Abstract
Background
The current mainstay of therapy for chronic obstructive pulmonary disease (COPD) is long-acting bronchodilators. To date, the effect of indacaterol, a β2-agonist, on activities of daily living in COPD patients is not well understood. The aim of this study was to evaluate the efficacy of indacaterol with regard to activities of daily living in patients with COPD.
Methods
In this nonrandomized open-label study, 23 patients with COPD were instructed to carry an accelerometer for 4 weeks without indacaterol therapy and then for another period of 4 weeks while receiving indacaterol therapy.
Results
The number of steps, duration of moderate or greater physical activity, and energy expenditure were significantly increased after treatment with indacaterol compared with baseline data in all patients with COPD; the metabolic equivalent of task was also significantly enhanced after treatment with indacaterol.
Conclusion
This study provides early evidence that indacaterol improves daily physical activity in patients with COPD.
Keywords: chronic obstructive pulmonary disease, indacaterol, long-acting β2-agonist, physical activity
Introduction
Chronic obstructive pulmonary disease (COPD) results from a chronic inflammatory process of the airways.1 The prevalence of COPD is very high, and the disease affects more than 10% of subjects aged over 40 years worldwide, and it is predicted that COPD will be the third leading cause of death by 2020.2 The main cause of the disease is cigarette smoking.2 The inflammatory process predominantly affects the small airways, is characterized by infiltration of neutrophils, macrophages, and CD8+ T cells, and leads progressively to fibrosis of the small bronchioles and destruction of the alveolar walls and lung parenchyma architecture.3 The functional consequences of these pathological changes are airflow limitation with air-trapping and dynamic hyperinflation, leading to reduced inspiratory capacity and increased end-expiratory lung volumes.3 Therefore, patients with COPD complain generally of dyspnea, particularly during exercise.2 This shortness of breath limits physical activity and worsens quality of life in these patients.
At present, there is no curative therapy for COPD, and the aim of treatment is to reduce symptoms and exacerbations and to improve quality of life.1 Treatment includes bronchodilators, rehabilitation, and home oxygen therapy. Recent studies have shown that the inhaled long-acting bronchodilators are very effective for improving symptoms, exercise capacity, and quality of life, as well as for prevention of exacerbations.4 The β2-agonist, indacaterol, is an ultra-long- acting bronchodilator with an established clinical efficacy and safety profile which needs to be administered only once daily.5,6 Indacaterol was approved for use in the clinic by the European Medicines Agency in 2009 and by the US Food and Drug Administration in 2011. Several clinical trials performed in patients with moderate to severe COPD have shown that indacaterol significantly improved symptoms, pulmonary function test results, and quality of life, and markedly reduced the use of rescue medication compared with a placebo control group.7–9 Recent studies suggest that indacaterol may also improve exercise endurance in patients with COPD, but its effect on daily physical activity in these patients is not well understood as yet.10,11
Physical activity refers to movement of the body produced by skeletal muscle contraction leading to energy expenditure.12 The level of physical activity during routine daily life has recently been recognized to be an important outcome parameter when evaluating the therapeutic response in patients with COPD.13–17 Limited physical activity has been reported to be associated with more hospitalizations because of exacerbations, and markers of physical activity have been shown to predict mortality in patients with COPD.18–21 Several studies have validated the use of accelerometers for objective measurement of physical activity in patients with the disease.15,20,22 In the present study, the effect of indacaterol on daily physical activity was evaluated in patients with COPD using a uniaxial accelerometer.
Materials and methods
Subjects
This study included 23 patients with COPD attending the outpatient department at Matsusaka Municipal Hospital. Table 1 shows the anthropometry, clinical staging, and results of pulmonary function testing in these subjects. All of the patients were ex-smokers and not on active smoking cessation pharmacotherapy, and had stable disease with no exacerbations before the beginning or during the study. Patients who were physically unable to move and those receiving home oxygen therapy or pulmonary rehabilitation were excluded. Three patients had comorbid arterial hypertension and three had hyperuricemia. In total, 24 patients were included in the study but one was excluded because of contusions and a sprained ankle following a traffic accident. Among the 23 eligible patients, 14 were receiving tiotropium 18 μg/day (by Handihaler) while nine were receiving no therapy; thus, during the entire study period, 14 cases received treatment with tiotropium together with indacaterol and nine with indacaterol alone. None of the patients received steroids or rescue with short-acting β-agonists during the study.
Table 1.
