Abstract
In the present study, we investigated the influence of HIV-1 subtype in the response to the dendritic cell (DC) therapeutic vaccine for HIV. HIV-1 viral load and TCD8+/TCD4+ cell counts for up to 48 weeks after vaccination. Out of 19 immunized subjects, 13 were infected by subtype B, 5 by subtype F, and 1 by subtype D. Overall, 42.1% (8/19) achieved a viral load decline of ≥ 1 log10 sustained up to 48 weeks after immunization. Such magnitude of viral load drop was seen in 80% (4/5) of subtype F infected patients, and in 23.0% (3/13) of the subtype B infected ones (p=0.08). Moreover, mean viral load decline was 1.32 log10, for subtype F infected individuals compared to 0.5 log10 among subtype B infected patients (p=0.01). The variation in TCD4+ cell count was not related to HIV-1 subtype. Larger studies are necessary to confirm the efficacy of this immunotherapy and the differential response according to the background genetic diversity of HIV-1.
Keywords: Dendritic cells, HIV-1 subtype, vaccine, immunotherapy.
INTRODUCTION
Progressive loss of dendritic cell function is considered to be one of the reasons for loss of immunological control of HIV-1 replication in chronically infected patients [1]. Dendritic cells (DCs) are the most important antigen-presenting cells (APCs) and regulators of the adaptative immune system. However, as the HIV-1 infection progresses, the virus disturbs several important DCs functions and induces significant levels of apoptosis in this cell population, thereby compromising the normal adaptative immune response [2]. It has been observed that in individuals with chronic HIV-1 infection, the antigen specific cytotoxic T-cell response against the virus is also progressively lost [3]. Therefore, in addition to viral adaptation and immune escape, it is likely that DC dysfunction in HIV-1 antigens specific presentation plays an important role in the evolution of anergic immune responses against the virus, thus allowing the selection of viral strains of higher fitness [1].
In an attempt to restore DC function in chronically infected patients, Lu et al. (2004) produced mature monocyte-derived DC pulsed with autologous inactivated virus in vitro and re-introduced them into a group of 19 HIV-1 chronically infected Brazilian patients as a form of immunotherapy [4]. The results of this approach after one year follow up were encouraging. All patients presented benefits as a decrease in viral loads and an increase of CD4 counts, where plasma viral load levels decreased by 80% (median) over the first 112 days following immunization. However, a half of the patients produced only moderate and short-lived virologic and immune responses, whereas the other half produced a controlled viral load and TCD4+ counts > 350 cels/mm3 lasting for one year.
The reasons for these different patterns of response to the DC immunotherapy are not completely understood. However, host and virus factors could be involved. It is not yet clear the impact of the genetic diversity of HIV in disease progression, antiretroviral response or pathways for selection of antiretroviral resistance, and these issues are relevant to developing countries. In Brazil, more than one HIV-1 subtypes co-circulate, being subtype B the more prevalent, followed respectively by of subtypes F and C and a variety of Unique and Circulating Recombinant forms [5, 6]. Using the Bayesian Markov chain Monte Carlo (BMCMC) method and the Reversible-jump MCMC method, it has been estimated that subtype B was introduced in Brazil in 1970, whereas subtype F was introduced in 1981, and subtype C in 1987 [7]. The maximum genetic variability in full length genomes of Brazilian subtypes B and F strains is 8.4% and 6.0%, respectively, and the mean variation between both subtypes ranges from 14.3% to 15.6% [8].
In this study, we present the impact of viral subtype on the efficacy of the dendritic cell immunotherapy, reported elsewhere by Lu et al. [4].
PATIENTS AND METHODS
Nineteen HIV infected patients aged 18 through 41 years (mean 27) were included in this study. Therefore, all participants in the clinical Phase 1 study, conducted by the Pernambuco Immunotherapy Research Institute (IPIPE) at the Keizo Asami Immunopathology Laboratory, Federal University of Pernambuco, Recife – Brazil were included in this analysis. Patients were immunized with autologous dendritic cells loaded with inactivated autologous HIV after September 2003 [4]. No patient received antiretroviral drugs throughout the 12-month follow-up period.
Patients were monitored by routine hematological exams, TCD4+ and TCD8+ cell counts, HIV-1, viral load, and clinical information. Virological response was classified as “successful” when a drop in viral load ≥ 1 log10 was sustained up to 48 weeks after immunization. Long term follow-up data and samples from above 48 weeks are not available from this group of patients.
Genomic DNA was extracted from the peripheral blood mononuclear cells (PBMC) collected before vaccination using the QIAGEN extraction procedure. Polymerase chain reaction (PCR) was used to amplify the pol region (RT/PR) of the provirus using specific primers [5], followed by DNA sequencing. The HIV-1 subtype of each sample was determined through phylogenetic analysis using the Kimura 2-parameter and neighbour-joining method [9]. Statistical analysis was performed using a two-tail Fisher exact test and the Mann-Whitney test.
