0.2–13.7 y). Sixty-eight patients experienced tumor progression and 5 had death as first event; 58 have died to date. Late disease progression occurring 5 years after treatment occurred in 7 patients, 6 of whom died. The mean age at initial diagnosis of those developing late tumor relapse was 6.8 years. Two relapsed at an age later than their age at diagnosis plus 9 months. Fifteen developed secondary tumors—of these, 11 occurred more than 5 years after diagnosis, and 9 patients died (see Table 2).
Table 2.
Secondary tumors
| Time of Secondary Tumor | Time after Treatmenta (y) | Regimen | Secondary Tumor Type | Life Status | Time since Last Seenb (y) |
|---|---|---|---|---|---|
| <5 y | 3.2 | B | Precursor T-cell lymphoblastic leukemia | Dead | 0.27 |
| 3.7 | A | Glioblastoma, NOS | Dead | 0.32 | |
| 4.7 | B | Basal cell carcinoma, NOS (Gorlin's) | Alive | 8.16 | |
| 4.8 | A | Spindle cell carcinoma | Alive | 1.67 | |
| >5 y | 5.3 | A | Glioma, malignant | Dead | 0.68 |
| 5.3 | A | Glioblastoma, NOS | Dead | 0.56 | |
| 5.7 | A | Osteosarcoma, NOS | Dead | 1.28 | |
| 5.8 | A | Myelodysplastic syndrome, NOS | Dead | 6.87 | |
| 6.4 | B | Myelodysplastic syndrome, NOS | Alive | 0.76 | |
| 6.5 | B | Pilocytic astrocytoma | Dead | 0.85 | |
| 8.2 | B | Papillary adenocarcinoma, NOS (thyroid) | Alive | 1.07 | |
| 9.2 | B | Glioblastoma multiforme | Dead | 2.37 | |
| 9.2 | B | Glioblastoma multiforme | Alive | 1.18 | |
| 10.1 | A | Papillary carcinoma, follicular (thyroid) | Alive | 2.79 | |
| 10.3 | A | Glioma, malignant | Dead | 0.51 |
Abbreviation: NOS, not otherwise specified.
aTime between initial diagnosis and development of the secondary tumor.
bTime between diagnosis of the secondary tumor and when last seen.
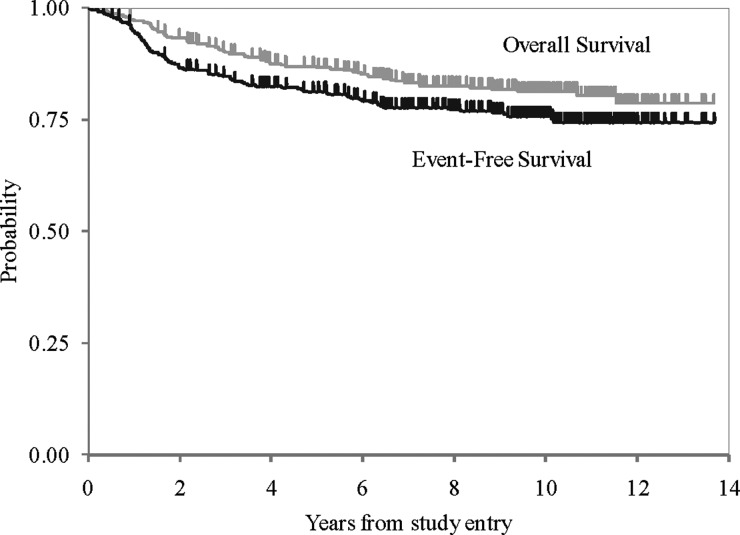
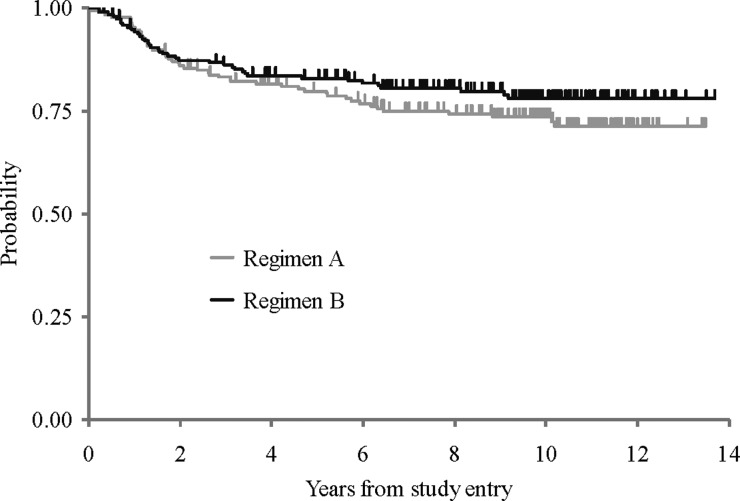
For the cohort of 379 patients, 5- and 10-year EFSs were 81 ± 2.0% and 75.8 ± 2.3%, respectively. Five- and 10-year OSs were 87 ± 1.8% and 81.3 ± 2.1%, respectively (see Fig. 2). As noted in the original article, EFS did not differ between patients treated with regimen A and those treated with regimen B (see Fig. 3)—10-year EFS for regimen A was 74 ± 3% compared with 78 ± 3.2% for regimen B (P = .24). Moreover, EFS and OS were not impacted by sex, race, age at diagnosis, gender, brainstem involvement, extent of resection, or histologic evidence of diffuse or focal anaplasia.
