Abstract
We investigated whether high levels of activated mitogen-activated protein kinase (p-MAPK) were associated with poor survival among patients with newly diagnosed glioblastoma during the temozolomide era. Nuclear p-MAPK expression of 108 patients with GBM was quantified and categorized in the following levels: low (0%–10%), medium (11%–40%), and high (41%–100%). Independent predictors of overall survival were determined using a multivariate Cox proportional hazards model. Our study included 108 patients with newly diagnosed GBM. Median age was 65 years, and 74% had high Karnofsky performance status (KPS ≥ 80). Median overall survival among all patients was 19.5 months. Activated MAPK expression levels of <10%, 11%–40%, and ≥41% were observed in 33 (30.6%), 37 (34.3%), and 38 (35.2%) patients, respectively. Median survival for low, medium, and high p-MAPK expression was 32.4, 18.2, and 12.5 months, respectively. Multivariate analysis showed 2.4-times hazard of death among patients with intermediate p-MAPK than low p-MAPK expression (hazard ratio [HR], 2.4; P = .02); high-expression patients were 3.9 times more likely to die, compared with patients with low p-MAPK (HR, 3.9; P = .007). Patients aged ≥65 years (HR, 2.8; P = .002) with KPS < 80 (HR, 3.1; P = .0003) and biopsy or partial resection (HR, 1.9; P = .02) had higher hazard of death. MGMT and PTEN expression were not associated with survival differences. This study provides quantitative means of evaluating p-MAPK in patients with GBM. It confirms the significant and independent prognostic relevance of p-MAPK in predicting survival of patients with GBM treated in the temozolomide era and highlights the need for therapies targeting the p-MAPK oncogenic pathway.
Keywords: EGFR, glioblastoma multiforme (GBM), IDH1, MGMT, overall survival, PTEN, p-MAPK
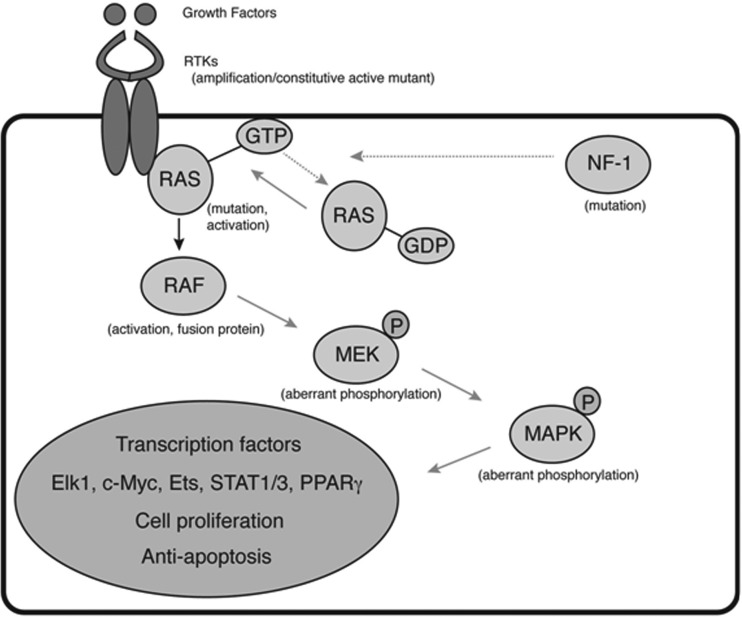
Glioblastoma multiforme (GBM) is the most common primary intracranial tumor, accounting for 54% of all gliomas.1 GBMs are aggressive, infiltrative tumors, and afflicted patients historically have had a median survival of just >1 year.2 Current standard treatment, which includes surgery followed by concurrent temozolomide and radiation therapy, extends median survival to 14.6 months.3 Because standard medical practice yields only a modest benefit, attention has shifted toward the identification and therapeutic targeting of various oncogenic pathways. Integrative analysis of GBMs by The Cancer Genome Atlas (TCGA) network has previously shown that most if not all glioblastomas have pathogenic alteration in (1) receptor tyrosine kinase (RTK/RAS/PI3K), (2) p53, and (3) retinoblastoma pathways.4 They report RTK/RAS/RAF and RTK/PI3K pathway alterations in 88% of GBM samples. One of the key transducers of aberrantly enhanced signaling is mitogen-activated protein kinase (MAPK), which works downstream of RAS and RAF (Fig. 1). Activated through phosphorylation, MAPK translocates into the nucleus and induces several transcriptional factors that govern proliferation, survival, and invasiveness.5–10 Because MAPK is one of the key downstream transducers of tyrosine kinase signaling, monitoring the degree of its phosphorylation can be an attractive marker of overall activity in the tyrosine kinase pathway. Data from several studies showed that a significant fraction of GBMs display high levels of phosphorylated MAPK (p-MAPK).11 However, practical aspects of evaluation of p-MAPK are not clearly established with respect to technical aspects of tissue processing and categorizing the extent of staining. Moreover, although prior to the standardization of treatment with temozolomide in 2005, patients with GBM with high expression of p-MAPK were shown to have worse survival, compared with patients with inactive MAPK,11,12 in the setting of a new treatment paradigm for GBM with concurrent radiation and temozolomide, it is unclear whether p-MAPK continues to be an important prognostic factor. Therefore, this study sought to determine whether expression of p-MAPK holds independent prognostic significance in patients with newly diagnosed GBM during the temozolomide era. In addition, our study provides a technical guideline for the application of p-MAPK assessment to the practice of neuro-oncology.
