Abstract
Background
Telemonitoring interventions featuring transmission of home glucose records to healthcare providers have resulted in improved glycemic control in patients with diabetes. No research has addressed the intensity or duration of telemonitoring required to sustain such improvements.
Purpose
The DiaTel study (10 January 2005 to 1 November 2007) compared active care management (ACM) with home telemonitoring (n=73) to monthly care coordination (CC) telephone calls (n=77) among veterans with diabetes and suboptimal glycemic control. The purpose of the DiaTel Extension was to assess whether initial improvements could be sustained with interventions of the same or lower intensity among participants who re-enrolled in a 6-month extension of DiaTel.
Methods
DiaTel participants receiving ACM were re-assigned randomly to monthly CC calls with continued telemonitoring but no active medication management (ACM-to-CCHT, n=23) or monthly CC telephone calls (ACM-to-CC, n=21). DiaTel participants receiving CC were re-assigned randomly to continued CC (CC-to-CC, n=28) or usual care (UC, ie, CC-to-UC, n=29). Hemaglobin A1c (HbA1c) was assessed at 3 and 6 months following re-randomization.
Results
Marked HbA1c improvements observed in DiaTel ACM participants were sustained 6 months after re-randomization in both ACM-to-CCHT and ACM-to-CC groups. Lesser HbA1c improvements observed in DiaTel CC participants were sustained in both CC-to-CC and CC-to-UC groups. No benefit was apparent for continued transmission of glucose data among DiaTel ACM participants or continued monthly telephone calls among DiaTel CC participants 6 months after re-randomization.
Conclusion
Significant improvements in HbA1c achieved using home telemonitoring and active medication management for 6 months were sustained 6 months later with interventions of decreased intensity in VA Health System-qualified veterans.
Clinical trial reg. no
Keywords: Clinical trials, diabetes, health services research, kidney disease, self-monitoring, telemedicine intervention studies, veterans
Diabetes mellitus affects approximately 8.3% of the US population, results in increased morbidity and mortality, and is associated with high medical costs.1 2 Intensive glycemic control has been shown to delay or prevent the development of diabetes-related microvascular complications.3 However, an estimated 43.2–55.6% of adults with diabetes do not meet the American Diabetes Association target for glycemic control (hemoglobin A1c (HbA1c) <7.0%).4 5 Factors that may contribute to suboptimal glycemic control include inadequate home glucose monitoring, non-compliance with medications or lifestyle changes, suboptimal patient education about the disease, and limited access to providers for diabetes management.6–9 In the absence of timely and accurate data on home blood glucose values, providers may be appropriately hesitant to escalate some oral hypoglycemic agents or insulin regimens aggressively, due to fear of hypoglycemia. Telemedicine or telehealth technologies could provide an effective approach for addressing education, compliance, monitoring and provider access issues. Glycemic control could be improved safely by basing medication changes on blood glucose readings obtained at home and transmitted in near real time to providers.
We reviewed 22 reports of clinical intervention studies involving the upload and direct transmission of capillary blood glucose data by patients with diabetes to providers via cellular telephone, telephone land line, or a web-based programme.10–32 The results of these studies were mixed, perhaps because many studies did not target participants with poor baseline glycemic control,12–15 18 23 27–29 31 or the interval between glucose transmission and follow-up was delayed10–17 19–21 23–26 29–31 or unspecified.13 22 27 Besides our Diabetes Telemonitoring (DiaTel) Study,32 only one study18 reported that providers reviewed glucose transmissions daily, which would facilitate more timely management of glycemia. None of these reports evaluated the intensity of intervention required to sustain achieved reductions in HbA1c after the implementation of home telemonitoring.
In the DiaTel Study, a telemonitoring intervention that included daily transmission of home glucose data and medication adjustments within 24–72 h (active care management; ACM) resulted in statistically significant reductions in HbA1c at 3 months (1.7% vs 0.7%) and 6 months (1.7% vs 0.8%; p<0.001 for each) compared with a monthly care coordination (CC) phone call offering diabetes self-management education and referral to the primary care provider (PCP) for medication adjustment.32 The purpose of the DiaTel Extension was to assess whether these initial improvements could be sustained with interventions of the same or lower intensity in DiaTel participants who re-enrolled in a 6-month extension of the study. In particular, we assessed the impact of continued data transmission, initial ACM, and continued monthly telephone calls.
