Abstract
The somatostatin analogs (SSAs) lanreotide Autogel/Depot and octreotide long-acting release are used to treat acromegaly and neuroendocrine tumors. The present study evaluated opinions on SSA injection devices, including a recently approved lanreotide new device (lanreotide-ND), among nurses in Europe and the USA. Nurses injecting SSAs for at least three patients per year (n = 77) were interviewed regarding SSA devices. Device attributes were rated via questionnaire; nurses were then timed administering test injections with lanreotide-ND and octreotide long-acting release. The most important delivery system attributes were easy/convenient preparation and injection (ranked in the top five by 70% of nurses), low clogging risk (58%), and high product efficacy (55%). Compared with the octreotide long-acting release device, lanreotide-ND scored higher on 15/16 attributes, had shorter mean preparation and administration time (329 versus 66 seconds, respectively; P ≤ 0.01) and a higher overall preference score (70 versus 114, respectively; P ≤ 0.01). The five most important lanreotide-ND attributes were: prefilled device, confidence a full dose was delivered, low clogging risk, easy/convenient preparation and injection, and fast administration. These device features could lead to improvements in clinical practice and benefit patients/caregivers who administer SSAs at home.
Keywords: nurse, somatostatin analog, device, lanreotide, octreotide
Introduction
Somatostatin analogs (SSAs) lower levels of growth hormone and insulin-like growth factor-1 and reduce clinical symptoms of acromegaly.1 In patients with gastroenteropancreatic neuroendocrine tumors, SSAs control symptoms by regulating gastrointestinal hormone secretion.2 Two long-acting SSA formulations are currently available: lanreotide Autogel (lanreotide Depot in the USA) (Somatuline® Autogel/Depot; Ipsen Pharma, Paris, France) and octreotide long-acting release (LAR) (Sandostatin® LAR; Novartis, Basel, Switzerland). Octreotide LAR (injection volume = 2.0 mL) is a slow-release formulation administered intramuscularly every 28 days using a 1.1 mm diameter needle. Lanreotide Autogel/Depot (injection volume = 0.3–0.5 mL) is administered by deep subcutaneous injection every 28 days using 1.2 mm (60 mg and 90 mg dose) or 1.4 mm (120 mg dose) needles or, in patients with acromegaly who achieve hormonal control with monthly injections, 120 mg may be given less frequently at the extended dose interval of 42 or 56 days (6 or 8 weeks).3 An extended dosing interval for octreotide LAR is not currently approved by regulatory authorities.4,5
Injection device usability, safety, and speed of administration are key factors for effective treatment delivery and efficient patient management. Preference studies in other therapeutic areas show that nurses prefer prefilled systems that avoid the need for reconstitution over vial systems; device attributes such as reliability and safety precautions are also important.6,7 The procedure for administering octreotide LAR requires reconstitution that can involve up to seven steps before injection,8 and must be carried out by a trained health professional, using vial and syringe. Lanreotide Autogel/Depot is supplied in a prefilled syringe that does not require reconstitution before injection, thereby supporting self- or partner administration.9,10 A new prefilled injection device for lanreotide Autogel/Depot, lanreotide new device (lanreotide-ND), has recently been developed. The device, which uses a 1.2 mm needle for all doses, includes a rigid needle cap, automatic needle guard to prevent needle-stick injuries, and a fully transparent delivery system.
The aim of this study was to gather the opinions, expectations, and perceptions of hospital and community nurses in Europe and the USA with respect to SSA injection devices, lanreotide-ND and octreotide LAR in particular.
Material and methods
Study design and participants
This multicenter opinion study was conducted in France, Germany, the UK, and the USA. It comprised two phases: a qualitative exploratory phase and a quantitative phase (described below).
Participants were nurses who had ≥3 years’ experience of injecting long-acting SSAs and were following/injecting at least three patients per year. The nurses were recruited from hospitals and community centers that treated high numbers of patients requiring long-acting SSAs. Eligibility was initially ascertained via phone interview and then confirmed in suitable candidates by a face-to-face interview with a study moderator.
Seventy-seven registered nurses practicing in Germany (n = 22), France (n = 19), the UK (n = 18), and the USA (n = 18) were recruited. This was the minimum number required to analyze statistical differences in mean product scores, with a margin of error of ±9% at a 90% confidence level.