Fig. 1.
Ras/Raf/MAPK pathway of oncogenesis in glioblastoma.
Materials and Methods
Patient Selection
Through a Research Electronic Data Capture (REDCap) database, we retrospectively reviewed 188 consecutive patients with newly diagnosed GBM treated at a single institution during 2007–2010. Patients with procedure-related mortality within 30 days after surgery and those without MAPK expression data were excluded. This yielded a final cohort of 108 patients with newly diagnosed GBM for the current analysis. All patients were treated with surgery (resection or biopsy). This study was approved by the Institutional Review Board of Cedars-Sinai Medical Center.
Data Variables
Patient age, sex, perioperative Karnofsky performance status (KPS) score, radiographic extent of resection, EGFR status, MAPK and PTEN expression, and overall survival data were collected. Patient age was stratified into ≤65 years of age or >65 years of age. Performance status was categorized as normal (80 ≤ KPS ≤ 100) or unable to perform normal activity (KPS < 80). Extent of resection (EOR) was determined on the basis of postoperative T1 imaging with contrast-enhanced MRI. EOR was categorized as biopsy, partial resection, near gross-total resection (>95% resection of enhancing component of tumor), or gross-total resection. Overall survival in months was calculated from the date of surgery to date of death or date of last follow-up in patients who were alive. Date of death was confirmed using the Social Security Death Index.
Immunohistochemical Detection of p-MAPK
Detection of activated (phosphorylated) MAPK involved using phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit monoclonal antibodies (Cell Signaling 20G11). This antibody detects both Erk1 and Erk2 MAP Kinase when dually phosphorylated at Thr202 and Tyr204 of Erk1 (or Thr185 and Tyr187 of Erk2) and mono-phosphorylated at Thr202 of Erk1. The antibody does not cross-react with the corresponding phosphorylated residues of either JNK/SAPK or p38 MAPK.
Analysis of pMAPK was performed prospectively on all cases of infiltrative gliomas, which were surgically resected at Cedars-Sinai Medical Center over a period of the study. Endothelial cells and reactive non-tumorous glia within the tumor were used as a positive control, see below.
Tumor tissue samples were procured from the biopsy sample obtained for intraoperative frozen section consultation. Half the pathological specimen was processed for actual frozen section, whereas the remainder of the tissue was immediately (within 20 min of resection/biopsy) fixed in 4% buffered formalin. The total length of fixation varied from 4 to 12 h. Subsequently, the tissue was paraffin-embedded using a PELORIS II automated tissue microprocessor (Leica Microsystems). Five-micron–thick sections were deparafinized, hydrated, and treated with an acidic antigen decloaker (Biocare Medical) aboard of a Ventana Benchmark Ultra CC1 stainer for 30min. The p-MAPK primary antibodies were applied in a 1:200 dilution, and the tissue sample was incubated for 32min in the Ventana Benchmark Ultra CC1 stainer. Detection used a VentanaUltraview DAB Detection Kit, followed by counterstaining with hematoxylin (Biocare Medical). Negative controls omitted the primary antibody and revealed no immunoreactivity. All cases were reviewed by 2 neuro-pathologists (S.I.B. and X.F.). Five years of experience with the antibody showed high day-to-day reproducibility of the assay.
Immunohistochemical Detection of PTEN
Evaluation of PTEN staining was performed using immunohistochemistry. The same biopsy blocks that were used for p-MAPK staining were stained for PTEN. Five-μm–thick paraffin sections were deparaffinized through xylene, rehydrated by graded alcohol, and pretreated with high-pH buffer on Leica Bond-III automated stainer station. Subsequently, a commercially available antibody to PTEN (Cascade Biosciences ABM-2052) was used at 1:250 dilution for 32 min incubation at room temperature followed by a detection with Leica Bond Refine DAB detection system. Nuclei were counterstained with hematoxylin (Biocare Medical). A negative control was performed by omitting the primary antibody, and this revealed no immunoreactivity.