Methods
Design and interventions: DiaTel
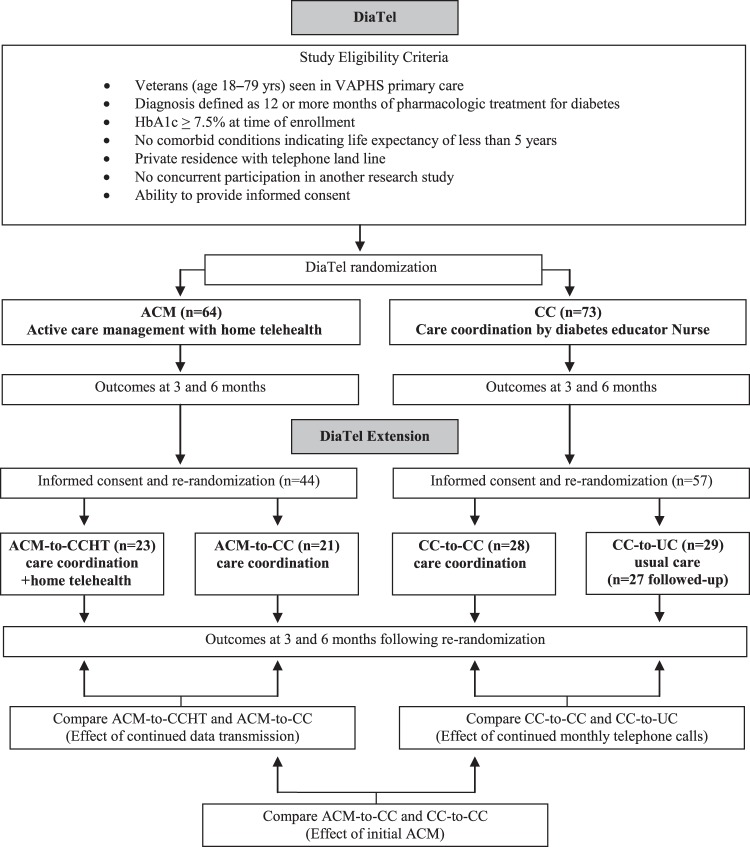
DiaTel was a randomized controlled trial of veterans with diabetes and suboptimal glycemic control managed by primary care services in the VA Pittsburgh Healthcare System (VAPHS). Participants were recruited from three VAPHS hospitals in Pittsburgh, Pennsylvania, and five affiliated community-based outpatient clinics in Pennsylvania and Ohio. Study eligibility criteria are summarized in figure 1. Mean HbA1c at randomization was 9.6% for ACM participants and 9.4% for CC participants (p=0.53). Patients were excluded if they did not have a telephone land line, as this was required by the telemonitoring device used in the study. Potentially eligible veterans were identified through a review of the VA electronic records and further evaluated by their PCP for appropriateness to participate in the study.
Figure 1.
Design of the DiaTel Study and the DiaTel Extension. ACM, active care management; VAPHS, VA Pittsburgh Healthcare System.
DiaTel participants were enrolled by the study nurses, and randomly assigned by the study coordinator using pseudo-random number sequences generated by the study statistician. Randomization was stratified on quartile of HbA1c within site and blocked on time in blocks of size four to six, with equal allocation to the two groups (ie, to receive 6 months of either home telemonitoring with ACM by a certified registered nurse practitioner (CRNP) or a less intensive CC intervention). All participants received baseline diabetes education from a certified diabetes educator registered nurse (CDE-RN), based on a VA publication.33
DiaTel participants randomly assigned to ACM received instruction in the use of the home-based Viterion 100 Monitor System (Viterion TeleHealthcare, Terrytown, New York, USA ) to transmit measurements of capillary blood glucose, blood pressure, and weight to the study CRNP at least once per day. The study CRNP reviewed participant measurements daily (Monday–Friday), contacted ACM participants with blood glucose levels greater than 300 mg/dl for 72 h or with any values less than 50 mg/dl, and made therapeutic adjustments by phone as indicated, after consultation with the study endocrinologist. ACM participants received more comprehensive and frequent education during the first 6 months of the study than did CC participants, as detailed in the 2010 Diabetes Care paper.32 ACM participants received daily messaging from the Viterion device, and monthly individualized educational phone calls from the study CRNP. DiaTel participants randomly assigned to CC received monthly monitoring by phone calls from the CDE-RN. Participants who reported any issues regarding their health or diabetes were directed to contact their PCP. The CDE-RN answered general questions about diabetes, diet, exercise, and medications during the monthly calls (or more frequently if a participant initiated contact regarding these topics). The methods and outcomes of the DiaTel Study have been reported elsewhere.32
Design and interventions: DiaTel Extension
In the DiaTel Extension, participants were re-consented, and re-assigned randomly to subsequent management at the same or lower intensity and followed for another 6 months (figure 1). Participants in the DiaTel ACM group were re-assigned randomly to receive either the lower intensity CC (ACM-to-CC) or CC with continued home telemonitoring (ACM-to-CCHT), which included continued daily Viterion data transmissions but no active medication management by the CRNP. Participants in the DiaTel CC group were re-assigned randomly to either continued CC (CC-to-CC) or VAPHS usual care (CC-to-UC), which consisted of visits to their PCP every 3–6 months.