Baseline demographic data for patients with chronic obstructive pulmonary disease
| Patients (n) | 23 |
| Age (years) | 69.74 ± 1.60 |
| Gender (M/F) | 21/2 |
| Body mass index | 21.59 ± 0.72 |
| GOLD scale (patients, n) | |
| 1 | 6 |
| 2 | 10 |
| 3 | 6 |
| 4 | 1 |
| Maximum inspiratory capacity (L) | 2.06 ± 0.088 |
| Forced vital capacity (L) | 3.319 ± 0.101 |
| FEV1 (% predicted) | 64.5 ± 4.3 |
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in one second.
The diagnosis of COPD was made according to the criteria of the American Thoracic Society.1 Pulmonary function tests were performed using a volume-type spirometer (Super Spiro, Discom-21FX III; Chest Corporation, Tokyo, Japan). The 6-minute walk test (6MWT) and the COPD Assessment Test (CAT) were performed based on a protocol and criteria previously described.23,24 Informed consent was obtained from all subjects before entry into the study.
Study design
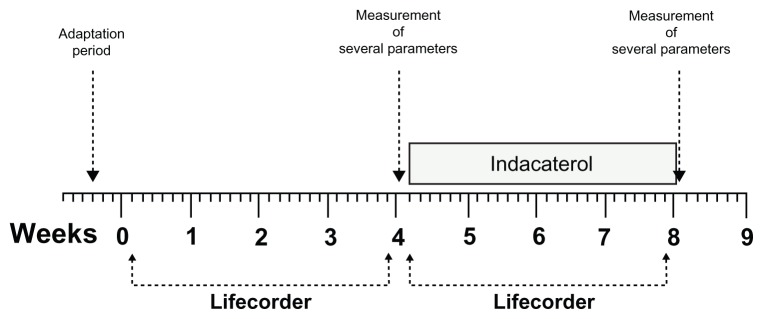
This was an open-label clinical trial with no randomization, no placebo group, and no blinding. To record physical activity, the patients were asked to carry a Lifecorder, a uniaxial accelerometry sensor (Suzuken Corporation, Nagoya, Japan),25 fixed to their belts for 4 weeks without receiving indacaterol therapy and then for another period of 4 weeks while receiving 150 μg of inhaled indacaterol (Figure 1). Physical activity before and after treatment with indacaterol was then compared. Before the trial was started, there was a period of adaptation during which each patient received instructions on how to fix and use the device correctly. Patients were instructed to fix the device every day upon waking and to remove it before going to sleep.
Figure 1.
Study design.
Notes: Patients fixed the Lifecorder to their belts for a period of 4 weeks without receiving indacaterol therapy and then for another period of 4 weeks while receiving 150 μg of inhaled indacaterol.
Statistical analysis
All data are expressed as the mean ± standard error of the mean. The difference between the means of two variables was assessed using the nonparametric Wilcoxon’s rank test. Statistical analyses were performed using the StatView 4.5 package for Macintosh (Abacus Concepts, Berkeley, CA). A P value less than 0.05 was considered to be statistically significant.
Results
Pulmonary function, 6MWT, and CAT
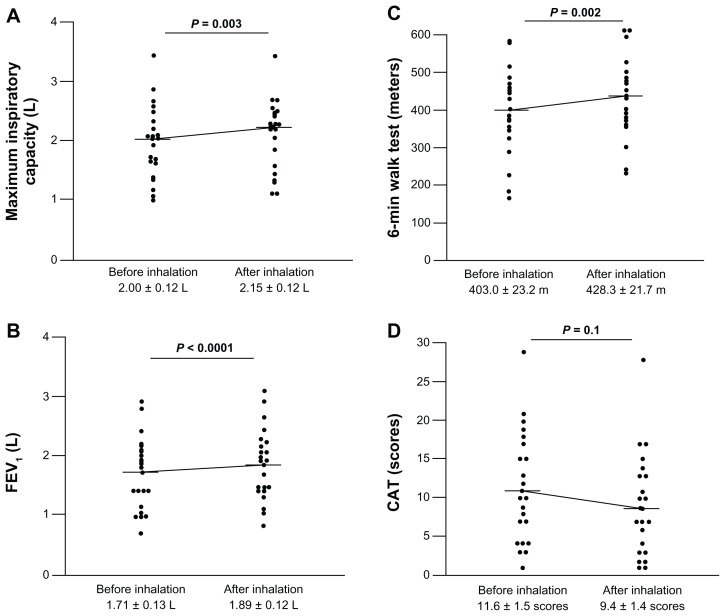
Forced expiratory volume in one second (FEV1) and 6MWT are important parameters when assessing the response to therapy in patients with COPD.1 Comparing these parameters before and after treatment showed a significant increase in FEV1 and in maximum inspiratory capacity after 4 weeks of treatment with indacaterol (Figure 2A and B). Similarly, the distance walked during the 6MWT was significantly increased after treatment compared with that before starting indacaterol (Figure 2C). The CAT score also tended to decrease after treatment with indacaterol but this did not reach a statistically significant level (Figure 2D).