Data collection
During the qualitative phase (July 2010), 13 hospital nurses were interviewed for 60 minutes on their opinions of the available SSA injection devices and their perceptions of lanreotide-ND. They also performed comparative test injections for octreotide LAR and lanreotide-ND. This interview was used to develop a list of key device attributes.
During the quantitative phase (October and November 2010), hospital and community nurses were interviewed for 45 minutes about their current practice patterns and SSA use. They then completed a questionnaire in which they rated 16 key device attributes (defined during the qualitative phase) on a scale of one (not important at all) to ten (extremely important), with the option to add additional attributes, and ranked the five most important from one (most important) to five (least important).
Following a demonstration and explanation of lanreotide-ND features, participants were asked to describe its most important characteristics. They were also timed while preparing and performing test injections of lanreotide-ND and octreotide LAR into an injection pad. For octreotide LAR, each nurse was provided with an unopened package and printed injection instructions, and performed the injection as per their usual practice. Prior to the drug- and manufacturer-blinded trial of lanreotide-ND, nurses received printed injection instructions and watched an instructional DVD on injection preparation and administration. For both devices, delivery of a single injection was timed, using a manual stopwatch, from opening the device packaging to completion of an injection.
Following the device try-out, nurses were asked to evaluate octreotide LAR and lanreotide-ND using the 16 device attributes considered earlier. They were also asked to rank, using a predefined list, the five device-specific attributes they considered most advantageous, and what they considered the main disadvantages of lanreotide-ND were compared with current devices.
Finally, participants were questioned on activities that could potentially be performed during the time freed up by using lanreotide-ND versus current devices.
Participants were assured that participation was voluntary and anonymous, and responses confidential. They received an honorarium for participation but were blind to the identity of the study sponsor. The study protocol and materials were reviewed and approved by the lead nurses.
Data analysis
Questionnaire results and data on administration time were analyzed using Modalisa statistical software (version 6.0, Kynos, Paris, France) and summarized using descriptive statistics. Mean evaluation scores were calculated for each SSA device attribute assessed in the questionnaire. Device scores for each attribute were summed to calculate a total preference score for each product. Statistical significance was evaluated at P < 0.05 and P < 0.01 using two-tailed tests.
Results
Participant characteristics
The study included a total of 77 nurses, whose characteristics, practice patterns, and categories of patient are shown in Table 1. Most nurses (57/77; 74%) were hospital-based and practiced in endocrinology departments (62/77; 81%). A total of 61/77 (79%) and 33/77 (43%) nurses injected patients for acromegaly and gastroenteropancreatic neuroendocrine tumors, respectively. They injected a mean of 6.6 patients per month.
Table 1.
Characteristics and practice patterns of 77 nurse participants
| Multiple choice possible | France (n = 22) | Germany (n = 19) | UK (n = 18) | USA (n = 18) | Total (n = 77) |
|---|---|---|---|---|---|
| Nurse practice setting | |||||
| Hospital | 22 | 15 | 17 | 3 | 57 |
| Office | – | 4 | – | 6 | 10 |
| Community health center | – | – | 1 | – | 1 |
| Outpatient clinic (not hospital) | – | – | – | 8 | 8 |
| Private practice (clinical research) | – | – | – | 2 | 2 |
| Independent contractor | – | – | – | 1 | 1 |
| Type of service/department | |||||
| Endocrinology | 17 | 13 | 16 | 16 | 62 (81) |
| Oncology | – | 5 | 4 | – | 9 (12) |
| Gastroenterology | 5 | – | – | – | 5 (6) |
| Cardiology | – | – | – | 3 | 3 (4) |
| Diabetes | 2 | – | – | 1 | 3 (4) |
| Other | 3 | 2 | 2 | 4 | 11 (14) |
| Patients seen per month | |||||
| Mean | 86 | 525 | 123 | 128 | 210 |
| Standard deviation | 81 | 294 | 83 | 96 | 240 |
| Patients injected with SSA per month in nurse practice/dept | |||||
| Mean | 12 | 17 | 15 | 10 | 13 |
| Standard deviation | 10 | 17 | 8 | 9 | 12 |
| Patients personally injected or trained to inject SSA by nurse | |||||
| Mean | 5.4 | 8.9 | 8.4 | 4.1 | 6.6 |
| Standard deviation | 6.2 | 12.9 | 7.4 | 2.4 | 8.2 |
| Nurses who personally inject SSA | |||||
| Acromegaly | 17 | 14 | 12 | 18 | 61 (79) |
| GEP-NET | 7 | 12 | 13 | 1 | 33 (43) |
| Carcinoid syndrome | – | – | – | – | 2 (3) |
| Chronic pancreatitis/pancreas | 2 | – | – | – | 2 (3) |
| Pituitary tumors | – | – | – | 2 | 2 (3) |
| Othera/don’t know | 3 | – | 1 | 1 | 5 (6) |
Notes: Data represent n (%);
gastrointestinal cancer, cirrhosis, gastrointestinal bleeding, other endocrine disorders.