Fig. 2.
Overall and event-free survival.
Fig. 3.
Event-free survival by regimen.
Pattern of Disease Relapse
The pattern of disease relapse in patients on this study is as noted in Table 1. In the 7 patients with late relapse, pattern of relapse, as determined by the treating institution, was local in 4, local plus supratentorial in 2, and supratentorial alone in 1. Of these patients at time of initial entry to study, 5 had “total” resections and 2 “subtotal” resections. Of the 2 with subtotal initial resections, 1 failed locally and distally, and the second distally alone. On central review, the patient with a supratentorial-alone relapse had findings (radiographic) consistent with infiltrating glioma; however, the patient was not biopsied at relapse. For the purposes of this report, the patient is still considered a “late” relapse, as patients were classified per treating-institution diagnosis, unless there was clear pathologic evidence to document a different histology. In contrast, patients who relapsed earlier than 5 years from diagnosis had predominantly at least some component of disseminated relapse, with only 16% of patients having local relapse alone compared with 57% of patients with late relapse (Fisher's exact test P = .029). Spinal involvement, either alone or in combination with local relapse, which was commonly seen in those relapsing before 5 years of age, was not seen in those relapsing later.
Table 1.
Pattern of relapse
| Type of Relapse | ≤5 y | >5 y |
|---|---|---|
| Local alone | 10 (16%) | 4 (57%) |
| Not local alone | 51 (84%) | 3 (43%) |
| Total | 61 | 7 |
Fisher exact test P = .029, percent of total cases in age range are given in parentheses.
Secondary Tumors
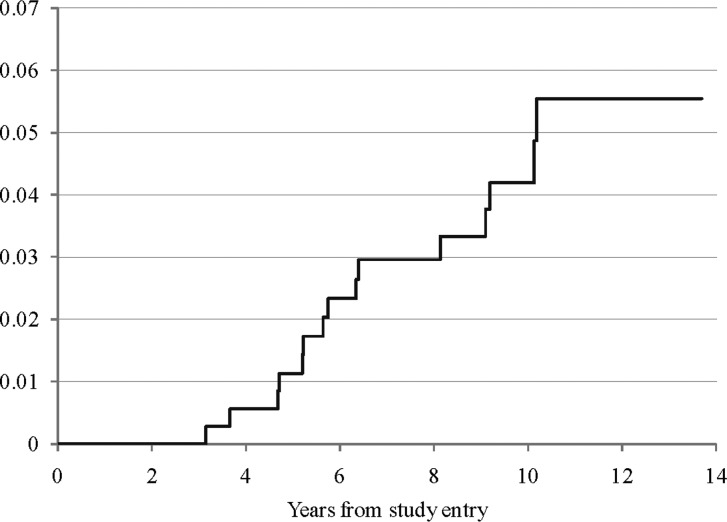
Fifteen patients experienced secondary tumors as a first event; 8 were on regimen A and 7 on regimen B. The median time to secondary tumor was 5.8 years; 4 occurred <5 years and 11 >5 years postdiagnosis, as shown in Table 2. Patients with secondary tumors were diagnosed at a median of 5.6 years postdiagnosis (range, 3.1–16.8 y). There was no significant difference in the incidence of secondary tumors in children older than 5 years at diagnosis compared with younger children. There was also no significant difference between the 2 randomized arms. The estimated cumulative incidence rate of secondary tumors at 5 and 10 years for the entire cohort was 1.1% (95% CI: 0.0%–2.3%) and 4.2% (95% CI: 1.9%–6.5%), respectively (see Fig. 4). Nine patients with secondary tumors died; 6 of the 9 were on regimen A (the CCNU-containing arm). One child with glioblastoma multiforme, who was alive at the time of this report, had been followed for 1.18 months and had been treated in the cyclophosphamide arm of the study. One child, diagnosed with a secondary “pilocytic astrocytoma” of the brainstem, died secondary to the tumor within 1 year of diagnosis (central histopathologic review was not performed). The child with basal cell carcinomas developing within the radiotherapy field was diagnosed with Gorlin's syndrome at time of development of the basal cell tumors. Of the 4 patients with non-CNS solid tumors, 2 had thyroid-region tumors, 1 had an osteosarcoma in the temporal bone, and 1 had a spindle cell sarcoma in the nasal region. Thus, all developed solid tumors in regions that would have received at least scatter radiation.