Figure 2.
Response to indacaterol. Maximum inspiratory capacity (A), FEV1 (B), and 6MWT (C) improved significantly after 4 weeks of treatment with indacaterol. The CAT score (D) tended to decrease after treatment with indacaterol.
Notes: Horizontal bars indicate the means. Statistical analysis was performed using the nonparametric Wilcoxon’s rank test.
Abbreviations: 6MWT, six-minute walk test; CAT, COPD Assessment Test; FEV1, forced expiratory volume in one second.
Physical activity parameters
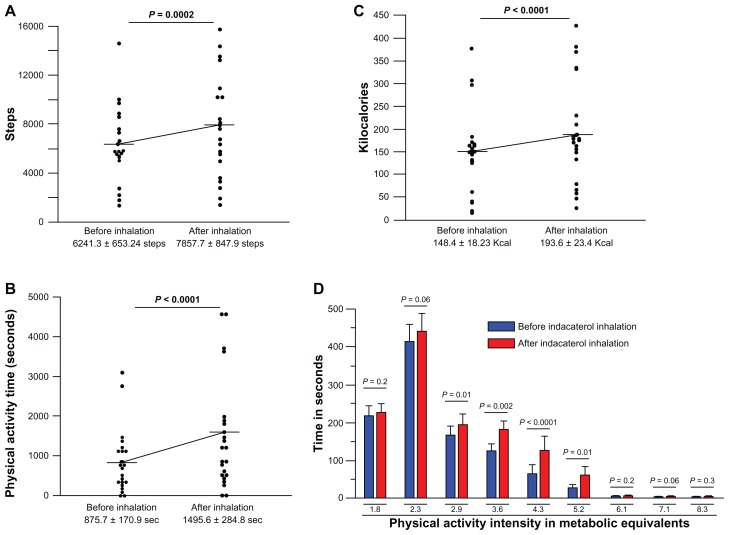
The Lifecorder was carried by all patients, and the number of steps, duration of moderate or higher degree exercise, energy expenditure, and metabolic equivalent of task were measured. The number of steps (Figure 3A), time in seconds of moderate or more activity (Figure 3B), and energy expenditure expressed in kilocalories (Figure 3C) were significantly increased after treatment compared with baseline in all patients. The metabolic equivalent of task was also significantly improved after treatment with indacaterol compared with baseline data (Figure 3D).
Figure 3.
Effect of indacaterol on physical activity parameters. Number of steps (A), duration of moderate or more exercise (B), energy expenditure (C) and metabolic equivalent of task (D) improved significantly after treatment with indacaterol in all patients.
Notes: Horizontal bars indicate the means. Statistical analysis was performed using the nonparametric Wilcoxon’s rank test.
Discussion
The results of this study show that the long-acting β2-agonist, indacaterol, significantly and effectively improved daily physical activity in patients with COPD and that the physical activity parameters recorded using the uniaxial accelerometry sensor are useful for monitoring the therapeutic response in this group of patients.
The mainstay of treatment for symptomatic improvement in patients with COPD is inhaled bronchodilators.1 Of these, the ultra-long-acting β2-adrenoreceptor agonist, indacaterol, and the long-acting antimuscarinic, tiotropium bromide, have been shown to be very effective for reducing symptoms, to increase exercise tolerance, to reduce the frequency of exacerbations, and to improve quality of life test scores.4 These drugs have a further advantage over previous long-acting bronchodilators, in that they can be used once daily, so are associated with fewer side effects.4,26,27 In the present study, we focused on the β2-agonist, indacaterol, and evaluated whether it can improve exercise capacity and FEV1 in patients with COPD treated for 4 weeks, and our results showed that this agent significantly improved the results of lung function testing and exercise endurance as measured by the 6MWT.