Abbreviations: GEP-NET, gastroenteropancreatic neuroendocrine tumor; SSA, somatostatin analog.
The nurses personally administered SSA injections to 514 patients. On a monthly basis (every 28 days), octreotide LAR was used by 62/77 (81%) nurses in 323/514 (63%) patients and lanreotide Autogel/Depot by 44/77 (57%) nurses in 93/514 (18%) patients. Most nurses (52/77; 68%) had not received guidelines on SSA injection procedures or devices. Of those who mentioned the existence of guidelines (n = 25), treatment protocols or injection instructions (n = 12; 48%) and guidelines provided by the manufacturer or pharmaceutical company (n = 9; 36%) were the most common.
The majority of nurses (53/77; 69%) had experienced clogging with SSAs during the last 3 years or had heard about such episodes from a colleague or patient; almost all episodes (440/443; 99%) occurred with octreotide LAR.
Current injection device attributes
Table 2 presents key attribute ratings for available SSA devices during the quantitative phase (from the list defined during the qualitative phase). Confidence that a full dose had been delivered received the highest mean score (9.5), followed by high product efficacy (9.4), good safety features (9.4), low risk of clogging (8.8), and easy/convenient preparation and injection (8.8). The three attributes ranked most often among the top five were easy/convenient preparation and injection (ranked by 70% of nurses), low clogging risk (58%), and high product efficacy (55%); those ranked least often among the top five were device transparency (14%), sturdy plunger (13%), and short/thin needle (13%).
Table 2.
Relative importance of somatostatin analog device attributes among 77 nurses
| Somatostatin analog device attribute | Mean score (scale: 1–10) | Proportion (%) of nurses who rated attribute 9 or 10 (scale: 1–10) | Proportion (%) of nurses who ranked attribute in top 5 |
|---|---|---|---|
| Confidence that a full dose has been delivered | 9.5 | 87 | 42 |
| High product efficacy | 9.4 | 84 | 55 |
| Good safety | 9.4 | 83 | 42 |
| Low risk of clogging | 8.8 | 73 | 58 |
| Easy/convenient preparation and injection | 8.8 | 70 | 70 |
| Easy to teach | 8.3 | 57 | 25 |
| Low risk of needle-stick injuries | 8.2 | 62 | 23 |
| Prefilled device | 8.1 | 49 | 30 |
| Fast administration (preparation/injection) | 7.9 | 43 | 36 |
| Calm environment for patient | 7.9 | 52 | 25 |
| Sturdy plunger | 7.9 | 40 | 13 |
| Transparent device | 7.8 | 44 | 14 |
| Comfortable to hold | 7.7 | 44 | 16 |
| Short/thin needle | 7.6 | 43 | 13 |
| Depth of injection (IM versus deep SC) | 7.2 | 44 | 16 |
| Self-injection possible | 6.8 | 36 | 19 |
Notes: Nurses were provided with a predefined list of device attributes and asked to rate each attribute on a scale of one to ten (one = not important at all; ten = most important).
Abbreviations: SC, subcutaneous; IM, intramuscular.