Evaluation of PTEN immunostaining was performed in at least 3 separate fields of the tumor, each containing ≥50 tumor cells. Endothelial cells in the tumor were used as a positive control, with a rank of staining assigned as 3+. Negative or weak staining (0+ or 1+) was considered as a loss of expression. A moderate, uniform cytoplasmic staining of tumor cells, to a degree lower than that of endothelial cells was assigned 2+ intensity and considered to be preserved. Variation from one field to another was generally low in most cases of GBM.
Evaluation of EGFR amplification by fluorescence in situ hybridization (FISH) was done using a commercially available kit (Vysis), according to the manufacturer's instructions. Dual-probe analysis was performed with a locus-specific probe for both EGFR (the gene is located on chromosome 7) and compared with second chromosome enumeration probe (CEP7). The tumors with an EGFR/CEP7 signal ratio of >2.00 were classified as having EGFR amplification. Tumors with >10% nuclei with ≥3 CEP7 signals were classified as having gain of chromosome 7. Both gain of chromosome 7 and EGFR amplification frequently occur together in gliomas.
Morphometric Analysis
Two experienced neuropathologists (S.I.B. and X.F.) performed prospective analysis and quantification of all pathological stains. We first examined whether antibody to p-MAPK can be applied to examination of formalin fixed and paraffin embedded tissue in a reliable and reproducible fashion. Because of the quantitative nature of this investigation, we sought to evaluate whether non-tumorous tissue, including vasculature, normal cellular constituents of the brain, such neurons, astrocytes, oligodendroglial, or microglial cells express any appreciable levels of p-MAPK. To this end, we evaluated a pattern of p-MAPK staining in normal brain as resected for treatment of intractable seizures, brain afflicted by variously aged infarcts, and demyelinating conditions and infections. We found significant expression of p-MAPK in reactive astrocytes and in endothelial cell with brain infarcts and abscesses, but not in the control specimens. No expression of cytoplasmic or nuclear p-MAPK was detected in neurons and in oligodendroglia. There was no definitive expression by microglial cells.
To test the effect of timing of tissue fixation on immunohistochemical detection of p-MAPK, we performed a paired quantitative comparison of p-MAPK levels obtained from the same viable tumor but processed in a way that would mimic a commonly seen perioperative condition. All surgically excised tissue was collected on saline-soaked Telfa pad and fixed in 4% buffered formalin either at ∼20min after resection (time of completion of frozen section consultation) or at ∼60 min after resection (time of completion of surgery). Immunohistochemical evaluation of corresponding specimens revealed that specimens fixed at 20 min tended to have a more uniform distribution of positive cells throughout the section, whereas specimens with delayed fixation had a nonuniform patchy staining, with a tendency to have hot spots in a peripheral distribution (presumably in the areas wherein formalin infiltration was the fastest). However, quantitative analysis of paired specimens revealed that the levels detected at 20 min were very similar and never actually exceeded the levels seen at the hot spots found in the specimens obtained at 60 min. Because of the technical limitation inherent in perisurgical handling of brain tumors, we operationally defined the true biological level of p-MAPK as one seen in 20 min after resection specimens.