This study was IRB approved and patients signed the informed consent.
RESULTS
The distribution of HIV-1 subtypes in the study participants were 68.4% B (13/19), 26.3% F (5/19), and 5.3% D (1/19).
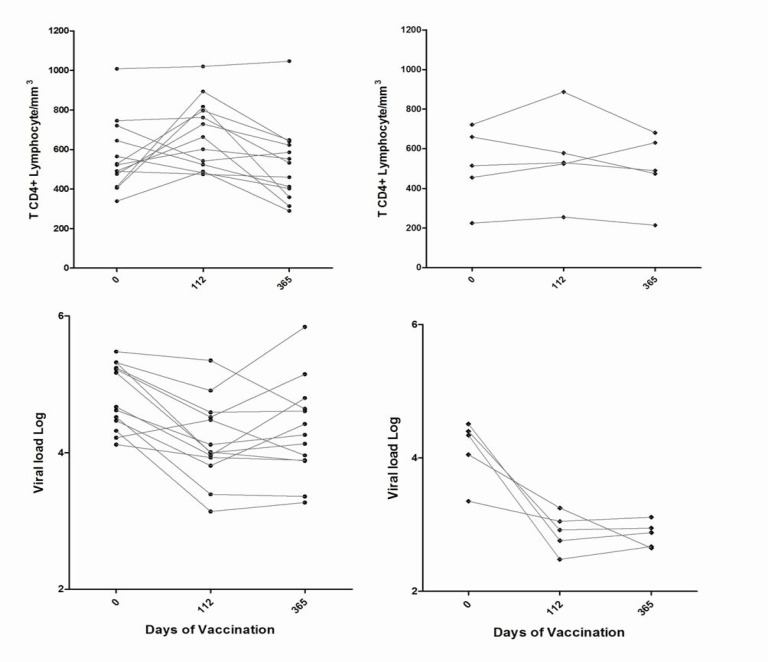
Overall, 42.1% (8/19) achieved a viral load decline of ≥ 1 log10 sustained up to 48 weeks after immunization. Such magnitude of viral load drop was seen in 80% (4/5) of subtype F infected patients, and in 23.0% (3/13) of the subtype B infected ones (p=0.08). Moreover, as seen in Fig. (1), mean viral load decline was 1.32 log10, for subtype F infected individuals compared to 0.5 log10 among subtype B infected patients on day 365 (p=0.01).
Fig. (1).
T CD4+ Lymphocytes and viral load variation over time after dendritic cell vaccination among subtype B and subtype F infected individuals.
Forty-eight weeks after DC treatment, HIV-1 viral load increased in none of the patients, was maintained stable in seven patients (4, 9, 11, 16, 27, 29, 32), decreased more than 0.5 log10 but less than 1 log10 in three patients (17, 21, 23), and the remaining eight patients (2, 3, 10, 13, 14, 17, 18, 24) decreased more than 1 log10 (Table 1).
Table 1.
HIV-1 Viral Load, TCD4+ and TCD8+ Cell Counts Up to 52 Weeks After Dendritic Cell Immunotherapy, According to Viral Subtype
| TCD4+ (mm3) | TCD8+ (mm3) | HIV-1 Viral Load (Log) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Viral Subtype | Day | Day | Day | ||||||
| 0 | 112 | 365 | 0 | 112 | 365 | 0 | 112 | 365 | ||
| 2 | F | 660 | 578 | 474 | 1006 | 714 | 1188 | 4.4 | 2.92 | 2.95 |
| 3 | B | 490 | 475 | 460 | 793 | 680 | 760 | 4.52 | 3.39 | 3.36 |
| 4 | B | 339 | 489 | 290 | 688 | 720 | 835 | 4.22 | 4.48 | 3.96 |
| 9 | F | 225 | 255 | 214 | 1587 | 1700 | 1185 | 3.35 | 3.05 | 3.11 |
| 10 | F | 515 | 530 | 490 | 429 | 368 | 425 | 4.51 | 2.76 | 2.88 |
| 11 | B | 522 | 602 | 553 | 860 | 831 | 721 | 4.67 | 3.96 | 4.8 |
| 13 | B | 412 | 894 | 641 | 849 | 1409 | 755 | 5.32 | 4 | 4.13 |
| 14 | F | 455 | 524 | 631 | 725 | 491 | 1021 | 4.34 | 2.48 | 2.67 |
| 16 | B | 645 | 524 | 413 | 510 | 491 | 659 | 5.22 | 4.52 | 5.15 |
| 17 | B | 1009 | 1021 | 1047 | 967 | 1061 | 1275 | 5.17 | 4.01 | 3.88 |
| 18 | F | 722 | 887 | 681 | 650 | 621 | 661 | 4.05 | 3.25 | 2.65 |
| 19 | B | 406 | 817 | 359 | 1447 | 1670 | 1502 | 5.24 | 4.59 | 4.61 |
| 21 | D | 270 | 683 | 569 | 682 | 1161 | 1009 | 4.98 | 4.71 | 4.07 |
| 24 | B | 476 | 729 | 623 | 1112 | 1544 | 1404 | 4.32 | 3.14 | 3.27 |
| 27 | B | 565 | 482 | 403 | 746 | 803 | 611 | 4.47 | 3.81 | 4.42 |
| 29 | B | 528 | 797 | 648 | 746 | 731 | 624 | 4.62 | 4.12 | 4.26 |
| 31 | B | 492 | 664 | 314 | 1035 | 976 | 1288 | 5.32 | 4.91 | 5.84 |
| 32 | B | 721 | 542 | 586 | 1036 | 1000 | 1132 | 4.12 | 3.93 | 3.89 |
| 33 | B | 746 | 762 | 533 | 735 | 1007 | 1029 | 5.48 | 5.35 | 4.64 |
Mean TCD4+ cell count variation from baseline to 112 days and 48 weeks after immunization were, respectively, 112.3/mm3 (range -82 to 482) and 38.5/mm3 (range -186 to 229) and among those who had a viral load decline greater than 1 log10, compared to 105.2 (range -179 to 413) and -52.4 (range -232 to 299) among patients with inferior viral load decline (Table 1). All but one patient (#2) with virological success sustained their TCD4+ count above 75% of their original level. The variation in TCD4+ cell count kept no relation with HIV-1 subtype.
Fourteen patients, six from the virological success group (10, 13, 14, 17, 18, 24), and eight (4, 9, 11, 19, 21, 29, 31, 33) without virological success had an increase in TCD4+ cell counts up to 112 days with a subsequent decline at 48 weeks.