One hurdle that needs to be overcome in the management of patients with COPD is the assessment of response to therapy in terms of physical activity during routine daily life. Recent studies have validated the use of several accelerometers in the assessment of the level of activity in COPD patients.15,21 The results reported have shown a significant association between a low level of physical activity as measured by accelerometers and increased mortality and morbidity from COPD.21 In addition, less active patients are more likely to be hospitalized as a result of exacerbations.20 To the authors’ knowledge, the efficacy of indacaterol in daily life activity has not been evaluated until now. In the present study, we used a novel accelerometer, the sensitivity and efficacy of which has been previously validated.25 This novel accelerometer can measure several indicators of daily physical activity, including the number of steps, energy expenditure, and metabolic equivalent of task.25 The device is easy to use, cost-effective, and can record data on a real-time basis every 4 seconds and over a lengthy period of time. We carried out a prospective and open clinical trial to assess whether indacaterol can improve daily physical activity in patients with COPD. The number of steps, energy expenditure, and metabolic equivalent of task were significantly increased in all patients after treatment with indacaterol, suggesting that this β2-agonist improves routine daily activity in patients with COPD.
Our study has several limitations, including its open nature, a small patient population, lack of a placebo control group, and no randomization. In addition, although the patients were-not asked or motivated to perform any exercise other than their normal or routine daily physical activity, they knew that they were receiving the drug during the second period of the study and therefore they could have been psychologically motivated or encouraged to perform better during their daily physical activity. However, to avoid some of these confounding factors, the patients were allowed to have an adaptation period during which they received training about how to use the device and about the performance of lung function tests.
In brief, this study provides early evidence that indacaterol improves daily activity in patients with COPD. However, a larger patient population and a placebo control group should be included in future studies to validate the present findings.
Acknowledgment
The authors are very grateful to Shinobu Taniguchi, Mayumi Asai, and Miyuki Kadomatsu for their kind assistance and cooperation with the completion of this work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–494. doi: 10.2147/COPD.S32330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149. doi: 10.1186/1465-9921-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beier J, Beeh KM. Long-acting beta-adrenoceptor agonists in the management of COPD: focus on indacaterol. Int J Chron Obstruct Pulmon Dis. 2011;6:237–243. doi: 10.2147/COPD.S7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeage K. Indacaterol: a review of its use as maintenance therapy in patients with chronic obstructive pulmonary disease. Drugs. 2012;72:543–563. doi: 10.2165/11208490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Cope S, Zhang J, Williams J, Jansen JP. Efficacy of once-daily indacaterol 75 mug relative to alternative bronchodilators in COPD: a study level and a patient level network meta-analysis. BMC Pulm Med. 2012;12:29. doi: 10.1186/1471-2466-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro M, Chapman KR. Comparative efficacy of indacaterol in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:145–152. doi: 10.2147/COPD.S19805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi A, Centanni S, Cerveri I, et al. Acute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropium. Respir Med. 2011;106:84–90. doi: 10.1016/j.rmed.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Mroz RM, Minarowski L, Chyczewska E. Indacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patients. Adv Exp Med Biol. 2013;756:23–28. doi: 10.1007/978-94-007-4549-0_4. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell DE, Casaburi R, Vincken W, et al. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105:1030–1036. doi: 10.1016/j.rmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Casaburi R. Activity monitoring in assessing activities of daily living. COPD. 2007;4:251–255. doi: 10.1080/15412550701480158. [DOI] [PubMed] [Google Scholar]
- 13.Cazzola M, Segreti A, Stirpe E, Appodia M, Senis L, Matera MG. Energy expenditure and impact of bronchodilators in COPD patients. Respir Med. 2010;104:1490–1494. doi: 10.1016/j.rmed.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Heijdra YF. Physiologic limitations during daily life activities in COPD patients. Respir Med. 2010;104:1152–1159. doi: 10.1016/j.rmed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 16.Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106:522–530. doi: 10.1016/j.rmed.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 18.Benzo RP, Chang CC, Farrell MH, et al. Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration. 2010;80:10–18. doi: 10.1159/000296504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in COPD patients. Chest. 2012;142:338–346. doi: 10.1378/chest.11-2014. [DOI] [PubMed] [Google Scholar]
- 20.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 21.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 22.Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106:522–530. doi: 10.1016/j.rmed.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 23.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 24.Mackay AJ, Donaldson GC, Patel AR, Jones PW, Hurst JR, Wedzicha JA. Usefulness of the Chronic Obstructive Pulmonary Disease Assessment Test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185:1218–1224. doi: 10.1164/rccm.201110-1843OC. [DOI] [PubMed] [Google Scholar]
- 25.Kumahara H, Schutz Y, Ayabe M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–243. doi: 10.1079/BJN20031033. [DOI] [PubMed] [Google Scholar]
- 26.Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 27.Feldman G, Siler T, Prasad N, et al. Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med. 2010;10:11. doi: 10.1186/1471-2466-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]