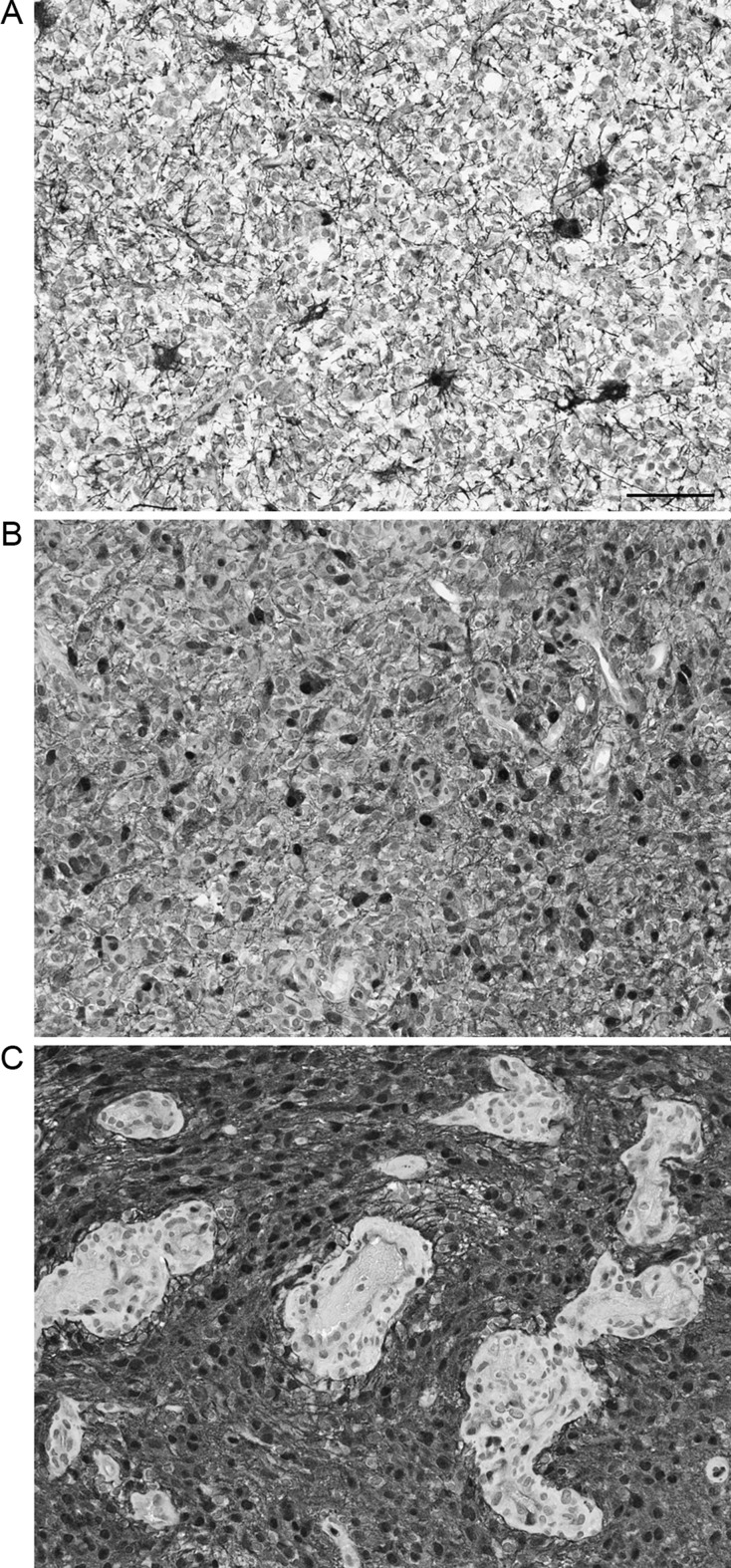
Evaluation of p-MAPK immunostaining was performed in at least 3 separate fields of the tumor, each containing ≥50 tumor cells. Immunoreactive nuclei were quantified with the mean and standard deviation of reactive nuclei in each of the 3 fields. Because of a frequent, characteristically graded, rapid decline in the staining intensity toward the center of the tissue in the block, areas with the strongest immunoreactivity were evaluated. Nuclear p-MAPK expression levels in 108 patients were quantified and later categorized in the following expression levels: low (0%–10%), moderate (11%–40%), and high (41%–100%) (Fig. 2). We specified the cutoffs based on the proportion of data in each category of p-MAPK expression. Although categorizing continuous variables typically involves higher uncertainty, our categorization of p-MAPK levels resulted from 2 separate explorations. First, an assessment of the distribution of p-MAPK levels showed a naturally skewed distribution at the most extreme (>40) and low (≤10) levels. Second, a correlation analysis of survival and p-MAPK expression as observed in the original data (ρ = −0.31, P = .009) and after categorization (ρ = −0.34, P = .0003) of p-MAPK showed a consistent relationship between these variables; these findings support true dichotomies in the discretization of p-MAPK and correspond to naturally occurring cohorts and qualitative staining features as depicted by Fig. 2.
Fig. 2.
Representative images of pMAPK staining in glioblastomas. A, Low (0%–10%) category. B, Intermediate (10%–40%). C, High (>40%). Inserts show 4× enlarged representative fields. Note “starburst” appearance of reactive gliosis on A, variable staining of endothelial cells on C. Original magnification 200×. Bar: 100 μ.
Statistical Analysis
The χ2 test was used to evaluate potential differences in median overall survival by MAPK expression and patient baseline characteristics. Differences in overall survival by MAPK expression were evaluated using Kaplan-Meier estimates (log-rank test). Multivariate Cox proportional hazards regression models were used to explore the predictive role of patient and tumor characteristics (age, KPS, extent of surgical resection, p-MAPK, MGMT, PTEN) on overall survival. All analyses were conducted using SAS, version 9.1, for Windows (SAS Institute).