Device try-out and device-specific attributes and preferences
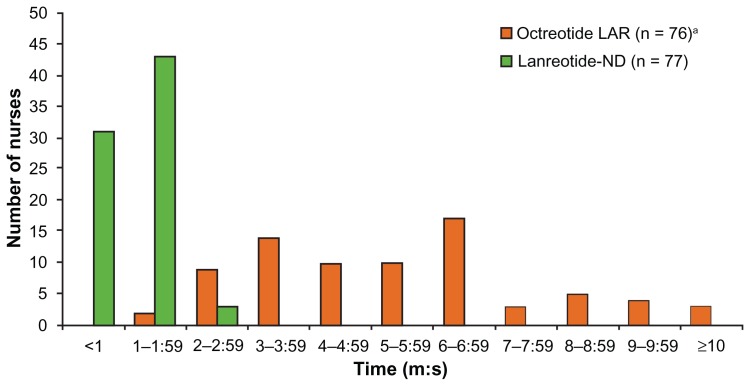
During the device try-out, injection preparation and administration time were significantly shorter with lanreotide-ND than octreotide LAR (mean [range]: 66 [28–140] versus 329 [106–812] seconds; P < 0.01). Figure 1 shows the distribution of times for both devices. Clogging occurred with two octreotide LAR, and no lanreotide-ND, injections. After trying lanreotide-ND, nurses spontaneously described its three most important characteristics as prefilled/ready to use (44%), automatic needle guard (35%), and device transparency (30%).
Figure 1.
Device try-out: time taken to prepare and administer test injections with octreotide LAR and lanreotide-ND.
Note:aOne nurse was unable to finalize the injection due to a clogging incident and was excluded.
Abbreviations: LAR, long-acting release; ND, new device.
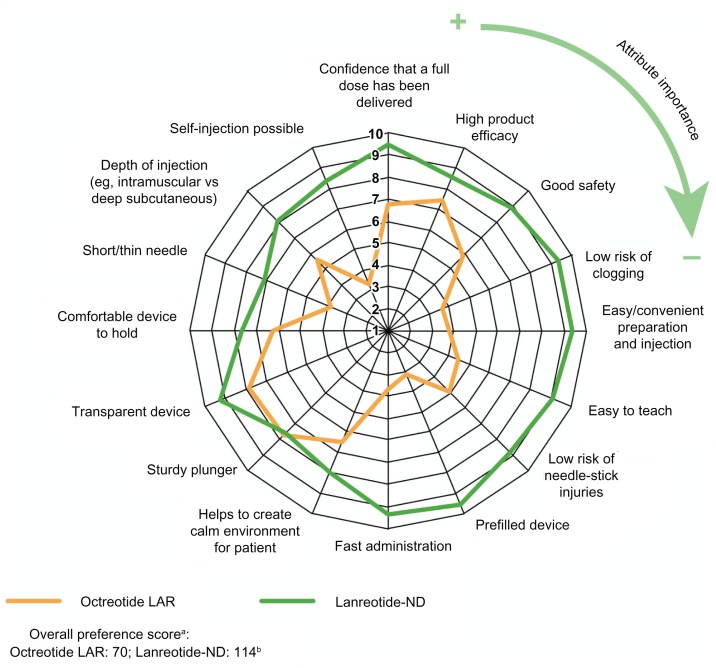
Mean evaluation scores were higher for lanreotide-ND than octreotide LAR for all 16 device attributes (P < 0.05) except plunger sturdiness (Figure 2). The highest-scoring attributes for lanreotide-ND were prefilled device and confidence that a full dose had been delivered (both 9.5), low clogging risk and easy/convenient preparation and injection (both 9.4), and fast administration (9.3).
Figure 2.
Evaluation of octreotide LAR and lanreotide-ND: mean evaluation scores for each device attribute (scale of one to ten).
Notes: Device attributes are labeled clockwise in order of importance to nurses (ie, confidence that a full dose has been delivered = most important and self-injection possible = least important) based on the mean evaluation score obtained in the first part of the interview. aAttributes weighted by importance to nurses; bdifference: P < 0.01.
Abbreviations: LAR, long-acting release; ND, new device.