DiaTel enrollment occurred between October 2005 and June 2006, with re-enrollment in the DiaTel Extension continuing to January 2007; the 6 month follow-up ended in July 2007. Because of administrative delays in the availability of funding for the DiaTel Extension, seven participants were re-assigned randomly between 13 and 50 days (median 31 days) after their 6-month DiaTel measurement visit. Consenting participants were re-assigned randomly by the study coordinator using pseudo-random binary sequences generated by the study statistician in Stata, with equal allocation within each initial DiaTel group. The sample size for the DiaTel Extension was limited by the number of re-consenting participants from DiaTel. A sample size of 25 participants per group provides 78% power to detect a pairwise difference of 1% in HbA1c based on a 0.01 two-sided t-test.
Because both studies involved the comparison of patient care interventions, it was not possible to blind participants, interventionists, or clinical investigators involved in making treatment decisions. Staff involved in the collection of laboratory data was blinded to randomization group. Both DiaTel and the DiaTel Extension were funded by the Department of Defense, and reviewed and approved by the VAPHS Institutional Review Board.
Measures
The intervention was evaluated in terms of the primary clinical outcome, HbA1c. The 6-month HbA1c from DiaTel was the baseline measure for the DiaTel Extension. Additional HbA1c measurements were obtained at clinic visits 3 and 6 months following re-randomization, ie, at approximately 9 and 12 months after the initial DiaTel randomization.
Analysis
Hypotheses regarding time-specific mean differences in HbA1c and differential changes over time were tested using two-sample t tests, with no adjustments for multiple comparisons. The following pairwise comparisons were made between the intervention groups: ACM-to-CCHT and ACM-to-CC, to evaluate the effect of continued data transmission; ACM-to-CC and CC-to-CC, to evaluate the effect of initial ACM; and CC-to-CC and CC-to-UC, to evaluate the effect of the continued monthly telephone calls associated with CC (figure 1). Changes over time within each intervention group were compared using paired t tests, and differential changes over time were compared using two-sample t tests on the difference scores. To account for the very small amount of missing HbA1c data (three measurements) and truncated data (one measurement), we used the mean of 10 imputations based on the fingerstick or other available HbA1c values. To assess the sensitivity of the results to the administrative delays in re-randomization, the primary analyses were re-run excluding the seven participants with delayed re-randomizations.
Results
Of the 137 (91.3%) participants who completed DiaTel, 101 (73.7%) consented to the DiaTel Extension (44/64 (68.8%) from the original ACM group, and 57/73 (78.1%) from the original CC group; figure 1). In the CC-to-UC group, two individuals were considered to be non-participants in subsequent analyses because no follow-up data were available. Follow-up at 3 and 6 months post re-randomization was otherwise complete, except for one CC-to-UC participant at 3 months and two CC-to-UC participants at 6 months (including one who had HbA1c assessed at a non-VA hospital). No statistically significant differences were observed between DiaTel Extension participants and non-participants within either DiaTel intervention group, although relatively more African-American than white individuals in ACM participated in the DiaTel Extension (table 1; p=0.06). No adverse events were experienced by any DiaTel Extension participant.
Table 1.
Sociodemographic characteristics of veterans who completed the DiaTel Study and did/did not enrol in the DiaTel Extension
| DiaTel ACM participants (n=64) | DiaTel CC participants (n=73) | |||||
| Refused (n=18) % | Re-enrolled (n=46) % | p Value | Refused (n=18) % | Re-enrollled (n=55) % | p Value | |
| Age, years | 0.27 | 0.11 | ||||
| <45 | 11.1 | 2.2 | 0.0 | 0.0 | ||
| 45–65 | 50.0 | 63.0 | 44.4 | 65.5 | ||
| >65 | 38.9 | 34.8 | 55.6 | 34.6 | ||
| Gender | 0.41 | |||||
| Male | 100.0 | 100.0 | 100.0 | 96.4 | ||
| Female | 0.0 | 0.0 | 0.0 | 3.6 | ||
| Race | 0.06 | 0.33 | ||||
| White, not Hispanic | 88.9 | 65.2 | 77.8 | 81.8 | ||
| African-American not Hispanic | 11.1 | 34.8 | 16.7 | 16.4 | ||