Results
A total of 108 patients with newly diagnosed GBM were included in this study. The median age was 65 years, and 64% were men (Table 1). Seventy-four percent (n = 80) of patients had KPS scores ≥80, 22% (n = 24) had scores of 50–70, and 4% (n = 4) had scores ≤40. Extent of resection was categorized as near-gross total or gross-total resection in 54%, and biopsy was performed in 23% of patients. EGFR was amplified in 62.6% and MGMT≥20% expression was observed in 40 patients (37.0%). PTEN expression loss was seen in 56.9% of patients. Standard therapy (radiation therapy with concurrent temozolomide) was completed in 79% of patients (Table 1). Median overall survival among all patients was 19.5 months (95% confidence interval [CI], 15.0–32.4 months).
Table 1.
Patient and tumor characteristics of 108 patients with newly diagnosed glioblastoma
| Characteristics | n | Percent |
|---|---|---|
| Total cases | 108 | |
| Age | ||
| Median | 65 | |
| Range | 18–92 | |
| Gender | ||
| Female | 39 | 36.1 |
| Male | 69 | 63.9 |
| Karnofsky Performance Status | ||
| 80–100 (normal activity) | 80 | 74.1 |
| 50–70 (requires assistance) | 24 | 22.2 |
| ≤40 (disabled) | 4 | 3.7 |
| Extent of Resection | ||
| Biopsy | 24 | 22.6 |
| Partial resection | 25 | 23.6 |
| Near gross-total resection | 18 | 1.7 |
| Gross-total resection | 39 | 36.8 |
| EGFR Expression | ||
| Normal | 34 | 37.4 |
| Abnormal | 57 | 62.6 |
| MGMT Immunohistochemical Expression | ||
| ≥20% | 40 | 37.0 |
| <20% | 68 | 63.0 |
| PTEN Expression | ||
| Loss | 58 | 56.9 |
| Retained | 44 | 43.1 |
| Standard Treatment* | ||
| Yes | 72 | 79.1 |
| No | 19 | 20.9 |
| Radiation | ||
| Yes | 98 | 90.7 |
| No | 10 | 9.3 |
*Standard treatment = Temozolomide and radiation.
EGFR (PTEN) expression data available in 91 (102) patients.
Activated p-MAPK expression levels of low (0%–10%), medium (11%–40%), and high (≥41%) were observed in 33 (30.6%), 37 (34.3%), and 38 (35.2%) patients, respectively. Patient and tumor characteristics stratified by p-MAPK expression level are presented in Table 2.
Table 2.
Patient and tumor characteristics of 108 patients with newly diagnosed glioblastoma stratified by p-MAPK expression
| All cases | pMAPK expression levels |
||||
|---|---|---|---|---|---|
| ≤10 | 10–40 | >40 | |||
| Characteristics | n | n (%) | n (%) | n (%) | P value |
| Total cases | 108 | 33 | 37 | 38 | |
| Age | |||||
| Median | 65 | 67 | 63 | 64 | .58 |
| Range | 18–92 | 31–85 | 37–88 | 18–92 | |
| Gender | .29 | ||||
| Female | 39 (36.1) | 11 (33.3) | 17 (46.0) | 11 (29.0) | |
| Male | 69 (63.9) | 22 (66.7) | 20 (54.0) | 27 (71.0) | |
| Tumor Location | .52 | ||||
| Infratentorial | 1 (0.9) | 1 (3.0) | 0 | 0 | |
| Supratentorial | 106 (98.2) | 32 (97.0) | 37 (100) | 37 (97.4) | |
| Both | 1 (0.9) | 0 (0) | 0 | 1 (2.6) | |
| Karnofsky Performance Status | .35 | ||||
| 80–100 (normal activity) | 80 (74.1) | 26 (78.8) | 29 (78.4) | 25 (65.8) | |
| 50–70 (requires assistance) | 24 (22.2) | 5 (15.2) | 8 (21.6) | 11(28.9) | |
| ≤40 (disabled) | 4 (3.7) | 2 (6.1) | 0 | 2 (5.3) | |
| Extent of Resection | .38 | ||||
| biopsy | 24 (22.6) | 9 (28.1) | 6 (16.2) | 9 (24.3) | |
| partial resection | 25 (23.6) | 7 (21.9) | 8 (21.6) | 10 (27.0) | |
| near gross-total resection | 18 (17.0) | 2 (6.3) | 8 (21.6) | 8 (21.6) | |
| gross-total resection | 39 (36.8) | 14 (43.8) | 15 (40.5) | 10 (27.0) | |
| EGFR Expression | .67 | ||||
| normal | 34 (37.4) | 13 (43.3) | 9 (32.1) | 12 (36.4) | |
| abnormal | 57 (62.6) | 17 (56.7) | 19 (67.9) | 21 (63.6) | |
| MGMT Immunohistochemical Expression | .02 | ||||
| ≥20% | 40 (37.0) | 6 (18.8) | 22 (59.5) | 19 (50.0) | |
| <20% | 68 (63.0) | 26 (81.2) | 15 (40.5) | 19 (50.0) | |
| PTEN Expression | .32 | ||||
| loss | 58 (56.9) | 21 (36.2) | 19 (32.8) | 18 (31.0) | |
| retained | 44 (43.1) | 10 (22.7) | 16 (36.4) | 18 (40.9) | |
| Standard Treatment* | .02 | ||||
| yes | 72 (79.1) | 29 (87.9) | 31 (83.8) | 29 (76.3) | |
| no | 19 (20.9) | 4 (12.1) | 6 (16.2) | 9 (23.7) | |
*Standard treatment = Temozolomide and radiation.