The five main advantages of lanreotide-ND chosen from the predefined list of characteristics were the prefilled ready-to-use formula (87%), automatic needle guard (70%), potential for self-administration (64%), time for preparing and injecting (55%), and low injection volume (52%). When considering disadvantages, 25% mentioned the plunger being difficult to press, 25% found the plunger lacked sturdiness, 21% were concerned with needle thickness/size, and 16% found the device big/bulky; 12% reported no disadvantages.
When questioned directly, 90% (69/77) of nurses considered administration time an advantage of lanreotide-ND, citing seeing more patients (49%) and having more time to talk to patients (45%) as benefits of the time saved. The opportunity to perform blood tests and blood pressure measurements (9%), make telephone calls (9%), administer other injections/treatments (7%), and perform other clinic tasks (6%) were also mentioned.
The overall preference score was 63% higher for lanreotide-ND than for octreotide LAR (114 versus 70, respectively; P ≤ 0.01).
Discussion
This study, the first to evaluate preference for SSA devices among nurses in Europe and the USA, found that ease of administration, low clogging risk, and product efficacy were the most important device factors. Preparation and administration of lanreotide-ND was significantly shorter than that for octreotide LAR, with potential time-saving implications for clinical practice. When the two injection devices were evaluated via questionnaire, lanreotide-ND received a significantly higher evaluation than octreotide LAR.
The majority of nurses considered ease of administration one of the most important attributes of an SSA delivery device, and nearly one-quarter considered it the most important. In a similar evaluation of devices for subcutaneous administration of human growth hormone, “ease of use” and “number of steps in preparation” were ranked among the five most important device attributes by physicians, nurses, patients, and parents,7 underscoring the importance of simple, efficient drug delivery systems. In the present study, the majority of nurses had previously experienced clogging during SSA delivery, particularly with octreotide LAR, and low clogging risk was ranked among the five most important device attributes along with high product efficacy, both mentioned by over half the nurses. Confidence that a full dose had been delivered and safety features were also ranked highly, while physical features of the device (transparency, needle size, and plunger sturdiness) were least important.
Recently, early phase trials of an oral formulation of octreotide, Octreolin™, demonstrated therapeutic levels of octreotide and effective growth hormone suppression in patients with acromegaly, raising the possibility of a twice-daily oral form of octreotide.11 The results of an ongoing Phase III safety and efficacy trial should indicate whether this is a viable therapeutic option. Patient opinions on the desirability of a once-monthly injection versus a twice-daily tablet will be highly relevant if the drug is approved.
During a test injection, participants took, on average, five times longer (>4 minutes longer) to prepare and inject octreotide LAR than lanreotide-ND. The present data accord with those of other studies with SSAs,12 and other injected therapies such as luteinizing hormone-releasing hormone agonists,6 finding that significantly less time was needed to reconstitute and inject the therapeutic agent when using a ready-to-use depot system than syringe and vial. A European cost–consequence study based on administration time and clogging data from the present study suggests these factors may contribute to cost savings with prefilled devices such as lanreotide-ND.13
Lanreotide-ND scored significantly higher than octreotide LAR on all device attributes except for plunger sturdiness, with one-quarter of participants mentioning difficulties pressing the plunger or apparent lack of sturdiness. There was also a significant difference in mean score for ease/convenience of preparation and injection, ranked among the most important attributes by 70% of nurses, for lanreotide-ND (9.4) and octreotide LAR (3.8). Nurses appreciated the shorter administration time with lanreotide-ND, which freed them up to perform other clinical tasks, and proposed it would also be appreciated by patients.
There are several study limitations to consider. First, there is the potential for selection bias due to nurses’ voluntary participation and results may be influenced by practice differences between hospital nurses and community nurses (represented by fewer participants). Second, questionnaire results may have been influenced by cultural or linguistic differences between countries. Finally, conclusions based on the injection simulation, which differs from real-life clinical practice, should be interpreted accordingly.
Conclusion
The most important device attributes for nurses performing SSA injections were ease of administration, safety (including low risk of clogging), and product efficacy. Device features such as needle size and plunger sturdiness were considered less important. The new SSA device was well accepted by interviewees, who appreciated the shorter preparation and injection time with lanreotide-ND and expressed an overall preference for this new device over octreotide LAR. Conceivably, the short administration time, confidence that a full dose has been delivered, and perceived ease-of-use of the new device could lead to improvements in clinical practice, and provide benefit to patients and caregivers when administering SSAs at home. Further studies are needed to confirm these findings in a clinical setting.