| Asian or Pacific Islander | 0.0 | 0.0 | 0.0 | 1.8 | ||
| American Indian or Alaskan Native | 0.0 | 0.0 | 5.6 | 0.0 | ||
| Employment status | 0.85 | 0.32 | ||||
| Employed full-time | 11.1 | 6.5 | 27.8 | 23.6 | ||
| Employed part-time | 11.1 | 13.0 | 0.0 | 14.6 | ||
| Full-time homemaker | 0.0 | 4.4 | 0.0 | 1.8 | ||
| Retired | 55.6 | 58.7 | 66.7 | 47.3 | ||
| Unemployed | 22.2 | 17.4 | 5.6 | 12.7 | ||
| Marital status | 0.38 | 0.76 | ||||
| Single, never married | 5.6 | 13.0 | 22.2 | 14.6 | ||
| Married or living as married | 38.9 | 54.4 | 44.4 | 58.2 | ||
| Widowed | 16.7 | 8.7 | 5.6 | 5.5 | ||
| Separated or divorced | 38.9 | 23.9 | 27.8 | 21.8 | ||
| Living arrangement | 0.76 | 0.42 | ||||
| Lives alone, private residence | 38.9 | 34.8 | 33.3 | 23.6 | ||
| Lives with other, private residence | 61.1 | 65.2 | 66.7 | 76.4 | ||
| Education | 0.55 | 0.76 | ||||
| Grade school (years 1–8) or less | 0.0 | 4.4 | 0.0 | 3.6 | ||
| Some high school | 5.6 | 8.7 | 11.1 | 7.3 | ||
| Completed high school or GED | 33.3 | 37.0 | 44.4 | 40.0 | ||
| Some post-high school education | 27.8 | 30.4 | 22.2 | 14.6 | ||
| Completed technical or vocational | 11.1 | 13.0 | 16.7 | 18.2 | ||
| Completed college or more | 22.2 | 6.5 | 5.6 | 16.4 | ||
| Comorbidities | ||||||
| Coronary artery disease | 38.9 | 39.1 | 0.99 | 38.9 | 30.9 | 0.53 |
| Congestive heart failure | 22.2 | 19.6 | 0.81 | 16.7 | 10.9 | 0.52 |
| Chronic obstructive pulmonary disease | 11.1 | 4.4 | 0.31 | 16.7 | 5.5 | 0.13 |
The two DiaTel CC-to-UC participants who re-enrolled but did not participate in the DiaTel Extension are counted as refused in this table.
ACM, active care management; CC, care coordination; GED, General Education Diploma; UC, usual care.
In the DiaTel Extension, six participants changed insulin status between 6 and 12 months (three started on insulin and three stopped taking insulin). For those on insulin, average increases in insulin dose were modest for three subgroups (between 3 and 8 IU) and 18 IU for the CC-to-UC subgroup (data not tabled).
Table 2A–C shows the time-specific means and standard deviations for HbA1c by the DiaTel Extension intervention group, with each table showing pairwise comparisons of the means of the designated groups. The largest, although not significant, pairwise differences were between the ACM-to-CC and CC-to-CC groups (0.59% at 6 months; p=0.11, reflecting DiaTel differences); 0.49% at 9 months, p=0.19; and 0.55% at 12 months, p=0.11. None of the remaining pairwise differences approached statistical significance at 6, 9, or 12 months (p>0.30 for each). Figure 2A–C shows the corresponding dotplots for each hypothesis. No significant differences are attributable to the continued data transmission in DiaTel ACM participants (figure 2A), initial ACM among DiaTel Extension CC participants (figure 2B), or continued monthly calls among DiaTel CC participants (figure 2C).
Table 2.
Time-specific means and standard deviations for HbA1c, by DiaTel Extension intervention group
| (a) HbA1c (%) | ACM-to-CCHT (n=23) | ACM-to-CC (n=21) | DiffACM-to-CC−ACM-to-CCHT | p Value for difference | |||
| Mean | SD | Mean | SD | Mean | SE | ||
| 6 Months | 7.77 | 0.82 | 7.97 | 1.41 | 0.20 | 0.34 | 0.57 |
| 9 Months | 7.93 | 0.96 | 8.04 | 1.34 | 0.12 | 0.35 | 0.74 |
| 12 Months | 8.03 | 1.03 | 8.16 | 1.03 | 0.14 | 0.31 | 0.67 |
| (b) HbA1c (%) | ACM-to-CC (n=21) | CC-to-CC (n=28) | DiffCC-to-CC−ACM-to-CC | p Value for difference | |||
| Mean | SD | Mean | SD | Mean | SE | ||
| 6 Months | 7.97 | 1.41 | 8.56 | 1.14 | 0.59 | 0.36 | 0.11 |
| 9 Months | 8.04 | 1.34 | 8.53 | 1.22 | 0.49 | 0.37 | 0.19 |
| 12 Months | 8.16 | 1.03 | 8.71 | 1.25 | 0.55 | 0.34 | 0.11 |
| (c) HbA1c (%) | CC-to-CC (n=28) | CC-to-UC (n=27) | DiffCC-to-UC−CC-to-CC | p Value for difference | |||
| Mean | SD | Mean | SD | Mean | SE | ||
| 6 Months | 8.56 | 1.14 | 8.53 | 1.18 | −0.03 | 0.31 | 0.92 |
| 9 Months | 8.53 | 1.22 | 8.87 | 1.25 | 0.34 | 0.33 | 0.31 |
| 12 Months | 8.71 | 1.25 | 8.84 | 1.38 | 0.13 | 0.35 | 0.72 |
p Values are given for tests of time-specific differences between the corresponding pairs of means, ie, ACM-to-CCHT and ACM-to-CC are compared in (a), ACM-to-CC and CC-to-CC are compared in (b), and CC-to-CC and CC-to-UC are compared in (c). The 6-month measurement is from DiaTel; the 9 and 12 months measurements are taken during the DiaTel Extension.