DISCUSSION
It is conceivable that the genetic diversity of HIV may impair immune responses to vaccine candidates, cause false-negative results in diagnostic/monitoring laboratory tests (especially those involving nucleic acids) [10,11] and lead to differences in the disease progression [12,13]. In the extremes of HIV phylogeny we have HIV-1 and HIV-2, and it is well known that disease progression is faster, and transmissions rates and viral loads are higher in HIV-1 as compared to HIV-2 [14, 15]. It has also even been speculated that faster progression on HIV-2 infected individuals may be related to the genetic diversity of the strains [16]. Interestingly, one retrospective analysis of a prospective study demonstrated that clade F infected individuals presented a poorer virologic response to antiretrovirals as compared to clade B infected individuals among subjects treated with zidovudine, lamivudine and non-boosted indinavir [17]. It has been suggested that this decreases response to ART might be related to the decreased susceptibility to PI caused by the L89M polymorphism present at the protease of clade F strains [18]., although this effect has not been observed when boosted PIs were used [19]. However, there is no data available on differential disease progression between among individuals infected by subtypes B or F.
In the present study the patients with subtype F presented a higher viral load reduction than did those with subtype B (p=0.01), although no significant differences between the growth characteristics of each virus or relative loading efficiencies of the different subtypes into the dendritic cells, neither differences in the maturation level of the dendritic cells have been identified (data not shown). Additionally, these results cannot be explained by distinct viability of the dendritic cells after loading the autologous viruses in the cultures.
A clear difference in the TCD4+ and TCD8+ absolute counts for both B and F subtype groups was not observed in the short term follow-up period of this study. As it has been demonstrated that the Th1 response may act in the viremia control independently to the TCD4+ absolute counts, it could be speculate that the immunotherapy using the HIV-1 subtype F may induce a more strong Th1 response than subtype B. Equally, the role of specific CTLs in this selective response also deserves further evaluation.
Another interesting feature about the Brazilian subtype F viruses is its tendency to recombine with subtype B, and therefore, it has been hard to find a pure subtype F strain among HIV infected individuals [8]. Therefore, it has been hypothesized that the immune pressure against subtype F may be stronger than the one exerted against subtype B.
We recognize that the small number of samples and the partial genomic characterization of this set of samples constitute a limitation of the present study, specially the number of non-subtype B infected individuals. However, our data suggest the immunization seems to contribute to the control of viramia and the consequence delay in disease progression, representing a promising intervention strategy for controlling chronic HIV-1 infection, and the subtype of the virus may influence the response to this therapy. Therefore, these results emphasize the necessity of larger studies in order to confirm the efficacy of this immunotreatment vis a vis the genetic diversity of HIV-1.