Acknowledgments
Operational implementation of the study was delegated by the sponsor to a contract research organization (Cegedim Strategic Data, Boulogne Billancourt, France), which was responsible for designing the study protocol, recruiting participating nurses, data collection and management, and statistical analysis. The authors wish to thank Sarah Hopwood, PhD (Scinopsis Medical Writing) and Helen Marshall (Watermeadow Medical) for editing assistance in the preparation of this manuscript, funded by Ipsen Pharma. This study was sponsored by Ipsen Pharma.
Footnotes
Disclosure
Daphne Adelman is currently a member of the Speaker’s Bureau and Nurse Advisory Board for Somatuline, and a member of the Pfizer Nurse Advisory Board. Andrea Burgess and Philippa Davies declare no conflicts of interest in this work.
References
- 1.Melmed S. Medical progress acromegaly. N Engl J Med. 2006;355(24):2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 2.Grozinsky-Glasberg S, Grossman AB, Korbonits M. The role of somatostatin analogues in the treatment of neuroendocrine tumours. Mol Cell Endocrinol. 2008;286(1–2):238–250. doi: 10.1016/j.mce.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Somatuline® Depot (lanreotide) injection [prescribing information] Paris: Ipsen Pharma; 2011. [Google Scholar]
- 4.Biermasz NR, van den Oever NC, Frolich M, et al. Sandostatin LAR in acromegaly: a 6-week injection interval suppresses GH secretion as effectively as a 4-week interval. Clin Endocrinol (Oxf) 2003;58(3):228–295. doi: 10.1046/j.1365-2265.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- 5.Turner HE, Thornton-Jones VA, Wass JA. Systematic dose-extension of octreotide LAR: the importance of individual tailoring of treatment in patients with acromegaly. Clin Endocrinol (Oxf) 2004;61(2):224–231. doi: 10.1111/j.1365-2265.2004.02084.x. [DOI] [PubMed] [Google Scholar]
- 6.Morgan G, Cooley C. Injection systems for two luteinising hormone-releasing hormone agonists: a comparative assessment of administration times and nurses’ perceptions. Eur J Oncol Nurs. 2005;9(4):334–340. doi: 10.1016/j.ejon.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Dumas H, Panayiotopoulos P, Parker D, Pongpairochana V. Understanding and meeting the needs of those using growth hormone injection devices. BMC Endocr Disord. 2006;6:5. doi: 10.1186/1472-6823-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novartis AG. Sandostatin® LAR®: mixing. 2012. [Accessed September 4, 2012]. Available from: http://www.sandostatin.com/about-sandostatin-lar/sandostatin-lar-mixing.jsp?lightbox=global-hcp.
- 9.Bevan JS, Newell-Price J, Wass JA, et al. Home administration of lanreotide Autogel by patients with acromegaly, or their partners, is safe and effective. Clin Endocrinol (Oxf) 2008;68(3):343–349. doi: 10.1111/j.1365-2265.2007.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvatori R, Nachtigall LB, Cook DM, et al. Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naive patients with acromegaly. Pituitary. 2010;13(2):115–122. doi: 10.1007/s11102-009-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuvia S, Atsmon J, Teichman SL, et al. Oral octreotide absorption in human subjects: comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J Clin Endocrinol Metab. 2012;97(7):2362–2369. doi: 10.1210/jc.2012-1179. [DOI] [PubMed] [Google Scholar]
- 12.Schweinsberg K, Smith S, Kirshner LS. Ease of administration of somatostatin analogs, octreotide LAR versus lanreotide [abstract] Endocr Rev. 2011;32(03_Meeting Abstracts):P1–P451. [Google Scholar]
- 13.Marty R, Roze S, Kurth H. Decision-tree model for health economic comparison of two long-acting somatostatin receptor ligand devices in France, Germany, and the UK. Medical Devices (Auckl) 2012;5:39–44. doi: 10.2147/MDER.S30913. [DOI] [PMC free article] [PubMed] [Google Scholar]