EGFR/PTEN expression data available in 91/102 patients.
EGFR amplification was not significantly correlated with p-MAPK expression. Sixty-four percent and 57% of patients with EGFR amplification had high and low p-MAPK expression, respectively (P = .67). Patients with low (≤10) p-MAPK expression also had low MGMT (<20%) expression (P = .02). PTEN expression showed a mild negative correlation with p-MAPK status (ρ = −0.20, P = .05).
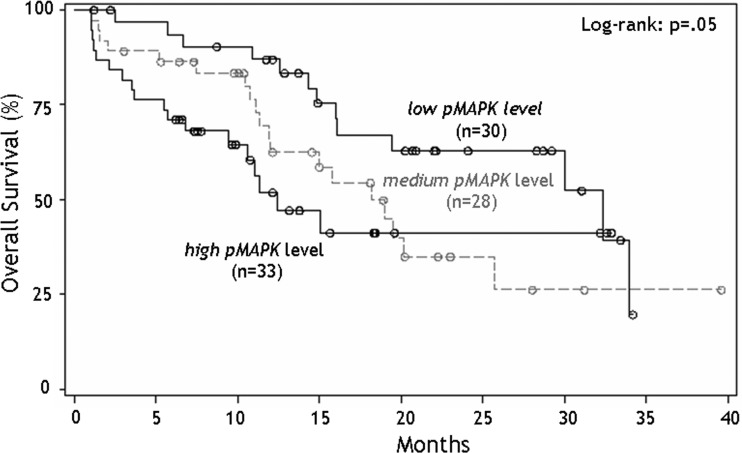
Univariate analysis showed that increasing p-MAPK expression was associated with shorter survival. Median survival for low, medium-, and high- p-MAPK expression levels was 32.4, 18.2, and 12.5 months, respectively. Fig. 3 illustrates the Kaplan-Meir survival curves for overall survival stratified by the 3 p-MAPK expression levels. One-year survival was 87%, 63%, and 52% in the low, medium, and high p-MAPK groups, respectively.
Fig. 3.
Overall survival for 108 patients with newly diagnosed glioblastoma stratified into 3 groups according to varying levels of MAPK expression.
The multivariate proportional hazards model showed that older patients (≥65 years; hazard ratio [HR], 2.8; P = .002) with KPS < 80 (HR, 3.10; P = .0003) and who had biopsy or partial resection (HR, 1.94; P = .02) had significantly increased hazard of death (Table 3). In addition, the hazard of death among patients with intermediate p-MAPK expression was 2.4 times that of patients with low p-MAPK expression (HR, 2.4; 95% CI, 1.1–4.9; P = .02). Finally, patients with high expression of p-MAPK were 3.9 times more likely to die than were patients with low p-MAPK expression (HR, 3.9; 95% CI, 1.6–7.5; P = .007). PTEN, MGMT, and EGFR status were not statistically significant predictors of survival in the multivariate model.
Table 3.
Hazard ratio (HR) for death among 108 patients with newly diagnosed glioblastoma, 95% confidence intervals (CI), and corresponding P values
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age (ref: age<65) | |||
| age 65+ | 2.75 | 1.46–5.18 | .002 |
| Karnofsky Performance Status (ref: KPS > 80) | |||
| KPS ≤ 80 | 3.10 | 1.68–5.65 | .0003 |
| Extent of Resection (ref: NGTR/GTR) | |||
| Partial Resection/Biopsy | 1.94 | 1.10–3.44 | .02 |
| p-MAPK Expression Level (ref: expression ≤10) | |||
| level 2 (10<expression ≤40) | 2.37 | 1.15–4.88 | .02 |
| level 3 (expression>40) | 3.90 | 1.78–8.55 | .007 |
*MGMT status was not a significant predictor of survival.
*p-MAPK by PTEN interactions were evaluated and found to be not significant.