ACM, active care management; CC, care coordination; CCHT, care coordination with continued home telemonitoring; HbA1c, hemoglobin A1c; UC, usual care.
Figure 2.
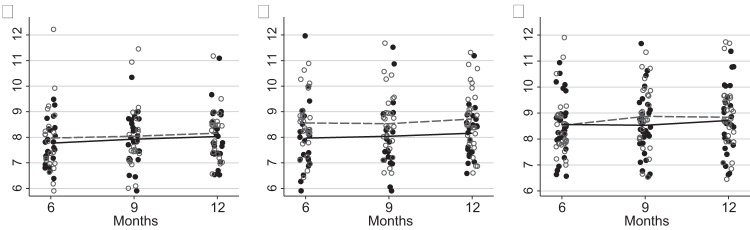
Pairwise comparisons of hemoglobin A1c (HbA1c) at 6, 9, and 12 months to test each hypothesis. The 6-month measurement is from DiaTel; the 9 and 12 months measurements are taken during the DiaTel Extension. In each plot, a solid dot denotes the data points and a solid line connects the time-specific means for the ‘more intensive’ intervention, and a hollow circle denotes the data points and a dotted line connects the time-specific means for the ‘less intensive’ intervention. The ‘more intensive’ intervention is ACM-to-CCHT in (a), ACM-to-CC in (b), and CC-to-CC in (c). The corresponding ‘less intensive’ intervention is ACM-to-CC in (a), CC-to-CC in (b), and CC-to-UC in (c). (a) ACM-to-CCHT versus ACM-to-CC (continued data transmission), (b) ACM-to-CC versus CC-to-CC (initial active care management) (c) CC-to-CC versus CC-to-UC (continued monthly calls). ACM, active care management; CC, care coordination; CCHT, care coordination with continued home telemonitoring; UC, usual care.
Table 3A–C summarizes within-group changes in HbA1c over time. The only within-group change that approached statistical significance was an increase in HbA1c of 0.35% in the CC-to-UC group between 6 and 9 months (p=0.06, not shown; p>0.20 for all other within-group comparisons over time). Except possibly for a differential change of 0.37% from 6 to 9 months between the CC-to-CC and CC-to-UC groups (p=0.09), figure 2 and table 3 show little evidence of differential change over time between any of the other pairwise comparisons considered (p>0.50 for each).
Table 3.
Within-group changes over time in HbA1c by DiaTel Extension intervention group
| (a) HbA1c Change (%) over time | ACM-to-CCHT (n=23) | ACM-to-CC (n=21) | DiffACM-to-CCHT−DiffACM-to-CC | p Value for difference | |||
| DiffACM-to-CCHT | SD | DiffACM-to-CC | SD | Mean | SE | ||
| 6–9 Months | −0.15 | 0.86 | −0.07 | 1.01 | −0.08 | 0.28 | 0.78 |
| 6–12 Months | −0.25 | 0.91 | −0.19 | 0.82 | −0.06 | 0.26 | 0.82 |
| 9–12 Months | −0.10 | 0.64 | −0.12 | 0.81 | 0.019 | 0.22 | 0.93 |
| (b) HbA1c Change (%) over time | ACM-to-CC (n=21) | CC-to-CC (n=28) | DiffACM-to-CCHT−DiffCC-to-CC | p Value for difference | |||
| DiffACM-to-CCHT | SD | DiffCC-to-CC | SD | Mean | SE | ||
| 6–9 Months | −0.07 | 1.01 | 0.03 | 0.71 | −0.10 | 0.25 | 0.68 |
| 6–12 Months | −0.19 | 0.82 | −0.15 | 1.16 | −0.04 | 0.30 | 0.90 |
| 9–12 Months | −0.12 | 0.81 | −0.18 | 1.01 | 0.06 | 0.27 | 0.81 |
| (c) HbA1c Change (%) over time | CC-to-CC (n=28) | CC-to-UC (n=27) | DiffCC-to-CC-−DiffCC-to-UC | p Value for difference | |||
| DiffCC-to-CC | SD | DiffCC-to-UC | SD | Mean | SE | ||
| 6–9 Months | 0.03 | 0.71 | −0.35 | 0.91 | 0.37 | 0.22 | 0.09 |
| 6–12 Months | −0.15 | 1.16 | −0.31 | 1.43 | 0.16 | 0.35 | 0.65 |
| 9–12 Months | −0.18 | 1.01 | 0.03 | 1.38 | −0.22 | 0.33 | 0.51 |
p Values are given for differential changes over time between pairs of intervention groups, ie, ACM-to-CCHT and ACM-to-CC are compared in (a), ACM-to-CC and CC-to-CC are compared in (b), and CC-to-CC and CC-to-UC are compared in (c). The 6-month measurement is from DiaTel; the 9 and 12 months measurements are from the DiaTel Extension.