Fig. 4.
Cumulative incidence of secondary tumors.
Discussion
The long-term results seen in this group of patients receiving radiotherapy and adjuvant chemotherapy, during and following radiotherapy, are both reassuring and cautionary. Ten-year EFS and OS rates of ∼75%–80% are encouraging and compare favorably with survival rates reported in series utilizing radiation therapy alone or preradiation chemotherapy.2,4,5,9 Prospective randomized trials comparing radiation therapy alone to radiation plus chemotherapy have not been performed; however, the best reported survival rates at 5 and 10 years for children with nondisseminated medulloblastoma receiving radiotherapy alone have ranged between 50% and 65%, even with the use of higher doses of craniospinal radiation (3600 cGy).4,7,10 Studies utilizing preradiation chemotherapy followed by higher doses of craniospinal radiation have disclosed 5-year survival rates of ∼60%–65%.9,11 Also reassuring is the stability of the survival curves after the multimodal treatment used in this study, which included a “reduced dose” of 2400 cGy of craniospinal radiation. In the few series that have reported long-term survival in children with medulloblastoma treated predominantly with radiation therapy alone, there has been no clear-cut plateauing of the survival curve, with some reporting a 10%–20% fall in survival between years 5 and 10.7,10 The data from this randomized prospective study show few relapses after 5 years, possibly due to the addition of adjuvant chemotherapy. The majority of relapses in our series occurred within 2 years of diagnosis, with approximately one-third of relapses occurring in years 3 to 5, but only in 7 of 68 after year 5.
The pattern of relapse also differed in those children who relapsed within the first 5 years of diagnosis compared with those who relapsed later. Excluding the 1 child who was considered to have an isolated supratentorial relapse by the treating institution and, in retrospect, may have had an infiltrating cortical glioma, all “late” relapses occurred with some component of local disease; none had spinal disease either in isolation or as a component of initial relapse. A similar pattern was reported by von Hoff for the HIT99 trial.12 Children in the Children's Oncology Group study who relapsed <5 years postdiagnosis overwhelmingly were likely to have some component of disease dissemination, as only 10 of the 61 had local relapse alone. Relapse outside the primary tumor site within 5 years of diagnosis, without any evidence of local relapse, occurred in 24 patients (40%), including 7 with spinal disease alone. This disseminated dominant pattern of failure with “early” relapse has also been found by others.12–14 There does not seem to be a strong rationale, given these results, to continue surveillance studies of the spine in children who have survived >5 years with medulloblastoma treated with radiation and 1 of the 2 chemotherapeutic regimens used in this study. However, although surveillance studies after 5 years of disease control are unlikely to show recurrent disease, the increasing incidence of secondary tumors gives more credence to their use. A limitation of our data is that it is unknown whether the 7 children with relapse >5 years postdiagnosis were symptomatic at time of relapse or were identified solely by surveillance studies.