Discussion
Our study found that activated MAPK was a strong independent prognostic marker for poor overall survival among patients with GBM in the temozolomide era. Survival among these patients decreased with the degree of p-MAPK expression in a graded fashion, with 1-year survival of low, medium, and high p-MAPK groups being 87%, 63%, and 52%, respectively. Compared with patients with low p-MAPK expression, intermediate p-MAPK expression increased the hazard of death by >2-fold and high expression of p-MAPK increased the odds of death by almost 4 times. Older age, low KPS, and extent of resection were also significant predictors of poor survival. In addition, we found that EGFR status was not statistically correlated to p-MAPK expression. Previous studies in the pre-temozolomide era have investigated p-MAPK as a prognostic factor.11,12 Higher p-MAPK expression has been shown to correlate with higher grade and increased proliferation (Mawrin 2003, Mizoguchi 2006).11,13 In addition, Pelloski et al.12 demonstrated that high p-MAPK expression was associated with poor survival and tumor resistance to radiation therapy in patients with GBM during the pre-temozolomide era. They evaluated p-MAPK as a binary outcome (normal/faint or high) and reported a 1.5–2-fold increase in the hazards of death in the high p-MAPK group. In comparison, our analysis and categorization showed a much higher graded effect (HR, 3.9) of MAPK expression on survival in the temozolomide era. This could suggest a possibility of MAPK-dependent resistance of GBM to our current standard therapies. The fact that the high MAPK group had median survival of only 12 months raises a question of whether this group with poor prognosis should be treated differently beyond the standard treatment paradigm.
Here, we describe a reliable, quantitative method of evaluating p-MAPK expression in formalin-fixed and paraffin-embedded specimens from human GBM. The study by Mawrin et al.11 was the only investigation that had previously applied quantitative analysis of p-MAPK levels to evaluate individual tumor specimens. Similar to our findings, they reported a relatively high prevalence of p-MAPK–positive GBMs (69%).11 In congruence with the Pelloski et al.12 study, we found that all the biopsies performed at our institution and fixed at 20 min after resection time had at least some (1%–10%) p-MAPK staining, which the Pelloski study described as faint or patchy. Of interest, in our experience, up to one-fourth of our outside consultation cases (not included in this study) were entirely negative for p-MAPK. This finding is in agreement with our observation of decay in MAPK phosphorylation with delay in formalin fixation. This phenomenon in effect may limit the clinical applicability of phosphorylation-specific MAPK antibodies and emphasize the need to fix the tissue in formalin within 20–60min after resection of the tumor specimen.
MAPK pathway
The RAS/RAF/MAPK intracellular signaling pathways are a critical regulator of several vital cell functions, including growth, differentiation, and survival (Fig. 1).14 This signaling pathway serves as an intracellular link between external molecular signals and regulation of transcription within the cell. It has various downstream targets within the cytosol and nucleus, including several proteins that mediate the transcription and, thus, expression of mitogenic regulatory proteins within the cell.8 Therefore, it is not surprising that mutations within RAS/RAF/MAPK pathways have been implicated in many cancers, including colorectal and hematopoietic malignancies14–17 Of interest, in spite of being a common mutagenic site in a variety of somatic cancers, mutations in RAS protein are rarely observed in GBM.8 Instead, abnormal MAP kinase expression is likely to be a result of mutation in upstream signaling domains, downstream effectors, and other regulatory pathways that interact with the RAS/RAF/MAPK pathway, such as the PI3k/Akt pathway.15–17
In the RAS/RAF/MAPK pathway, receptor tyrosine kinases activate Ras, which triggers the phosphorylation of RAF. RAF propagates the signaling pathway by phosphorylating MEK, which continues on to activate MAP kinase by converting it to p-MAP kinase (Fig. 1). This activated MAP kinase is then able to regulate the transcription of mitogenic signals crucial for cell growth, proliferation and apoptosis. As excessive proliferation remains one of the defining characteristics of GBM, it appears that deregulation of the MAPK pathway plays a critical role in enabling the increased malignancy of these tumors. In congruence, a study by Mizoguchi et al.13 found higher MAPK activation in patients with GBM when compared to grade III anaplastic astrocytoma (AA). Furthermore, studies have reported a correlation between the co-activation of MAPK and Akt with progression of AA to secondary GBM, suggesting that activation of these proteins contributed to the phenotypic progression of these tumors and, perhaps, to their significantly worse survival.13,18
MAPK and EGFR