ACM, active care management; CC, care coordination; CCHT, care coordination with continued home telemonitoring; HbA1c, hemoglobin A1c; UC, usual care.
Figure 3 shows patterns in mean HbA1c by randomization group for DiaTel Extension participants during both studies. DiaTel Extension participants in each intervention group demonstrated a decline in HbA1c between the DiaTel baseline and 12 months, and the improvements observed at 6 months in DiaTel were attenuated only slightly when the intensity of each intervention was decreased (or maintained, for the CC-to-CC group) in the DiaTel Extension. Similar results were obtained when the seven participants re-assigned randomly more than 12 days after their 6-month DiaTel visits were excluded (one in ACM-to-CCHT, one in ACM-to-CC, three in CC-to-CC, and two in CC-to-UC).
Figure 3.
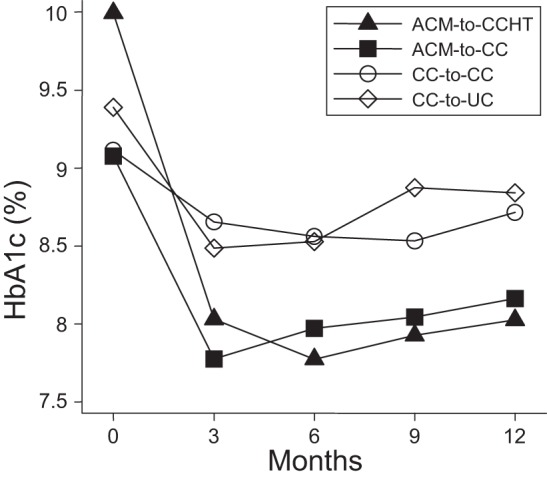
Profile plot of mean hemoglobin A1c (HbA1c) over time by DiaTel Extension intervention group. Participants were re-assigned randomly at 6 months, so the measurements at 0, 3 and 6 months reflect the initial DiaTel intervention for these DiaTel Extension participants.
Discussion
In DiaTel, intensive telemonitoring with active medication management intervention in veterans with diabetes and poor glycemic control was effective in rapidly reducing HbA1c, with the greatest reductions obtained by 3 months and sustained at 6 months.32 Our DiaTel Extension data show that the gains achieved by the end of DiaTel were attenuated only slightly by 6 months after re-randomization.
Telemedicine interventions offer the potential to engage patients and healthcare providers in safe, effective and efficient management of glycemia. Telemedicine may result in significant economic savings, by reducing the time and travel costs for patients with diabetes and facilitating analysis of glucose data and treatment decisions by providers.34 Several studies on the economic costs and benefits of such interventions suggest that patients benefit from telemedicine in terms of the reduced time and travel costs relative to routine care, but that telemonitoring interventions require more provider time than does traditional diabetes management.12 14 17 18 24 Consequently, disseminating such interventions will require not only a reimbursement mechanism but some consideration of cost-effective ways to employ telemonitoring in patients with diabetes.
The ACM-to-CCHT and ACM-to-CC comparisons failed to show benefits from continuing the use of home telemonitoring in the DiaTel Extension. These and other subgroup comparisons in the DiaTel Extension suggest that the same or lower intensity contact could be as effective as higher intensity contact in maintaining achieved improvements in glycemic control, at least over a 6-month period. However, because participants in the ACM-to-CC group continued to receive monthly CC phone calls, the DiaTel Extension does not address the value of a short-term, high-intensity telemonitoring intervention followed by a return to conventional primary care. While our results are not definitive, they certainly suggest that consideration should be given to the intensity and duration of telemonitoring interventions such as the ones we employed. Additional research is needed to confirm our results, examine the duration and intensity of contact required for long-term maintenance of glycemic control, and assess the cost-effectiveness of these approaches.
The DiaTel Extension builds on a concurrent randomized clinical trial to address a question that has received little attention in the literature. A major strength is the re-randomization, which allows unbiased comparisons within the two DiaTel intervention arms to assess the impact of the continued data transmission and the continued monthly calls.