The 4.2% 10-year cumulative incidence of secondary tumors is quite worrisome, although the confidence intervals range between 2% and 6.5%. After closure of the database, another secondary presumed high-grade glioma of the brainstem (unbiopsied at the treating physician's discretion) occurred in a 9-year survivor. Direct comparison with other series is difficult because in most series, information was not gathered prospectively but rather was obtained from retrospective reviews and registries. There seems to be no question that radiotherapy is associated with increased relative risk for development of secondary tumors in children with brain tumors and leukemia, especially secondary malignant brain tumors >5 years from diagnosis and treatment.15–18 In our series, all solid non-CNS secondary tumors occurred either within the radiation therapy portal or in regions where scatter radiation was likely (thyroid, nasal region, and temporal bone). However, the exact incidence of these secondary tumors is difficult to glean from studies, and for children with medulloblastoma, the incidence has been estimated to be in the 1%–2% range.2,10 In retrospective reviews, the incidence of secondary tumors has been noted to be somewhat less after radiation therapy alone (in the 1% range at 10 years) or is not mentioned at all.4,7,10 In a recent prospective series from Germany of 280 patients administered either sandwich pre- and postradiation chemotherapy or postradiation chemotherapy, 12 patients developed secondary tumors, including 3 with high-grade gliomas;12 8 of the 12 tumors were noted in patients who received the more aggressive sandwich chemotherapy, using similar drugs to those used in this series. In an analysis of the Surveillance Epidemiology and End Results data, a higher incidence of secondary tumors was noted in children surviving brain tumors treated after 1985 compared with those treated between 1979 and 1984, even when controlling for the use of radiation.15 The authors suggest that this might be due to the use of more aggressive chemotherapy in the later eras. The Childrens Cancer Survivor Study found a trend but not a statistically significant relationship between an increased occurrence of secondary tumors and treatment in the later era, compared with those treated earlier.16 It should be noted that although chemotherapy was used to some extent in the early 1980s, it has been increasingly employed since and is now considered by most a standard component of treatment for all children >3 years of age with medulloblastoma. Also, chemotherapeutic regimens employing potentially mutagenic alkylating agents, including in some cases etoposide, have been intensified over the past decade, raising the possibility that more secondary tumors may occur.3,12 On the other hand, those same studies used lower-dose craniospinal radiotherapy, and increased total doses of radiotherapy have been related to a higher incidence of secondary brain cancer.16 It remains to be seen whether the use of more focused radiotherapeutic techniques, such as proton beam irradiation, will in the future reduce the incidence of radiation-associated non-CNS secondary tumors.
Younger age at time of radiation has been related to a higher likelihood of development of a secondary tumor, but the results of this study do not show a relationship.16 Patients specifically developing high-grade gliomas were a median of 5.8 years of age at initial diagnosis (range, 3.7–10.8 y).
In the cohort of patients treated in this study, the majority of secondary tumors, especially those occurring >5 years postdiagnosis, have been highly aggressive, with 5 malignant gliomas, 1 osteosarcoma, and 2 myelodysplastic syndromes. The literature and our experience would suggest that those patients with high-grade gliomas will rarely respond to treatment or survive, making occurrence of this complication even more devastating.12,18 Diagnosis of secondary malignant brain tumors in children with medulloblastoma is challenging, especially when they occur in the brainstem or similar deep-seated areas, and distinction between tumor recurrence and a secondary tumor can be impossible without histologic confirmation. Complicating diagnosis further is the difficulty of distinguishing small-cell gliomas from medulloblastomas that have undergone extensive glial differentiation, even when tissue is available for analysis. With all these considerations, it is impossible to determine whether this worrisome incidence of secondary tumors in this and other series evaluating patients with medulloblastoma receiving radiotherapy and chemotherapy is due to a true rise in incidence or better ascertainment. Also, in the present series, no meningiomas have been noted, and it is likely that as the survivor cohort ages, this tumor type will become prevalent.16,19,20
In conclusion, the updated results of this study demonstrate that the vast majority of children with nondisseminated medulloblastomas treated with radiation and receiving the chemotherapeutic regimens used in this study, during and after radiation therapy, will survive relapse-free. A small proportion of patients will relapse ≥5 years postdiagnosis, and in almost all, relapse will occur at the primary site. Patients are also at risk for development of secondary tumors including, but not limited to, tumors of the central nervous system, and long-term follow-up strategies must take this into account.
Conflict of interest statement. None declared.
Funding
This work was supported by the Chair's Grant U10 CA98543-08 Statistics and Data Center Grant U10 CA98413-08 of the Children's Oncology Group from the National Cancer Institute (NCI), National Institutes of Health (NIH), Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH.