Overexpression or mutation of upstream receptors is one plausible explanation for higher expression of p-MAPK in an oncogenic state. One such receptor that has been exhaustively studied is the epithelial growth factor receptor (EGFR), which has been found to be amplified in up to 50% of GBMs.19 Mutation and amplification of EGFR and has been shown to result in sustained MAPK activation.20 EGFR is a membrane-bound receptor that plays a role in activating several intracellular signaling cascades, including the JAK/STAT pathway, the PI3k/Akt pathway, and the RAS/RAF/MAPK pathway. Furthermore, overexpression and mutation of wild-type EGFR has been associated with deregulation of these pathways.8,17 Our study showed that EGFR amplification status alone did not correlate with p-MAPK expression. Among the alternative mechanisms of direct EGFR activation is EGFRvIII mutation, which makes activation of the protein ligand independent. Our patients were not evaluated for EGFRvIII mutation in their tumors. The absence of a simple positive correlation between EGFR amplification status and p-MAPK status suggests a complex relationship between EGFR and p-MAPK. Similar to our findings, Mizoguchi et al.13 did not find a correlation between EGFR status and p-MAPK. This can be explained by the fact that receptor tyrosine kinases other than EGFR, such as PDGFR-alpha, FGFR, and IGFR, are also involved in MAPK pathway activation.8 In addition, significant interactions between the Akt and MAPK pathway have been reported that are independent of EGFR.8,12,21 For example, several in vitro experiments have implicated YKL-40 protein in constituent activation of Akt and MAPK pathways.21 Furthermore, Pelloski et al.12 have reported a positive correlation between YKL-40 and activated Akt and MAPK pathway intermediates in GBM specimens, demonstrating another EGFR-independent method of MAPK activation.
In the context of a new treatment paradigm for GBM with concurrent radiation and temozolomide, it was previously unclear whether MAPK continues to be an important prognostic factor. Our study has shown that expression of p-MAPK still holds a very significant, important, and independent prognostic significance in patients with GBM during the temozolomide era. In fact, our data show what appears to be a greater impact of p-MAPK expression on survival in the temozolomide era than in pretemozolomide studies.12,13 These findings suggest a role of p-MAPK–dependent resistance of GBM to our current standard therapies and support the efforts that are under way to develop therapeutics that target this pathway.
Acknowledgments
We thank Sherry Brandon for her expert help with figures, tables, and administrative support.
Conflict of interest statement. The authors report no conflict of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 6.Bouterfa HL, Sattelmeyer V, Czub S, Vordermark D, Roosen K, Tonn JC. Inhibition of Ras farnesylation by lovastatin leads to downregulation of proliferation and migration in primary cultured human glioblastoma cells. Anticancer Research. 2000;20:2761–2771. [PubMed] [Google Scholar]
- 7.Lakka SS, Jasti SL, Gondi C, et al. Downregulation of MMP-9 in ERK-mutated stable transfectants inhibits glioma invasion in vitro. Oncogene. 2002;21:5601–5608. doi: 10.1038/sj.onc.1205646. [DOI] [PubMed] [Google Scholar]
- 8.Nakada M, Kita D, Watanabe T, et al. Aberrant Signaling Pathways in Glioma. Cancers. 2011;3::3242–3278. doi: 10.3390/cancers3033242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MJ, Park IC, Hur JH, et al. Modulation of phorbol ester-induced regulation of matrix metalloproteinases and tissue inhibitors of metalloproteinases by SB203580, a specific inhibitor of p38 mitogen-activated protein kinase. Journal of Neurosurgery. 2002;97:112–118. doi: 10.3171/jns.2002.97.1.0112. [DOI] [PubMed] [Google Scholar]
- 10.Van Brocklyn J, Letterle C, Snyder P, Prior T. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Letters. 2002;181:195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 11.Mawrin C, Diete S, Treuheit T, et al. Prognostic relevance of MAPK expression in glioblastoma multiforme. Int J Oncol. 2003;23:641–648. [PubMed] [Google Scholar]
- 12.Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2006;12:3935–3941. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 13.Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65:1181–1188. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 14.Poulogiannis G, Luo F, Arends MJ. RAS signalling in the colorectum in health and disease. Cell Communication & Adhesion. 2012;19(1):1–9. doi: 10.3109/15419061.2011.649380. [DOI] [PubMed] [Google Scholar]
- 15.Guha A, Feldkamp MM, Lau N, Boss G, Pawson A. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene. 1997;15:2755–2765. doi: 10.1038/sj.onc.1201455. [DOI] [PubMed] [Google Scholar]
- 16.Knobbe CB, Reifenberger J, Reifenberger G. Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol. 2004;108:467–470. doi: 10.1007/s00401-004-0929-9. [DOI] [PubMed] [Google Scholar]
- 17.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 19.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 20.Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 21.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. The Biochemical Journal. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]