ACKNOWLEDGEMENTS
We thank Drs. A. Tanuri, R. Brindeiro and their research group, at the Universidade Federal do Rio de Janeiro for the assistance with the execution of nucleotide sequencing. This work was supported in part by research grants from Ministry of Health of Brazil.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1. Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 2. Beuria P, Chen H, Timoney M, Sperber K. Impaired accessory cell function in a human dendritic cell line after human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2005;12:453–64. doi: 10.1128/CDLI.12.3.453-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salerno-Goncalves R, Lu W, Andrieu JM. Quantitative analysis of the antiviral activity of CD8(+) T cells from human immunodeficiency virus-positive asymptomatic patients with different rates of CD4(+) T-cell decrease. J Virol. 2000;74:6648–51. doi: 10.1128/jvi.74.14.6648-6651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–65. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 5. Soares MA, De Oliveira T, Brindeiro RM, et al. A specific subtype C of human immunodeficiency virus type 1 circulates in Brazil. AIDS. 2003;17:11–21. doi: 10.1097/00002030-200301030-00004. [DOI] [PubMed] [Google Scholar]
- 6. De Sa Filho DJ, Sucupira MC, Caseiro MM, Sabino EC, Diaz RS, Janini LM. Identification of two HIV type 1 circulating recombinant forms in Brazil. AIDS Res Hum Retroviruses. 2006;22:1–13. doi: 10.1089/aid.2006.22.1. [DOI] [PubMed] [Google Scholar]
- 7. Leal E ML, Janini LM, Diaz RS. Evolutionary dynamics of HIV-1 BF and CB recombinants and its parental counterparts in South America. Retrovirology. 2008;1:1–14. [Google Scholar]
- 8. Sanabani S, Neto WK, de Sa Filho DJ, et al. Full-length genome analysis of human immunodeficiency virus type 1 subtype C in Brazil. AIDS Res Hum Retroviruses. 2006;22:171–6. doi: 10.1089/aid.2006.22.171. [DOI] [PubMed] [Google Scholar]
- 9. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 10. Apetrei C, Loussert-Ajaka I, Descamps D, et al. Lack of screening test sensitivity during HIV-1 non-subtype B seroconversions. AIDS. 1996;10:F57–60. doi: 10.1097/00002030-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 11. Burgisser P, Vernazza P, Flepp M, et al. Performance of five different assays for the quantification of viral load in persons infected with various subtypes of HIV-1. Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2000;23:138–44. doi: 10.1097/00126334-200002010-00005. [DOI] [PubMed] [Google Scholar]
- 12. Kaleebu P, Ross A, Morgan D, et al. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS. 2001;15:293–9. doi: 10.1097/00002030-200102160-00001. [DOI] [PubMed] [Google Scholar]
- 13. Kanki PJ, Hamel DJ, Sankale JL, et al. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis . 1999;179:68–73. doi: 10.1086/314557. [DOI] [PubMed] [Google Scholar]
- 14. De Cock KM, Brun-Vezinet F. Epidemiology of HIV-2 infection. AIDS. 1989;3(Suppl 1 ):S89–95. doi: 10.1097/00002030-198901001-00013. [DOI] [PubMed] [Google Scholar]
- 15. Matheron S, Mendoza-Sassi G, Simon F, Olivares R, Coulaud JP, Brun-Vezinet F. HIV-1 and HIV-2 AIDS in African patients living in Paris. AIDS. 1997;11:934–6. [PubMed] [Google Scholar]
- 16. Fusuma EE, Caruso SC, Lopez DF, et al. Duplication of perikappaB and NF-kappab sites of the first human immunodeficiency virus type 2 (HIV-2) transmission in Brazil. AIDS Res Hum Retroviruses. 2005;21:965–70. doi: 10.1089/aid.2005.21.965. [DOI] [PubMed] [Google Scholar]
- 17. Accetturi CA, Pardini R, Novaes Pinto GH, Turcato G, Jr, Lewi DS, Diaz RS. Effects of CCR5 genetic polymorphism and HIV-1 subtype in antiretroviral response in Brazilian HIV-1-infected patients. J Acquir Immune Defic Syndr. 2000;24:399–400. doi: 10.1097/00126334-200008010-00016. [DOI] [PubMed] [Google Scholar]
- 18. Calazans A, Brindeiro R, Brindeiro P, et al. Low accumulation of L90M in protease from subtype F HIV-1 with resistance to protease inhibitors is caused by the L89M polymorphism. J Infect Dis. 2005;191:1961–70. doi: 10.1086/430002. [DOI] [PubMed] [Google Scholar]
- 19. Diaz RS, Vasconcelos L, Hayden RL, et al. Similar efficacy of lopinavir/ritonavir-containing regimens among clades B and F HIV-1-Infected individuals in Brazil. J Acquir Immune Defic Syndr. 2008;47:399–401. doi: 10.1097/qai.0b013e31815b0d48. [DOI] [PubMed] [Google Scholar]