References
- 1.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4203. doi: 10.1200/JCO.2006.06.4980. doi:10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 2.Oyharcabal-Bourden V, Kalifa C, Gentet JC, et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology study. J Clin Oncol. 2005;23(19):4726–4734. doi: 10.1200/JCO.2005.00.760. doi:10.1200/JCO.2005.00.760. [DOI] [PubMed] [Google Scholar]
- 3.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. doi: 10.1016/S1470-2045(06)70867-1. http://oncology.thelancet.com. Published online September 7, 2006. doi:10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PRM, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuroaxis irradiation. J Clin Oncol. 2000;18(16):3004–3011. doi: 10.1200/JCO.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 study. J Clin Oncol. 2003;21(8):1581–1591. doi: 10.1200/JCO.2003.05.116. doi:10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 6.Kortmann R-D, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys. 2000;46(2):269–279. doi: 10.1016/s0360-3016(99)00369-7. doi:10.1016/S0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 7.Hughes EN, Shillito J, Sallan S, et al. Medulloblastoma at the Joint Center for Radiation Therapy between 1968 and 1984. The influence of radiation dose on the patterns of failure and survival. Cancer. 1998;61:1992–1998. doi: 10.1002/1097-0142(19880515)61:10<1992::aid-cncr2820611011>3.0.co;2-j. doi:10.1002/1097-0142(19880515)61:10<1992::AID-CNCR2820611011>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.Modha A, Vassilyadi M, George A, et al. Medulloblastoma in children—the Ottawa experience. Child's Nerv Syst. 2000;16:341–350. doi: 10.1007/s003810050529. doi:10.1007/s003810050529. [DOI] [PubMed] [Google Scholar]
- 9.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 10.Park TS, Hoffman HJ, Hendrick EB, et al. Medulloblastoma: clinical presentation and management. Experience at the Hospital for Sick Children, Toronto, 1950–1980. J Neurosurg. 1983;58:543–552. doi: 10.3171/jns.1983.58.4.0543. doi:10.3171/jns.1983.58.4.0543. [DOI] [PubMed] [Google Scholar]
- 11.Bailey CC, Gnekow A, Wellek S, et al. Prospective randomized trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol. 1995;25:166–178. doi: 10.1002/mpo.2950250303. doi:10.1002/mpo.2950250303. [DOI] [PubMed] [Google Scholar]
- 12.von Hoff K, Hinkes B, Gerber NU, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomized multicentre trial HIT'91. Eur J Cancer. 2009;45:1209–1217. doi: 10.1016/j.ejca.2009.01.015. doi:10.1016/j.ejca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Hong TS, Mehta MP, Boyett JM, et al. Patterns of treatment failure in infants with primitive neuroectodermal tumors who were treated on CCG-921: a phase III combined modality study. Pediatr Blood Cancer. 2005;45:676–682. doi: 10.1002/pbc.20184. doi:10.1002/pbc.20184. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga-Johnson Ni, Lee JH, Sandler HM, et al. Patterns of failure following treatment for medulloblastoma: is it necessary to treat the entire posterior fossa? Int J Radiat Oncol Biol Phys. 1998;42(1):143–146. doi: 10.1016/s0360-3016(98)00178-3. doi:10.1016/S0360-3016(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 15.Neglia JP, Meadows AT, Robison LL, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;325:1330–1336. doi: 10.1056/NEJM199111073251902. doi:10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- 16.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. doi:10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 17.Peterson KM, Shao C, McCarter R, et al. An analysis of SEER data of increasing risk of secondary malignant neoplasms among long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2006;47:83–88. doi: 10.1002/pbc.20690. doi:10.1002/pbc.20690. [DOI] [PubMed] [Google Scholar]
- 18.Romeike BFM, Kim Y-J, Steudel W-I, et al. Diffuse high-grade gliomas as second malignant neoplasms after radio-chemotherapy for pediatric malignancies. Childs Nerv Syst. 2007;23:185–193. doi: 10.1007/s00381-006-0199-z. doi:10.1007/s00381-006-0199-z. [DOI] [PubMed] [Google Scholar]
- 19.Jenkin D, Greenberg M, Hoffman H, et al. Brain tumors in children: long-term survival after radiation treatment. Int J Radiat Oncol Biol Phys. 1995;31(3):445–451. doi: 10.1016/0360-3016(94)00393-Y. doi:10.1016/0360-3016(94)00393-Y. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha RS, Pascoe EM, Rushing EJ, et al. Meningiomas in children and adolescents: a meta-analysis of individual patient data. doi: 10.1016/S1470-2045(11)70275-3. http://www.thelancet/oncology. Published online November 16, 2011. doi:10.1016/S1470-2045(11)70275-3. [DOI] [PubMed] [Google Scholar]