This study also has several limitations. First, power is limited because the sample size was restricted to those DiaTel participants who consented to be re-assigned randomly. Second, this study did not include a group re-assigned randomly to ACM, so the effect of continued active medication management cannot be assessed. Third, we could not assess potential carry-over effects from the patient education and medication management in the initial DiaTel study; DiaTel ACM participants on insulin tended to be on somewhat higher doses of insulin by 6 months. Fourth, because of the time delays between the end of DiaTel and re-randomization, DiaTel treatment was continued for somewhat longer than 6 months for seven participants. However, our results were not sensitive to this administrative delay. Fifth, although participants and non-participants in the DiaTel Extension appear to be similar demographically, DiaTel Extension participants may be more highly motivated to improve their glycemic control than the typical diabetes patient with poor glycemic control. Also, some participants did not have poor glycemic control by the start of the DiaTel Extension. Finally, sociodemographic differences between veterans in the VA Healthcare System and the US civilian population with diabetes may limit the generalizability of the study findings.
Conclusion
Marked improvements in glycemic control among ACM participants with diabetes at 6 months were largely sustained for 6 months following re-randomization to an intervention that did not include either active medication management by a CRNP or home telemonitoring. There were no apparent benefits of continued transmission of glucose data by a home telemedicine device. Smaller initial improvements in glycemic control among CC participants during the DiaTel study were also sustained, even after return to UC. Ongoing monitoring of HbA1c provides an opportunity to identify patients who may, despite an initial course of intensive management and improvement, need additional intensive management at some time in the future. Our results suggest that improvements in HbA1c achieved using home telemonitoring and active medication management for 6 months could be sustained for an additional 6 months following re-randomization with interventions of decreased intensity in a population of VA-qualified veterans that has a high level of access to care.
Acknowledgments
The authors would like to thank VAPHS primary care providers and clinic nurses for assistance in recruiting patients into the study; Carol Franko and Rebecca A Anglin, for delivering the telemonitoring and care coordination interventions; Julie Heinzl, for nutrition counseling; and D Scott Obrosky, for data management. The contents of this article do not represent the views of the Department of Veterans Affairs of the United States government.
Footnotes
Contributors: RAS and MAS developed the protocol and wrote the manuscript; RHR developed the protocol, provided medical oversight, and reviewed and edited the manuscript; DSM developed the protocol and reviewed and edited the manuscript; CC and SK analyzed and interpreted data; LJH managed the study and reviewed and edited the manuscript; FRD designed the study, developed the protocol, provided scientific oversight of the study, and reviewed and edited the manuscript.
Funding: This work was supported by award W81XWH-04-2-0030 from the US Air Force, administered by the US Army Medical Research Acquisition Activity, Fort Detrick, Maryland, and by resources and the use of facilities at the VA Pittsburgh Healthcare System. A portion of the telemonitoring and other equipment costs were supported by Viterion TeleHealthcare, LLC; Tarrytown, New York. The funding agencies played no role in the conduct or reporting of this study.
Competing interests: None.
Ethics approval: Ethics approval was provided by the institutional review board of the VA Pittsburgh Healthcare System.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: VA Pittburgh Healthcare System institutional review board restrictions and the language included in the signed informed consent used with this study preclude the sharing of patient-level data.
References
- 1. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National Diabetes Statistics Fact Sheet: General Information And National Estimates on Diabetes in the United States, 2005. Bethesda, MD: US Department of Health and Human Services, National Institute of Health, 2005 [Google Scholar]
- 2. Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. http://diabetes.niddk.nih.gov/dm/pubs/statistics (accessed 29 Apr 2011). [Google Scholar]
- 3. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group (DPPRG) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoerger TJ, Segel JE, Gregg EW, et al. Is glycemic control improving in US adults? Diabetes Care 2008;31:81–6 [DOI] [PubMed] [Google Scholar]
- 5. Sperl-Hillen JM, Solberg LI, Hroscikoski MC, et al. The effect of advanced access implementation on quality of diabetes care. Prev Chronic Dis. http://www.cdc.gov/pcd/issues/2008/jan/06_0177.htm (accessed 5 May 2011). [PMC free article] [PubMed] [Google Scholar]
- 6. Karter AJ, Parker MM, Moffet HH, et al. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care 2006;29:1757–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiu CJ, Wray LA. Factors Predicting glycemic control in middle-aged and older adults with type 2 diabetes. Prev Chronic Dis 2010;7 http://www.cdc.gov/pcd/issues/2010/jan/08_0250.htm (accessed 3 May 2011). [PMC free article] [PubMed] [Google Scholar]
- 8. Rhee MK, Slocum W, Ziemer DC, et al. Patients' adherence improves glycemic control. Diabetes Educ 2005;31:240–50 [DOI] [PubMed] [Google Scholar]
- 9. Khattab M, Khader YS, Al-Khawaldeh A, et al. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications 2010;24:84–9 [DOI] [PubMed] [Google Scholar]
- 10. Ahring KK, Ahring JP, Joyce C, et al. Telephone modem access improves diabetes control in those with insulin-requiring diabetes. Diabetes Care 1992;15:971–5 [DOI] [PubMed] [Google Scholar]
- 11. Benhamou PY, Melki V, Boizel R, et al. One-year efficacy and safety of web-based follow-up using cellular phone in type 1 diabetic patients under insulin pump therapy: the PumpNet study. Diabetes Metab 2007;33:220–6 [DOI] [PubMed] [Google Scholar]
- 12. Bergenstal RM, Anderson RL, Bina DM, et al. Impact of modem-transferred blood glucose data on clinician work efficiency and patient glycemic control. Diabetes Technol Ther 2005;7:241–7 [DOI] [PubMed] [Google Scholar]
- 13. Bellazzi R, Larizza C, Montani S, et al. A telemedicine support for diabetes management: the T-IDDM project. Comput Methods Programs Biomed 2002;69:147–61 [DOI] [PubMed] [Google Scholar]
- 14. Biermann E, Dietrich W, Rihl J, et al. Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial. Comput Methods Programs Biomed 2002;69:137–46 [DOI] [PubMed] [Google Scholar]
- 15. Billiard A, Rohmer V, Roques MA, et al. Telematic transmission of computerized blood glucose profiles for IDDM patients. Diabetes Care 1991;14:130–4 [DOI] [PubMed] [Google Scholar]
- 16. Cadario F, Binotti M, Brustia M, et al. Telecare for teenagers with type 1 diabetes: a trial. Minerva Pediatr 2007;59:299–305 [PubMed] [Google Scholar]
- 17. Chase HP, Roberts MD, Pearson JA, et al. Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care 2003;26:1475–9 [DOI] [PubMed] [Google Scholar]
- 18. Cho JH, Song KH, Chang SA, et al. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability. Diabetes Care 2006;29:2625–31 [DOI] [PubMed] [Google Scholar]
- 19. Farmer AJ, Hayton PM, Gibson OJ, et al. A randomized controlled trial of the effect of real-time telemedicine support on glycemic control in young adults with type 1 diabetes. Diabetes Care 2005;28:2697–702 [DOI] [PubMed] [Google Scholar]
- 20. Gay CL, Chapuis F, Bendelac N, et al. Reinforced follow-up for children and adolescents with type 1 diabetes and inadequate glycemic control: a randomized controlled trial intervention via the local pharmacist and telecare. Diabetes Metab 2006;32:159–65 [DOI] [PubMed] [Google Scholar]
- 21. McCarrier KP, Ralston JD, Hirsch IB, et al. Web-based collaborative care for type 1 diabetes: a pilot randomized trial. Diabetes Technol Ther 2009;11:211–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMahon GT, Hu TMJ, Gomes HE, et al. Web-based care management in poorly controlled diabetes. Diabetes Care 2005;28:1624–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marrero DG, Vandagriff JL, Kronz K, et al. Using telecommunication technology to manage children with diabetes: the Computer-Linked Outpatient Clinic (CLOC) study. Diabetes Educ 1995;21:313–19 [DOI] [PubMed] [Google Scholar]
- 24. Montori VM, Helgemoe PK, Guyatt GH, et al. Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care 2004;27:1088–94 [DOI] [PubMed] [Google Scholar]
- 25. Quinn CC, Clough SS, Minor JM, et al. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther 2008;10:160–8 [DOI] [PubMed] [Google Scholar]
- 26. Ralston JD, Hirsch IB, Hoath J, et al. Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care 2009;32:234–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 2006;13:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shea S, Weinstock RS, Teresi JA, et al. A randomized trial comparing telemedicine care management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc 2009;16:446–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vahatalo MA, Virtamo HE, Viikari JS, et al. Cellular phone transferred self blood glucose monitoring: prerequisites for positive outcome. Pract Diab Int 2004;21:192–4 [Google Scholar]
- 30. Welch G, Sokolove M, Mullin C, et al. Use of modem equipped blood glucose meter augmented with biweekly educator telephone support lowers HbA1c in type 1 diabetes. Diabetes 2003;52(Suppl 1):A100 [Google Scholar]
- 31. Liesenfeld B, Renner R, Neese M, et al. Telemedical care reduces hypoglycemias and improves glycemic control in children and adolescents with type 1 diabetes. Diabetes Technol Ther 2000;2:561–7 [DOI] [PubMed] [Google Scholar]
- 32. Stone R, Rao R, Sevick M, et al. Acute care management supported by home telemonitoring in Veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care 2009;33:478–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Department of Veterans Affairs, United States of America Guideline Toolkit: Survival skills for the Person with Diabetes. Washington, DC: US Department of Veterans Affairs, 2004 [Google Scholar]
- 34. Azar M, Gabbay R. Web-based management of diabetes through glucose uploads: has the time come for telemedicine? Diabetes Res Clin Pract 2009;83:9–17 [DOI] [PubMed] [Google Scholar]